The new role of 1,1-diamino-2,2-dinitroethylene (FOX-7): two unexpected reactions†
Tianhong
Zhou
a,
Yanfeng
Li
a,
Kangzhen
Xu
 *ab,
Jirong
Song
a and
Fengqi
Zhao
c
*ab,
Jirong
Song
a and
Fengqi
Zhao
c
aSchool of Chemical Engineering, Northwest University, Xi'an 710069, China. E-mail: xukz@nwu.edu.cn; Tel: +86 29 88307755
bDepartment of Electrical and Computer Engineering, Missouri of University, Columbia 65211, USA
cXi'an Modern Chemistry Research Institute, Xi'an 710065, China
First published on 22nd November 2016
Abstract
Two novel derivatives of FOX-7, 2-methyl-5-nitro-1,2,3-triazole-4-amine (MNTzA) and 1,5-bis(1-amino-2,2-dinitrovinyl)carbonohydrazide (BADCh), were synthesized by two unexpected reactions of FOX-7 and hydrazino compounds, and the two reaction processes were analyzed. The reaction to form MNTzA is unusual among the syntheses of 2,4,5-trisubstituted 1,2,3-triazole compounds. The structures of the two compounds were studied by NMR, single crystal X-ray crystallography and theoretical calculations. MNTzA crystallizes in the monoclinic P2(1)/n space group with four molecules per unit cell, and each molecule exhibits good coplanarity. However BADCh shows serious structural distortion giving it a ‘W’ shape and possesses two highly polarized C–C double bonds. The DSC analyses and detonation properties of the two compounds were compared with those of FOX-7 and RDX, and the results indicate that MNTzA is an available intermediate for the synthesis of other complicated 2,4,5-trisubstituted 1,2,3-triazole compounds, and BADCh is an excellent energetic material and exhibits good thermal stability (thermal decomposition peak temperature >250 °C), with lower sensitivity (impact sensitivity >19.6 J and friction sensitivity 16%) and similar detonation properties (detonation pressure 32.1 GPa and detonation velocity 8.6 km s−1) when compared to FOX-7 and RDX.
1. Introduction
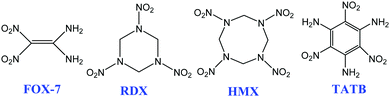
The design and synthesis of new high-energy insensitive compounds is still an important research direction in energetic materials.1 1,1-Diamino-2,2-dinitroethylene (FOX-7), as a new high-energy insensitive material, has been considered for applications as a component of insensitive ammunition and solid propellants in the future, possessing a density of 1.885 g cm−3, a heat of formation of 133.7 kJ mol−1, the same insensitivity as 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) and a similar energy-density to 1,3,5-trinitro-1,3,5-triazinane (RDX) and cyclotetramethylenetetranitramine (HMX) (Scheme 1).2 Much research on FOX-7 has been reported every year since it was first synthesized by Latypov in 1998.2a,3 Recently, Zhou et al.4 studied the mechanical anisotropy of the FOX-7 crystal, Lempert et al.5 explored the energy potential of solid composite propellants based on FOX-7 and Wang et al.6 also studied the configuration and stability of FOX-7 embedded in graphene.FOX-7 is a special compound due to its surprising chemical reactivity. Though the molecular composition and structure of FOX-7 are very simple, its chemical reactivity is abundant, including acid–base reactions, coordination reactions, nucleophilic substitution reactions, acetylation reactions, oxidation reactions, reduction reactions and electrophilic addition reactions.7 More than 130 derivative compounds of FOX-7 have been reported through the above reactions. These reactions were systematically summarized and analyzed in two reviews just published this year.7
Lately, Vo et al. reported their exciting discovery of the first cations based on FOX-7 and its derivative 1-amino-1-hydrazino-2,2-dinitroethylene, and the amphoteric properties of the two compounds were demonstrated.8 Gao et al. also reported some complicated multinitro derivatives of FOX-7, and those compounds exhibit good thermal stabilities, high densities, positive HOFs, acceptable oxygen balances and excellent detonation properties.9 He et al. theoretically studied two potential derivatives of FOX-7, 5-(dinitromethylene)-1,4-dinitramino-tetrazole (NNAT) and 1,1′-dinitro-4,4′-diamino-5,5′-bitetrazole (DNABT), and found they all present good detonation properties.10 The study of the reactivity of FOX-7 is still an active field in energetic materials.
In this paper, we report two unexpected reactions of FOX-7, and the structures and properties of the two reaction products have been meticulously studied. This work further enriches the research on FOX-7.
