Reversible piezofluorochromism of a triphenylamine-based benzothiazole derivative with a strong fluorescence response to volatile acid vapors†
Yong
Zhan
*,
Peng
Yang
,
Gang
Li
,
Yuanyuan
Zhang
and
Ying
Bao
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China. E-mail: zhanyong2046@126.com; Tel: +86-024-23986449
First published on 21st November 2016
Abstract
A novel D–π–A type triphenylamine modified benzothiazole derivative TVBT was synthesized, and it could emit strong fluorescence in solution and in the solid state. It should be noted that TVBT revealed reversible piezofluorochromic behaviors upon grinding and fuming with DCM vapor/heating treatment on account of the reversible transformation between the crystalline and amorphous states. The single crystal X-ray diffraction data revealed that the intermolecular interactions of C–H⋯N (2.54 Å, 2.68 Å), C–H⋯S (2.58 Å, 2.74 Å) and C–H⋯π (2.83 Å, 2.88 Å) would lead to the increased rigidity of the molecular skeleton, which would give rise to a loose packing motif, yielding piezofluorochromism. Interestingly, TVBT also exhibited reversible and remarkable acid/base-induced fluorescence switching properties in both solution and the solid state. In particular, the ground film of TVBT rapidly responded to TFA vapor. For example, the fluorescence gradually quenched with the increase of the concentration of TFA. When the concentration of TFA vapor was 459 ppm, the quenching efficiency reached 95.3%. In addition, the ground film of TVBT could also detect other volatile acid vapors, such as HCl and HNO3. Therefore, the ground film of TVBT could act as a fluorescence chemosensor with high performance for the naked-eye detection of volatile acid vapors by color and emission changes.
Introduction
Detection of volatile acid vapors has gained considerable importance in recent years, both for environmental and industrial purposes.1–3 In particular, the rapid sensing of volatile acid vapors is of significance for air quality monitoring. Although some approaches for detecting volatile acid vapors, such as liquid or gas chromatography,4 titration methods,5 and near-infrared spectroscopy,6 have been developed, the fluorescent sensors for detecting volatile acid vapors have received much attention because of their high selectivity, high sensitivity, local observation and high signal-to-noise ratio.7 Much effort has been made in developing fluorescent sensors for probing volatile acid vapors due to their sensitivity. For example, Yu and co-workers have reported AIE luminogen-functionalised mesoporous nanomaterials for the efficient detection of volatile acid gases by fluorescence changes.8 Jiang and co-workers have reported a fluorescent sensing film based on triphenylamine functionalized BODIPY for detecting HCl vapor with high stability.9 Our group developed emitting fiber-based films fabricated from L-phenylalanine derivatives with a quinoxaline group for sensing materials to detect acid vapor.10 We have also reported strong emissive nanofibers constructed from a benzothiazole modified carbazole derivative for the detection of volatile acid vapors.11 These organic molecules with a strong fluorescence emission in the solid state have attracted attention in organic electronics, photovoltaic devices and solid lasers,12 and they show switchable solid state luminescence upon exposure to external stimuli such as light, heat, chemical, electrical, and mechanical stimuli.13 Among them, piezofluorochromic (PFC) materials are particularly important because of their fundamental relevance and potential for application in sensors, optical information storage devices, optoelectronic devices and security inks.14 Although a few PFC materials have been developed, the investigation of the PFC materials is still in the initial stages. Most reported PFC materials consist of nonplanar π-conjugated molecules, such as 9,10-diphenylanthracene, organoboron compounds, tetraphenylethene, and triphenylacrylonitrile.15 Relatively few reports have focused on the PFC materials with benzothiazole groups. Moreover, most of the reported studies are usually confined to the description of the phenomenon and research of the mechanism, the sensing properties of PFC materials are rarely reported, especially, the quantitative study of the fluorescence properties of the volatile acid vapors, such as color change and fluorescence intensity, is still pending as an important and tough topic due to the lack of effective research methods.16 On the one hand, triphenylamine (TPA) has electron-donating characteristics and a propeller-like molecular structure with high steric hindrance,17 which can prevent interaction or close stacking in the solid state, and some TPA-based compounds with PFC properties have