2. Experimental section
2.1. Materials
FOX-7 was obtained from the Xi'an Modern Chemistry Research Institute, and its purity was over 99%. Methylhydrazine aqueous solution and carbohydrazide were purchased from commercial sources and used as received.2.2. Physical measurements
NMR spectra were recorded with a AV500 NMR spectrometer (Bruker). IR spectra were determined on a Nexus 870 (Thermo Nicolet) with KBr pellets. Elemental analyses were performed on a Vario EL III elemental analyzer (Elementar). MS spectra were recorded on a GCMS-QP2010 mass spectrometer (Shimadzu). SEM images were recorded using a Zeiss Sigma scanning electron microscope. DSC experiments were performed using a DSC200 F3 apparatus (Netzsch, Germany) under a nitrogen atmosphere at a flow rate of 80 mL min−1. TG–DTG experiments were performed using SDT-Q600 apparatus (TA, USA) under a nitrogen atmosphere at a flow rate of 100 mL min−1. The combustion energy was determined by using an IKA C5000 oxygen-bomb calorimeter (Germany). The impact and friction sensitivities were determined by using a ZBL-B impact sensitivity instrument (Nachen, China) and an MGY-2 friction sensitivity instrument (Nachen, China), respectively. The mass of the drop hammer was 5.0 kg. The swing angle and gauge pressure were 50° and 0.6 MPa. The mass for each test was 30 mg. All other reagents were purchased from commercial sources and used without further purification.2.3. Crystal structure determinations
Single-crystal X-ray diffraction data were collected on a Bruker Smart APEX CCD X-ray diffractometer using graphite-monochromated Mo-Kα radiation (λ = 0.071073 nm). The structures were solved by direct methods (SHELXTL-97) and refined by the full-matrix-block least-squares method on F2 with anisotropic thermal parameters for all non-hydrogen atoms.11 The hydrogen atoms were added according to the theoretical models.2.4. Theoretical calculation investigation
A molecular unit of BADCh generated with the Chem3D software was optimized by minimizing its energy with the MOPAC software repeatedly until it did not change, and this was then selected as the initial model, while the B3LYP/6-31+G method in the Gaussian 03 package was used to further optimize the structure and compute its vibrational frequencies at different temperatures.12 Meanwhile, the optimized parameters were further used to compute the molecular volume 100 times, and the average value was regarded as the credible molecular volume. All the convergence criteria were the system default values, and all the calculations were carried out on a Lenovo T260 server.2.5. Preparation
2-Methyl-5-nitro-1,2,3-triazole-4-amine (MNTzA) was prepared according to the following method: FOX-7 (0.02 mol, 2.96 g) was dispersed in 12 mL of water and to it methylhydrazine aqueous solution (about 0.05 mol, 18 mL) was added dropwise. After reacting with stirring at 100 °C for 1.5 h, the resulting solution was slowly cooled to ambient temperature, then diluted with water, filtered, washed with water and dried under vacuum to obtain pale yellow crystals of MNTzA. These pale yellow crystals appear regularly rodlike as shown in Fig. 1. Yield 1.78 g (62%). M.p. 167–169 °C. IR (KBr, ν, cm−1), 3440(vs), 3321(vs), 3237(w), 3188(m), 3047(w), 2685(w), 1643(vs), 1574(vs), 1473(w), 1448(w), 1354(m), 1299(s), 1207(m), 1114(w), 1070(m), 866(m). 13C NMR (DMSO, 500 MHz, δ, ppm) 149.745, 137.845, 43.472. 1H NMR (DMSO, 500 MHz, δ, ppm) 4.057, 6.773. MS (m/z, %) 143 (M+, 60), 53 (M+, 35), 43 (M+, 100), 30 (M+, 30). Anal. calcd for C3H5O2N5 (%): C 25.18, N 48.94, H 3.52. Found C 25.11, N 48.87, H 3.59.1,5-Bis(1-amino-2,2-dinitrovinyl)carbonohydrazide (BADCh) was prepared according to the following method: FOX-7 (0.01 mol, 1.48 g) was dispersed in 30 mL of H2O and to it carbohydrazide (0.02 mol, 1.82 g) was added. After reacting at 105 °C for 28 h, the resulting solution was slowly cooled to ambient temperature and diluted with the proper amount of water. Many brown solids were formed, which were filtered, washed with water, and dried under vacuum, giving a yield of 0.472 g (26.8%). Fig. 1 shows that the crystalline solids of BADCh have an irregular blocky structure. Our attempts to make single crystals all failed. 1H NMR (DMSO, 400 MHz, ppm), δ = 8.788, 3.346, 2.509. 13C NMR (DMSO, 400 MHz, ppm), δ = 43.477, 137.005, 149.745. IR (KBr) ν: 3421, 2360, 1635 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1396, 1132, 752, 619 cm−1. MS [m/z (%), 351 (M+, 30), 307 (M+, 100), 263 (M+, 35), 105 (M+, 30)]. Anal. calcd for C5H8N10O9: C 17.05, H 2.290, N 39.77%; found: C 17.09, H 2.263, N 39.49%.
O), 1396, 1132, 752, 619 cm−1. MS [m/z (%), 351 (M+, 30), 307 (M+, 100), 263 (M+, 35), 105 (M+, 30)]. Anal. calcd for C5H8N10O9: C 17.05, H 2.290, N 39.77%; found: C 17.09, H 2.263, N 39.49%.