been recently reported.18 On the other hand, benzothiazole is an attractive building block to construct π-conjugated functional organic materials,19 because of its electron-withdrawing character and high physical and chemical stability. In addition, D–π–A conjugated compounds usually exhibited strong ICT emission, which was favorable to form PFC materials. Molecules containing nitrogen atoms, for example, were found to be able to respond to acids because of the easy availability of the lone pair of nitrogen atom for proton binding. With these in mind, herein, we designed and synthesized a new D–π–A type triphenylamine modified benzothiazole derivative (TVBT, Scheme 1). It was found that TVBT emitted strong fluorescence in solution and the solid state; at the same time TVBT exhibited strong ICT emission due to the strong electron-donating ability of triphenylamine and the electron-accepting ability of benzothiazole. It is interesting that reversible PFC behaviors of TVBT were observed upon grinding/fuming or heating treatment. We deemed that the reversible piezofluorochromism was derived from the transformation between crystalline and amorphous states. Moreover, the compound TVBT displayed acid–base fluorescence switching behaviors in solution and in the solid state, the fluorescence of which in solution could be quenched by TFA and restored by TEA. The fluorescence quenching should be related to the protonation of the TVBT molecule by acid treatment. Notably, the strong emission of the ground film of TVBT was obviously quenched upon exposure to gaseous acids, and the ground film could detect acid vapors, such as TFA, HCl and HNO3. Therefore, such a PFC compound could construct a multi-stimuli responsive and recyclable fluorescence switching system.Results and discussion
Synthesis
The synthetic route to TVBT is shown in Scheme 1. Firstly, N-phenyl-N-(4-vinylphenyl)benzenamine 120 and 2-(4-bromophenyl)benzo[d]thiazole 221 were prepared according to the reported methods. Then, the Heck reaction between 1 and 2 catalyzed by Pd(OAc)2 afforded the target molecule TVBT in a yield of 65%. TVBT was synthesized as a yellow platy crystal. All the intermediates and the target molecule were purified by column chromatography on silica gel, and characterized by 1H-NMR, 13C-NMR, FT-IR, and MALDI-TOF mass spectrometry. In the 1H-NMR spectrum of TVBT, the coupling constant of peaks at 7.17 ppm and 7.05 ppm was 16.0 Hz, indicating that the vinyl group had undergone a transformation. TVBT was soluble in common organic solvents, such as THF, DCM, CHCl3 and DMF, but showed poor solubility in alcohols (such as ethanol and methanol) and aliphatic hydrocarbon solvents (such as n-hexane and cyclohexane) at room temperature.Photophysical properties in solutions
The UV-vis absorption and fluorescence emission spectra of TVBT in different solvents (1.0 × 10−5 M) are shown in Fig. 1, and the corresponding photophysical data are listed in Table S1 (ESI†). From Fig. 1a, it is clear that TVBT showed two obvious absorption bands at ca. 308 nm and 400 nm in different solvents, and the former band might be ascribed to the π–π* transitions, whereas the latter one was derived from an intramolecular change transfer (ICT) transition, which could be confirmed by the solvent-dependent fluorescence emission spectra. From Fig. 1b, we find that TVBT emitted blue luminescence in cyclohexane, and the emission gave a vibrational structure (442 nm and 470 nm). The fluorescence emission bands of TVBT red-shifted obviously with increasing solvent polarity. For example, the emission peaks were located at 442 nm in cyclohexane and 547 nm in acetonitrile. Thus, the Stokes shifts increased clearly with increasing solvent polarity, and they were 2692 cm−1 and 7035 cm−1 in cyclohexane and acetonitrile, respectively. Moreover, the fluorescence quantum yield (ΦF) of TVBT decreased significantly with increasing solvent polarity. The ΦF was 0.98 in cyclohexane and decreased to 0.31 in acetonitrile using 9,10-diphenylanthracene in benzene (ΦF = 0.85) as the standard (Table S1, ESI†). Therefore, the significant red-shifts of the emission band, the large Stokes shifts, as well as the decreased ΦF for TVBT with increasing solvent polarity suggested that the emission was originated from ICT transitions.22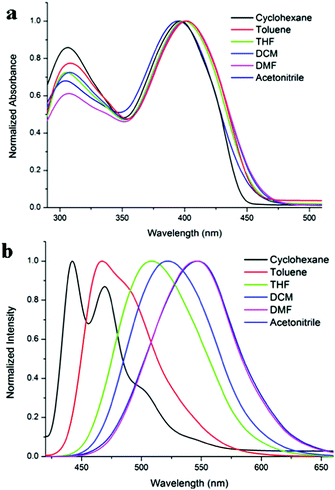 | ||
| Fig. 1 Normalized UV-vis absorption spectra of TVBT (a) and fluorescence emission spectra of TVBT (b, λex = 400 nm) in different solvents (1.0 × 10−5 M). | ||
Electrochemical properties