3. Results and discussion
3.1. Synthesis of MNTzA
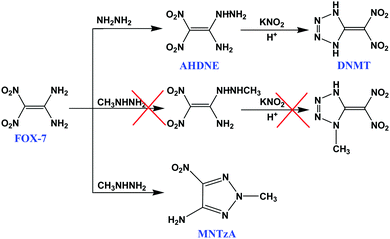
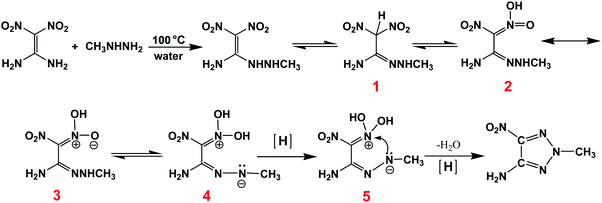
1-Amino-1-hydrazino-2,2-dinitroethylene (AHDNE), as a nucleophilic substitution derivative of FOX-7, exhibits high reactivity due to its special adjacent amino and hydrazino groups.7b,13AHDNE can react with potassium nitrite in organic acid solution to synthesize a nitrogen-rich compound, 5-(dinitromethylene)-tetrazole (DNMT) (Scheme 2).14 However, AHDNE and DNMT are unstable, and AHDNE can even self-explode or self-ignite at ambient temperature.9,13b,c Therefore we intended to substitute methylhydrazine for hydrazine to prepare a stable energetic compound. However, the results were unexpected. The intended compound was not obtained, but an unusual 2,4,5-trisubstituted 1,2,3-triazole compound, 2-methyl-5-nitro-1,2,3-triazole-4-amine, (MNTzA) was, surprisingly, synthesized (Scheme 2). 1,2,3-Triazole compounds have been extensively reported every year since the publication of “Click Chemistry” by Sharpless in 2001,15 and the main “Click Reaction” (cycloaddition) is the use of azides and acetylides with the action of catalysts to obtain 1,2,3-triazole or tetrazole compounds. However, obtaining 1,4,5-trisubstituted or 2,4,5-trisubstituted 1,2,3-triazole compounds needs much more complicated multistep reactions and the yields are very low.16 However, a 2,4,5-trisubstituted 1,2,3-triazole compound can be easily synthesized as described in this study, without involving “Click Chemistry”. Though the compound was reported by Zhang et al. in 2013,17 their synthesis method was very complicated and needed reactions with about 13 steps, using acetic anhydride as the initial raw material. MNTzA is not a complicated compound, but its amino group, nitro group and methyl group are active groups and can be further substituted or reacted to prepare other complicated 1,2,3-triazole compounds.17 The reaction to form MNTzA may be a good way to synthesize complicated 2,4,5-trisubstituted 1,2,3-triazole compounds, and MNTzA may be an available intermediate for synthesizing other complicated 2,4,5-trisubstituted 1,2,3-triazole compounds.The conjectural reaction process for MNTzA is shown in Scheme 3. Firstly, FOX-7 reacts with methylhydrazine to form an unstable intermediate, 1-amino-1-methylhydrazino-2,2-dinitroethylene. Secondly, 1-amino-1-methylhydrazino-2,2-dinitroethylene can exist in a variety of tautomers and resonances (1–3).3a–c Thirdly, with the action of the electron-donating CH3– group, the H atom in –NH– dissociates to the adjacent O− atom, so the middle N atom of the methylhydrazino group exhibits obvious electronegativity (4). Finally, the N− atom with its lone pair of electrons attacks the adjacent N+ atom to form a stable 1,2,3-triazole ring in the strongly reducing environment of methylhydrazine, along with the loss of the two hydroxyl groups to form water.
3.2. Synthesis of BADCh
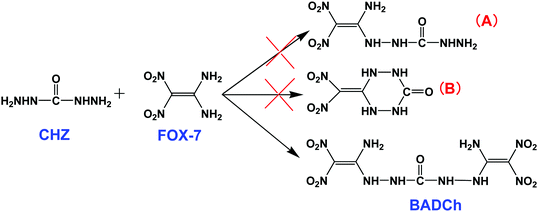
Based on the nucleophilic reactions of FOX-7,7a carbohydrazide (CHZ) was used as a nucleophile to prepare a new energetic compound. The results indicate that only the seemingly symmetrical compound 1,5-bis(1-amino-2,2-dinitrovinyl)carbonohydrazide (BADCh) was formed, through the reaction of one CHZ molecule and two FOX-7 molecules with a yield of 26.8%, but the other two expected compounds (A and B) were not found, regardless of how the reaction conditions were changed (Scheme 4). Compound B is attractive, and we ever doubted that the product would be compound B, because the compound possesses a stable six-membered ring and should have a big competitive advantage compared to the other two compounds. This rule was also found to hold for other double nucleophilic substitution derivatives.2b,7a However, the results of the 1H NMR (three chemical shifts: 8.788, 3.346, 2.509 ppm) and MS [351 (M+, 30)] investigations contradict our guess, and BADCh is the only product. Our attempts to obtain compound B all failed.3.3. Structural analysis of MNTzA
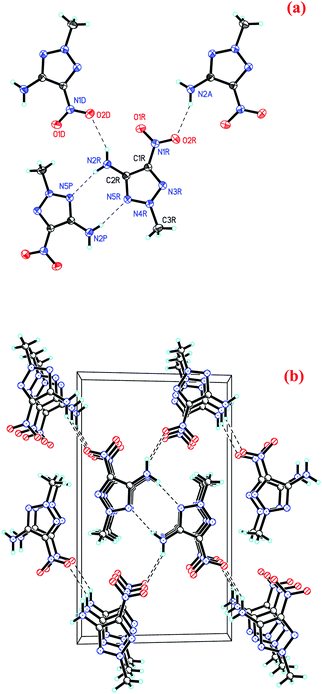
Single crystals of MNTzA were obtained when the reaction solution was cooled very slowly. The orderly rodlike crystals of MNTzA are pale yellow (Fig. 1), and their structures were found by single crystal X-ray crystallography. MNTzA crystallizes in the monoclinic P2(1)/n space group with four molecules per unit cell (ESI†). The structure of MNTzA is simple, consisting of a triazole ring and three small substituent groups as shown in Fig. 2(a). From selected bond lengths, the N2–C2 bond (1.346 Å) is a typical conjugated N–C double bond, but the N4–C3 bond (1.456 Å) is a typical C–N single bond. In the triazole ring, the N3–N4 bond (1.299 Å), N3–C1 bond (1.341 Å), N4–N5 bond (1.348 Å), N5–C2 bond (1.339 Å) and C1–C2 bond (1.403 Å) are all conjugated double bonds. Moreover, the N2–C2 bond is also involved in conjugation with the triazole ring to form a larger conjugated system, which reduces the energy and increases the stability of the molecule. From selected bond angles (N4–N3–C1, 102.667°; N3–N4–N5, 116.56°; N4–N5–C2, 103.82°; N3–C1–C2, 110.37° and N5–C2–C1, 106.58°), it can be seen that the triazole ring was greatly affected by the substituent groups, especially the amino group. Despite of the influence of the substituent groups, all the non-hydrogen atoms in the triazole ring and the three substituent groups have good coplanarity. The two biggest torsion angles are 177.86° (C1–N3–N4–C3) and −177.75° (C3–N4–N5–C2), and the two smallest are 0° (N4–N5–C2–C1) and −0.3° (N3–N4–N5–C2). Each MNTzA molecule contacts with two of the surrounding molecules by four hydrogen-bond interactions [of only two kinds; N2–H⋯N5#1 (d(D⋯A) = 3.102 Å, ∠DHA = 168.06°), and N2–H⋯O2#2 (d(D⋯A) = 3.313 Å, ∠DHA = 142.65°); symmetry transformations: #1: −x + 1, −y, −z + 2; #2: x + 1/2, −y + 1/2, z + 1/2] (Fig. 2(a)), which infinitely extend out to form an ordered 3D packing of MNTzA as shown in Fig. 2(b).3.4. Theoretical calculation results of BADCh
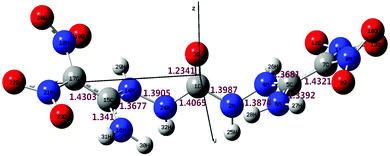
Analysis of the vibrational frequencies showed that the optimized structure lies at a minimum point on the potential energy surface, proving that the optimized structure is stable and the calculation results are reliable.12 The optimized structure of BADCh and some selected bond lengths are shown in Fig. 3. One CHZ molecule connects with two FOX-7 molecules by nucleophilic substitution to form a new bigger molecule with the loss of two amino groups. The new molecule (BADCh) shows serious structural distortion giving it a ‘W’ shape around the central CHZ group, which is very different from the similar but planar structure of the FOX-7 molecule and its highly ordered single-layer spatial construction.18 The structural distortion can also be supported by seemingly symmetric chemical bonds, such as bonds C5–C7 (1.431 Å) and C15–C17 (1.430 Å), C1–N24 (1.407 Å) and C1–N2 (1.399 Å), N14–N24 (1.391 Å) and N2–N4 (1.387 Å), and C15–N14 (1.368 Å) and C5–N4 (1.368 Å) (see ESI†). From the bond lengths, bonds C5–C7 and C15–C17 are still conjugated double bonds. Bonds C15–N14, C15–N16, C5–N4 and C5–N6 are conjugated double bonds. Comparing these with the related bond lengths of CHZ [C–N (1.348 Å) and N–N (1.411 Å)],19 the C–N bond was elongated, but the N–N bond was shortened in BADCh. Therefore, a large conjugated system forms from C7 to C17 including all the atoms in between, which should be the main reason for BADCh having better thermal stability than FOX-7. The distribution of the atomic net charges indicates that C5 (0.7581 e) and C15 (0.6476 e) have more positive charges than C7 (0.1377 e) and C17 (0.2604 e), also indicating that BADCh possesses two highly polarized C–C double bonds. The carbonyl group reduces the activities of the N–N bond and C–N bond on both sides, which may be another reason for BADCh having better thermal stability than FOX-7.3.5. DSC analysis
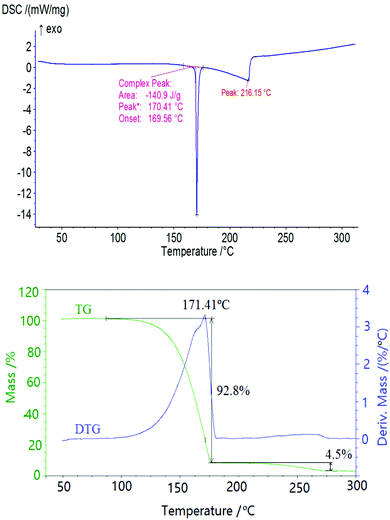
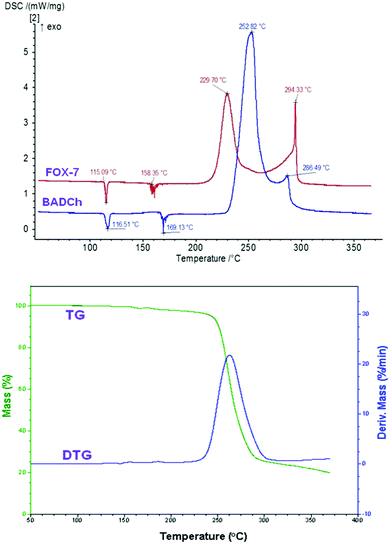
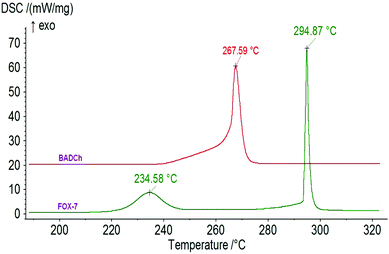