The electrochemical behaviors of the as-prepared triphenylamine-based benzothiazole derivative were investigated by cyclic voltammetry (CV) in CH2Cl2 (Fig. S1, ESI†), and the corresponding electrochemical data are listed in Table 1. It is clear that TVBT exhibited two well-defined reversible oxidation peaks with a first half-wave potential of 0.86 V vs. ferrocenium/ferrocene (vs. Fc/Fc+). The redox potential of Fc/Fc+, which possessed an absolute energy level of 4.8 eV relative to the vacuum level for calibration, was located at 0.41 eV in our case. As a result, the HOMO and LUMO energy levels were calculated using the empirical equations: EHOMO = −(Eox + 4.39) and ELUMO = EHOMO + Eg, in which Eg was estimated from the onset of the absorption spectrum (Eg = 1240/λonset). The HOMO and LUMO energy levels were determined to be −5.25 eV and −2.57 eV, respectively.| E ox (V) | E HOMO (eV) | E LUMO (eV) | E g (eV) | E HOMO (eV) | E LUMO (eV) |
|---|---|---|---|---|---|
| a E ox = potential versus Fc/Fc+. b E HOMO = −(Eox + 4.39); ELUMO = EHOMO + Eg. c Determined from the onset of absorption at the lower energy band edge (Eg = 1240/λonset). d Obtained from quantum chemical calculations using DFT/B3LYP/6-31G. | |||||
| 0.86 | −5.25 | −2.57 | 2.68 | −4.92 | −1.96 |
Theoretical calculations
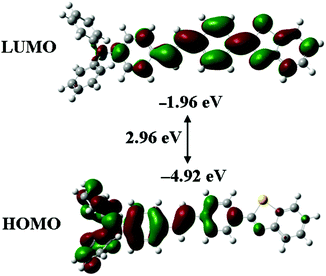
To better clarify the spectroscopic properties and electronic structure of TVBT, density functional theory (DFT) calculations were performed on the target molecule at the B3LYP/6-31G(d) level after optimizing its structure to the lowest energy conformation using the Gaussian 09W program. As shown in Fig. 2, it was found that the HOMO density for TVBT was mainly located on the donor triphenylamine moiety, while the LUMO density was primarily distributed in the acceptor benzothiazole unit. This result demonstrated that ICT occurred from the electron donor to the acceptor unit in TVBT. In addition, the HOMO and LUMO energy levels were also obtained by theoretical calculations. As shown in Table 1, the HOMO and LUMO energy levels were located at −1.96 eV and −4.92 eV, respectively. Moreover, the compound TVBT adopted a nonplanar conformation in its optimized lowest energy state (Fig. S2, ESI†). The introduction of a triphenylamine group in a π-conjugated system could enhance the degree of molecular distortion owing to the steric hindrance effect. This conformation disfavored close molecular packing in the solid state, leading to efficient fluorescence emission. Therefore, PFC activity is expected for TVBT in the solid state.Piezofluorochromic behaviors
As discussed above, the compound TVBT is nonplanar and possesses an electronic push–pull structure, and therefore PFC behaviors are expected.23 The fluorescence responses of TVBT toward grinding and fuming/heating processes are illustrated in Fig. 3. It is clear from the illustration of Fig. 3a that the as-prepared crystal of TVBT emitted bright green light under UV irradiation, and the as-prepared crystal could be easily changed into a yellowish green powder via simple grinding using a mortar and pestle, indicating the piezofluorochromism of TVBT. In addition, the fluorescence quantum yields (ΦF) of TVBT in the as-prepared crystal and the ground powder were 31.42% and 25.68%, respectively. Thus, TVBT could be used as a solid emitting material. As shown in Fig. 3b, we found that the emission of TVBT in the as-prepared crystal appeared at 494 nm, and red-shifted to 508 nm in the ground powder. When the ground powder was fumed with DCM vapor for 5 s or was heated at 100 °C for 5 s, the powder recovered its original emission. When the fumed or heated samples were reground, the emission color changed into yellowish green, and its emission band red-shifted to 507 nm again. As a result, the emission color of TVBT could be changed between bright green and yellowish green reversibly through grinding and CH2Cl2 fuming/heating treatment. The PFC behaviors of TVBT could be repeated multiple times, indicating reversibility and durability under external stimuli (Fig. S3, ESI†).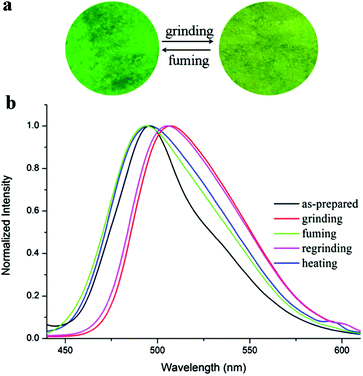 | ||
| Fig. 3 (a) Photograph of TVBT in the solid states under UV irradiation and (b) normalized fluorescence emission spectra of TVBT in different solid states excited at 400 nm. | ||
To gain an insight into the PFC mechanism of TVBT, powder wide-angle X-ray diffraction experiments were investigated in different states. As shown in Fig. 4, the as-prepared crystal exhibited many sharp and intense peaks, indicating an ordered arrangement. In contrast, the ground sample was amorphous because of very weak diffraction peaks. After fuming the ground powder with CH2Cl2 vapor, sharp and strong diffraction peaks similar to those of the crystal appeared again, implying the recovery of an ordered crystalline state. This result indicated that the reversibility of PFC behavior of TVBT was due to reversible phase transformation between crystalline and amorphous states.
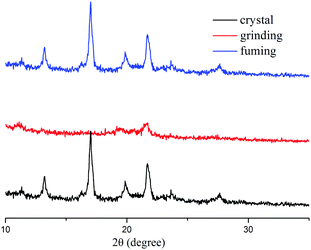 | ||
| Fig. 4 XRD patterns of TVBT in different solid-states: as-synthesized, and during grinding and fuming. | ||
To further reveal the effect of heating on the PFC properties, differential scanning calorimetry (DSC) measurements of TVBT were performed to study its thermal properties. The DSC curves of TVBT in different solid states are shown in Fig. S4 (ESI†). The as-prepared crystal of TVBT exhibited only a single endothermal peak at 258 °C, corresponding to its melting point. However, the ground powder of TVBT displayed an additional exothermic peak at 84 °C, which can be assigned to its cold-crystallization temperature. The ground sample in the amorphous phase is in a metastable state, which can be converted to a crystalline phase upon thermal annealing, indicating the phase transition from a poorly organized phase to a well-defined phase.
Crystal structure
In order to effectively understand piezofluorochromic behavior of the synthesized compound, we intended to obtain a single crystal of TVBT. The single crystal of TVBT was prepared by slow evaporation of its solutions in DCM/methanol. The collected crystal was good enough for single crystal X-ray diffraction analysis, and the data are summarized in Table S2 (ESI†). The single crystal study revealed that the crystal structures of TVBT belonged to the triclinic space group p![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and each cell contained four molecules (Z = 4). The molecular ORTEP drawing is displayed in Fig. 5a. The crystal structure revealed that the triphenylamine units in the molecular structure adopted a nonplanar orientation. As shown in Fig. S5 (ESI†), in the crystal packing of TVBT, the molecules adopted a stacking mode of J-type aggregation along the a axis. Moreover, the intermolecular interactions among molecules in TVBT are shown in Fig. 5b. The existence of weak intermolecular C–H⋯N (d = 2.54 Å, 2.68 Å), C–H⋯S (d = 2.58 Å, 2.74 Å) and C–H⋯π (d = 2.83 Å, 2.88 Å) interactions was observed. These intermolecular interactions facilitated the molecular packing, and the lack of an intense intermolecular acting force gave rise to a loose packing motif, which allowed slipping upon exposure to external mechanical stimuli.
, and each cell contained four molecules (Z = 4). The molecular ORTEP drawing is displayed in Fig. 5a. The crystal structure revealed that the triphenylamine units in the molecular structure adopted a nonplanar orientation. As shown in Fig. S5 (ESI†), in the crystal packing of TVBT, the molecules adopted a stacking mode of J-type aggregation along the a axis. Moreover, the intermolecular interactions among molecules in TVBT are shown in Fig. 5b. The existence of weak intermolecular C–H⋯N (d = 2.54 Å, 2.68 Å), C–H⋯S (d = 2.58 Å, 2.74 Å) and C–H⋯π (d = 2.83 Å, 2.88 Å) interactions was observed. These intermolecular interactions facilitated the molecular packing, and the lack of an intense intermolecular acting force gave rise to a loose packing motif, which allowed slipping upon exposure to external mechanical stimuli.