The typical DSC and TG/DTG curves in Fig. 4 indicate that the thermal behavior of MNTzA presents two obvious stages. The first is an intense endothermic melting-decomposition process with a mass loss of about 92.8%, and the onset temperature, peak temperature and decomposition enthalpy at a heating rate of 10 °C min−1 are 169.56 °C, 170.41 °C and 140.9 J g−1, respectively. The second is a slow endothermic decomposition process with a mass loss of only about 4.5%. The final residue at 300 °C is about 3.3%. No exothermic process can be found in the whole DSC curve of MNTzA. The introduction of a methyl group and the loss of a nitro group make the thermal behavior of the compound change greatly, compared with that of the parent compound (FOX-7) in Fig. 5.Fig. 5 indicates that the thermal behavior of BADCh can be divided into four stages. The first two stages are crystal phase transition processes, and the peak temperatures are 116.51 and 169.13 °C at the heating rate of 5.0 °C min−1. The last two stages are a continuous exothermic decomposition process with a mass loss of about 76%, and the peak temperatures are 252.82 and 286.49 °C at the heating rate of 5.0 °C min−1. It is surprising that the thermal behavior of BADCh is very similar to that of FOX-7 including two crystal phase transition processes, and the difference between the two compounds is that the thermal decomposition temperature of BADCh is higher than that of FOX-7 (229.70 and 294.33 °C). The same phenomenon can also be found in another derivative of FOX-7, 1-amino-1-(2,4-dinitrophenylhydrazinyl)-2,2-dinitroethylene (APHDNE),20 indicating that the 1-amino-2,2-dinitrovinyl group plays a key role in these molecules. The sequence of thermal stability for the three compounds is BADCh > APHDNE > FOX-7. The introduction of a stable group can raise the thermal stability of a compound. Moreover, the two continuous exothermic decomposition processes of BADCh can easily become one large and wide exothermic process with an increase in the heating rate (10 °C min−1), but FOX-7 still presents two exothermic decomposition processes (Fig. 6).
A multiple heating method was employed to obtain the kinetic parameters [the apparent activation energy (E) and pre-exponential factor (A)]. The DSC data and results obtained by the Kissinger method and Ozawa method for the decomposition process of BADCh are listed in Table 1.21 The apparent activation energy obtained by the Kissinger method agrees well with that by the Ozawa method. The linear correlation coefficients (r) are very close to 1. Therefore, the results are credible. Moreover, the apparent activation energy is low, indicating that BADCh easily decomposes at high temperatures.
| β/(°C min−1) | T e/°C | T p/°C | E k/(kJ mol−1) | log(Ak/s−1) | r k | E O/(kJ mol−1) | r O |
|---|---|---|---|---|---|---|---|
| Subscript k indicates data obtained by the Kissinger method, subscript O indicates data obtained by the Ozawa method. | |||||||
| 5.0 | 235.1 | 252.8 | 113.6 | 8.90 | 0.9905 | 116.5 | 0.9918 |
| 7.5 | 238.8 | 260.8 | |||||
| 10.0 | 255.3 | 267.6 | |||||
| 12.5 | 257.7 | 269.7 | |||||
The self-accelerating decomposition temperature (TSADT) and critical temperature of thermal explosion (Tb) are two important parameters required to ensure safe storage and process operations for energetic materials and then to evaluate their thermal stability. TSADT and Tb for BADCh are calculated as 213.7 and 222.1 °C respectively,22 which are much higher than those for FOX-7, 206.0 and 207.1 °C,3g also indicating that BADCh has higher thermal stability than FOX-7.
3.6. Detonation properties
The detonation properties of MNTzA and BADCh and the properties of FOX-7 and RDX for comparison are summarized in Table 2.2d,8MNTzA, as an energetic material, exhibits bad detonation properties when compared with FOX-7 and RDX, despite having a high nitrogen content (48.9%) and good insensitivity (>16.9 J, 24%). The reason should be the loss of one nitro group and the introduction of a methyl group, which make the energy and density of the compound decline greatly (the crystal density declines from 1.88 g cm−3 for FOX-7 to 1.58 g cm−3 for MNTzA).18 However, the detonation properties of BADCh are good, where the detonation velocity and detonation pressure are close to those of FOX-7 and RDX, but the sensitivity is much lower than that of RDX.| Sample | M /g mol−1 | T m /°C | d /g cm−3 | OBd/% | −ΔcHc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e/kJ g−1 e/kJ g−1 |
ΔfH0m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) f/kJ mol−1 f/kJ mol−1 |