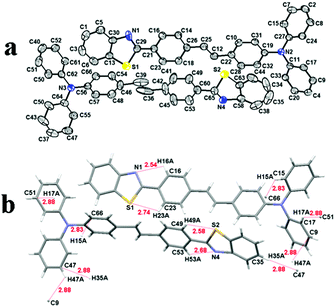 | ||
| Fig. 5 (a) ORTEP drawing of TVBT at the 30% probability level ellipsoids and (b) the intermolecular interactions in the single crystal of TVBT. | ||
Fluorescence sensing properties towards volatile acid vapors
In our previous work, we have found that the emission of the ground film fabricated from benzoxazole derivatives could be quenched significantly by gaseous acid vapors,24 so the sensing properties of the obtained ground film of TVBT towards volatile acid vapors were investigated. First of all, the response of TVBT to trifluoroacetic acid (TFA) in solution was studied. The absorption spectral change of TVBT towards TFA in CHCl3 solution (5.0 × 10−5 M) is shown in Fig. 6a. When TFA was gradually added to the CHCl3 solution of TVBT, the yellow solution gradually converted into a dark red one. As shown in Fig. 6a, the absorption band of TVBT from ca. 360 nm to ca. 430 nm in CHCl3 continuously decreased upon the addition of TFA, and a new band appeared at ca. 483 nm and intensified with increasing amounts of TFA addition. This peak results in a solution color change from yellow to red (inset of Fig. 6a). The isobestic point was obtained at ca. 435 nm, implying a reactive equilibrium of the two components.25 In other words, TVBT could bind a proton to form a cation TVBT-H+ (Fig. 7), which revealed a red-shifted absorption band because the protonation of benzothiazole strengthened its electron-withdrawing ability, and further promoted the ICT process from the triphenylamine moiety to the benzothiazole-H+ unit.26 The fluorescence of TVBT in CHCl3 solution could also respond to protons. The emission spectral changes of TVBT upon the addition of TFA are shown in Fig. 6b. It is clear that the emission band at ca. 502 nm decreased and red-shifted by degrees with an increase in the concentration of TFA. The strong fluorescence emission of TVBT could be quenched obviously by TFA. The emission was quenched by 65.5% under additional 20 equiv. TFA, at which point the fluorescence emission peak red-shifted to 506 nm. A further increase in the TFA amount quenched the solution emission, its fluorescence was reduced by almost 99.3% when exposed to additional 150 equiv. TFA. A high TFA concentration almost completely quenched the fluorescence of TVBT. The result suggested that TVBT-H+ was non-emissive. In addition, the change in the photophysical properties induced by TFA could be restored by the addition of triphenylamine (TEA). This process can be repeated multiple times (Fig. S6, ESI†). Finally, TVBT exhibited reversible and remarkable acid/base-induced fluorescence switching behaviors, implying that it could be used as a probe for sensing strong acids in solutions.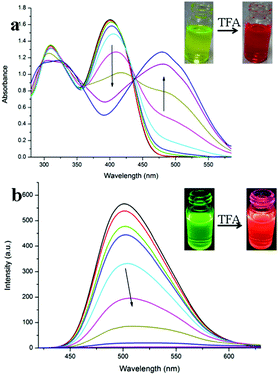 | ||
| Fig. 6 (a) UV-vis absorption and (b) fluorescence emission spectral changes of TVBT in CHCl3 (5.0 × 10−5 M) from 0 equiv. to 150 equiv. with additional TFA at room temperature (λex = 400 nm). | ||
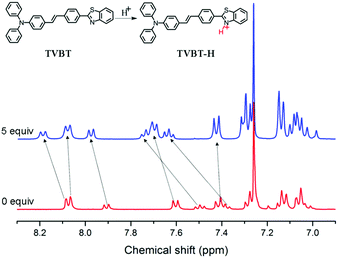
In order to better understand the interaction between TVBT and TFA, the 1H NMR spectra before and after the addition of TFA were investigated and compared. As shown in Fig. 7, we found that the addition of TFA caused the signals to show a significant downfield shift for all protons, particularly at the benzothiazole ring and an ambient benzene group. This result demonstrated that the proton binding of TVBT might be ascribed to protonation of the nitrogen atom in the benzothiazole moiety to form a cation TVBT-H+, which reduced the electron density around all protons, enhanced the electron withdrawing ability of the benzothiazole unit and caused the bathochromic shift in the absorption spectrum.27