ISg/J | FSh/% | P /GPa | D /km s−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| a Molecular mass. b Melting point. c Crystal density. d Oxygen balance. e Combustion energy. f Enthalpy of formation. g Impact sensitivity. h Friction sensitivity. i Detonation pressure based on the Kamlet–Jacobs (K–J) equations. j Detonation velocity based on the Kamlet–Jacobs (K–J) equations. k Theoretical calculation result. | ||||||||||
| MNTzA | 143.12 | 167.8 | 1.58 | −72.7 | 12.38 | −130.1 | 16.9 | 24 | 20.7 | 7.1 |
| BADCh | 352.18 | — | 1.75k | −22.7 | 8.11 | −260.9 | 19.6 | 16 | 32.1 | 8.6 |
| FOX-7 | 148.08 | — | 1.88 | −21.6 | 7.83 | −133.7 | 24.7 | 10 | 36.1 | 8.3 |
| RDX | 222.12 | 204.5 | 1.82 | −21.6 | 9.55 | 96.2 | 7.4 | 76 | 33.7 | 8.7 |
4. Conclusions
In conclusion, we have reported two new reactions of FOX-7 in this paper, and the two reaction products, 2-methyl-5-nitro-1,2,3-triazole-4-amine (MNTzA) and 1,5-bis(1-amino-2,2-dinitrovinyl)carbonohydrazide (BADCh), are unexpected. The reaction to form MNTzA is unusual among the syntheses of 2,4,5-trisubstituted 1,2,3-triazole compounds, and may be a suitable way to synthesize other complicated 2,4,5-trisubstituted 1,2,3-triazole compounds without involving well-known “Click Chemistry”. The 1-amino-2,2-dinitrovinyl group plays the key role in the thermal behavior of the compound, but the introduction of a methyl group and the loss of a nitro group make the thermal behavior of FOX-7 change greatly. MNTzA is not a good energetic compound due to its bad detonation properties, but BADCh still exhibits excellent thermal stability, low sensitivity and similar detonation properties to FOX-7 and RDX and has good potential for application as an energetic material. This work further enriches the reactivity of FOX-7.Acknowledgements
This investigation received financial assistance from the National Natural Science Foundation of China (No. 21673178 and 21241003) and the Chinese Scholarship Council (No. 201606975010).References
- (a) D. M. Badgujar, M. B. Talawar, S. N. Asthana and P. P. Mahulikar, Advances in Science and Technology of Modern Energetic Materials: An Overview, J. Hazard. Mater., 2008, 151, 289 CrossRef CAS PubMed; (b) H. Gao and J. M. Shreeve, Azole-based Energetic Salts, Chem. Rev., 2011, 111, 7377 CrossRef CAS PubMed.
- (a) V. N. Latypov and J. Bergman, Synthesis and Reactions of 1,1-Diamino-2,2-Dinitroethylene, Tetrahedron, 1998, 54, 11525 CrossRef; (b) A. J. Bellamy, P. Goede, C. Sandberg and N. V. Latypov, Substitution Reactions of 1,1-Diamino-2,2-Dinitroethylene (FOX-7), The Proceedings of the 33th International Annual Conference ICT, Karlsruhe, Germany, 2002; (c) B. Buszewski, M. Michel, S. Cudzilo and Z. Chylek, High Performance Liquid Chromatography of 1,1-Diamino-2,2-dinitroethene and Some Intermediate Products of Its Synthesis, J. Hazard. Mater., 2009, 164, 1051 CrossRef CAS PubMed; (d) Y. D. Tian, F. Q. Zhao and J. H. Liu, Handbook of Energetic Materials and the Related Compounds, National Defense Industry Press, Beijing, 2011 (in Chinese) Search PubMed.
- (a) G. Hervé, G. Jacob and N. Latypov, The Reactivity of 1,1-Diamino-2,2-dinitroethene (FOX-7), Tetrahedron, 2005, 61, 6743 CrossRef; (b) M. Anniyappan, M. B. Talawar, G. M. Gore, S. Venugopalan and B. R. Gandhe, Synthesis, Characterization and Thermolysis of 1,1-Diamino-2,2-dinitroethylene (FOX-7) and Its Salts, J. Hazard. Mater., 2006, 137, 812 CrossRef CAS PubMed; (c) G. Hervé, G. Jacob and N. Latypov, Novel Illustrations of the Specific Reactivity of 1,1-Diamino-2,2-dinitroethene (DADNE) Leading to New Unexpected Compounds, Tetrahedron, 2007, 63, 953 CrossRef; (d) C. R. Chang, K. Z. Xu, J. R. Song, B. Yan, H. X. Ma, H. X. Gao and F. Q. Zhao, Preparation, Crystal Structure and Theoretical Calculation of 1-Amino-1-hydrazino-2,2-dinitroethene, Acta Chim. Sin., 2008, 66, 1399 CAS; (e) K. Z. Xu, J. R. Song, F. Q. Zhao, H. X. Ma, H. X. Gao, C. R. Chang, Y. H. Ren and R. Z. Hu, Thermal Behavior, Specific Heat Capacity and Adiabatic Time-to-Explosion of G(FOX-7), J. Hazard. Mater., 2008, 158, 333 CrossRef CAS PubMed; (f) K. Z. Xu, F. Wang, Y. H. Ren, W. H. Li, F. Q. Zhao, C. R. Chang and J. R. Song, Structural Characterization and Thermal Behavior of 1-Amino-1-methylamino-2,2-dinitroethylene, Chin. J. Chem., 2010, 28, 583 CrossRef CAS; (g) H. X. Gao, F. Q. Zhao, R. Z. Hu, Q. Pan, B. Z. Wang, X. W. Yang, Y. Gao, S. L. Gao and Q. Z. Shi, Thermochemical Properties, Thermal Behavior and Decomposition Mechanism of 1,1-Diamino-2,2-dinitroethylene (DADE), Chin. J. Chem., 2006, 24, 177 CrossRef CAS; (h) S. Garg, H. X. Gao, Y. H. Joo, D. A. Parrish, Y. Huang and J. M. Shreeve, Taming of the Silver FOX, J. Am. Chem. Soc., 2010, 132, 8888 CrossRef CAS PubMed; (i) T. T. Vo, D. A. Parrish and J. M. Shreeve, 1,1-Diamino-2,2-dintroethene (FOX-7) in Copper and Nickel Diamine Complexes and Copper FOX-7, Inorg. Chem., 2012, 51, 1963 CrossRef CAS PubMed; (j) Z. Gao, J. Huang, K. Z. Xu, W. T. Zhang, J. R. Song and F. Q. Zhao, Synthesis, Structural Characterization, and Thermal Properties of a New Energetic Zinc-FOX-7 Complex, J. Coord. Chem., 2013, 66, 3572 CrossRef CAS; (k) T. T. Vo, J. Zhang, D. A. Parrish, B. Twamley and J. M. Shreeve, New Roles for 1,1-Diamino-2,2-dinitroethene (FOX-7): Halogenated FOX-7 and Azo-bis (diahaloFOX) as Energetic Materials and Oxidizers, J. Am. Chem. Soc., 2013, 135, 11787 CrossRef CAS PubMed; (l) X. Fang and W. G. Mcluckie, Laser Ignitibility of Insensitive Secondary Explosive 1,1-Diamino-2,2-dinitroethene (FOX-7), J. Hazard. Mater., 2015, 285, 375 CrossRef CAS PubMed; (m) H. M. Shin, H. S. Kim and K. K. Koo, Molecular Modeling on Supersaturation-Dependent Growth Habit of 1,1-Diamino-2,2-dinitroethylene, Cryst. Growth Des., 2015, 15, 1833 CrossRef; (n) M. Civis, S. Civis, K. Sovova, K. Dryahina, P. Spanel and M. Kyncl, Laser Ablation of FOX-7: Proposed Mechanism of Decomposition, Anal. Chem., 2011, 83, 1069 CrossRef CAS PubMed; (o) V. G. Kiselev and N. P. Gritsan, Unexpected Primary Reactions for Thermolysis of 1,1-Diamino-2,2-dinitroethylene (FOX-7) Revealed by Ab Initio Calculations, J. Phys. Chem. A, 2014, 118, 8002 CrossRef CAS PubMed; (p) M. M. Bishop, N. Velisavljevic, R. Chellappa and Y. K. Vohra, High Pressure–Temperature Phase Diagram of 1,1-Diamino-2,2-dinitroethylene (FOX-7), J. Phys. Chem. A, 2015, 119, 9739 CrossRef CAS PubMed.
- X. Zhou, Z. Lu, D. Chen, H. Li, F. Nie and C. Zhang, Mechanical Anisotropy of the Energetic Crystal of 1,1-Diamino-2,2-dinitroethylene (FOX-7): A Study by Nanoindentation Experiments and Density Functional Theory, J. Phys. Chem. C, 2016, 120, 13434 CAS.
- D. B. Lempert, E. M. Dorofeenko and Y. Shu, Energy Potential of Solid Composite Propellants Based on 1,1-Diamino-2,2-dinitroethylene, Russ. J. Phys. Chem. B, 2016, 10, 483 CrossRef CAS.
- Y. Q. Wang and G. X. Wang, Configuration and Stability of 1,1-Diamino-2,2-dinitroethylene (FOX-7) Embedded in Graphene, Bull. Korean Chem. Soc., 2016, 37, 1571 CrossRef CAS.
- (a) Y. Zhang, Q. Sun, K. Z. Xu, J. R. Song and F. Q. Zhao, Review on the Reactivity of 1,1-Diamino-2,2-dinitroethylene (FOX-7), Propellants, Explos., Pyrotech., 2016, 41, 35 CrossRef CAS; (b) H. Gao and J. M. Shreeve, Recent Progress in Taming FOX-7 (1,1-Diamino-2,2-dinitroethene), RSC Adv., 2016, 6, 56271 RSC.
- T. T. Vo and J. M. Shreeve, 1,1-Diamino-2,2-dinitroethene (FOX-7) and 1-Amino-1-hydrazino-2,2-dinitroethene (HFOX) as Amphotères: Bases with Strong Acids, J. Mater. Chem. A, 2015, 3, 8756 CAS.
- H. X. Gao and J. M. Shreeve, The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials, Angew. Chem., Int. Ed., 2015, 54, 6335 CrossRef CAS PubMed.
- P. He, J. G. Zhang, K. Wang, X. Yin, X. Jin and T. L. Zhang, Extensive Theoretical Studies on Two New Members of the FOX-7 Family: 5-(Dinitromethylene)-1,4-dinitramino-tetrazole and 1,1′-Dinitro-4,4′-diamino-5,5′-bitetrazole as Energetic Compounds, Phys. Chem. Chem. Phys., 2015, 17, 5840 RSC.
- (a) G. M. Sheldrick, SHELXS, University of Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXL, Program for X-ray Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Azyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision B.01., Gaussian. Inc., Pittsburgh, PA, 2003 Search PubMed.
- (a) A. J. Bellamy, FOX-7 (1, 1-Diamino-2, 2-dinitroethene), Struct. Bonding, 2007, 125, 1 CrossRef CAS; (b) A. J. Bellamy and A. E. Contini, 1-Amino-1-hydrazo-2,2-dinitroethene–a Hazard Warning, Propellants, Explos., Pyrotech., 2008, 33, 87 CrossRef CAS; (c) H. X. Gao, Y. H. Joo, D. A. Parrish, T. Vo and J. M. Shreeve, 1-Amino-1-hydrazino-2,2-dinitroethene and Corresponding Salts: Synthesis, Characterization, and Thermolysis Studies, Chem. – Eur. J., 2011, 17, 4613 CrossRef CAS PubMed.
- (a) J. N. She, K. Z. Xu, H. Zhang, J. Huang, F. Q. Zhao and J. R. Song, Preparation, Crystal Structure and Thermal Behavior of 1,4-Dihydro-5H-(dinitromethylidene)-tetrazole (DNMT), Acta Chim. Sin., 2009, 67, 2645 CAS; (b) K. Z. Xu, H. Zhang, P. Liu, J. Huang, Y. H. Ren, B. Z. Wang and F. Q. Zhao, Structural and Thermal Characterization of a Novel High Nitrogen Energetic Material:(NH4)2DNMT, Propellants, Explos., Pyrotech., 2012, 37, 653 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Click Chemistry: Diverse Chemical Function from a Few Good Reactions, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- (a) G. A. Romeiro and L. R. Pereira, A New and Efficient Procedure for Preparing 1,2,3-Triazoles, Tetrahedron Lett., 1997, 38, 5103 CrossRef CAS; (b) A. Krasinski, V. V. Fokin and K. B. Sharpless, Direct Synthesis of 1,5-Disubstituted-4-magnesio-1,2,3-triazoles, Revisited, Org. Lett., 2004, 6, 1237 CrossRef CAS PubMed; (c) Y. M. Wu, J. Deng, Y. Li and Q. Y. Chen, Regiospecific Synthesis of 1,4,5-Trisubstituted-1,2,3-triazole via One-Pot Reaction Promoted by Copper(I) Salt, Synthesis, 2005, 1314 CrossRef CAS; (d) Y. Liu, W. Yan, Y. Chen, J. L. Petersen and X. Shi, Efficient Synthesis of N-2-Aryl-1,2,3-triazole Fluorophores via Post-Triazole Arylation, Org. Lett., 2008, 10, 5389 CrossRef CAS PubMed; (e) D. Kumar, V. B. Reddy and R. S. Varma, A Facile And Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Tiazoles Uing Cick Cemistry, Tetrahedron Lett., 2009, 50, 2065 CrossRef CAS; (f) L. J. T. Danence, Y. Gao, M. Li, Y. Huang and J. Wang, Organocatalytic Enamide–Azide Cycloaddition Reactions: Regiospecific Synthesis of 1,4,5-Trisubstituted-1,2,3-triazoles, Chem. – Eur. J., 2011, 17, 3584 CrossRef CAS PubMed; (g) W. Liu, Y. Li, B. Xu and C. Kuang, Palladium-Catalyzed Olefination and Arylation of 2-Substituted 1,2,3-Triazole N-Oxides, Org. Lett., 2013, 15, 2342 CrossRef CAS PubMed; (h) S. Koguchi and K. Izawa, Ionic Liquid-Phase Synthesis of 1,5-Disubstituted 1,2,3-Triazoles, ACS Comb. Sci., 2014, 16, 381 CrossRef CAS PubMed; (i) L. J. Li, Y. Q. Zhang, Y. Zhang, A. L. Zhu and G. S. Zhang, Synthesis of 5-Functionalized-1,2,3-triazoles via a One-Pot Aerobic Oxidative Coupling Reaction of Alkynes and Azides, Chin. Chem. Lett., 2014, 25, 1161 CrossRef CAS.
- Y. Zhang, Damon A. Parrish and J. M. Shreeve, Derivatives of 5-Nitro-1,2,3-2H-Triazole–High Performance Energetic Materials, J. Mater. Chem. A, 2013, 1, 585 CAS.
- U. Bemm and H. Öestmark, 1,1-Diamino-2,2-dinitroethylene: a Novel Energetic Material with Infinite Layers in Two Dimensions, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 1997 Search PubMed.
- J. G. Zhang, T. L. Zhang and K. B. Yu, The Preparation, Molecular Structure, and Theoretical Study of Carbohydrazide (CHZ), Struct. Chem., 2006, 17, 249 CrossRef CAS.
- X. L. Ren, X. G. Zuo, K. Z. Xu, Y. H. Ren, J. Huang, J. R. Song, B. Z. Wang and F. Q. Zhao, Structural Characterization and Thermal Behavior of a Novel Energetic Material: 1-Amino-1-(2,4-dinitrophenylhydrazinyl)-2,2-dinitroethylene, Bull. Korean Chem. Soc., 2011, 32, 2267 CrossRef CAS.
- (a) H. E. Kissinger, Reaction Kinetics in Differential Thermal Analysis, Anal. Chem., 1975, 29, 1702 CrossRef; (b) T. Ozawa and A. New Method, of Analyzing Thermogravimetric Data, Bull. Chem. Soc. Jpn., 1965, 38, 1881 CrossRef CAS.
- (a) R. Z. Hu, S. L. Gao, F. Q. Zhao, Q. Z. Shi, T. L. Zhang and J. J. Zhang, Thermal Analysis Kinetics, Science Press, 2nd edn, 2008. (in Chinese) Search PubMed; (b) T. L. Zhang, R. Z. Hu, Y. Xie and F. P. Li, The Estimation of Critical Temperatures of Thermal Explosion for Energetic Materials Using Non-isothermal DSC, Thermochim. Acta, 1994, 244, 171 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, optimized bond lengths and bond angles, atomic net charges, and energies of combustion. CCDC 1044208. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6nj03370a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |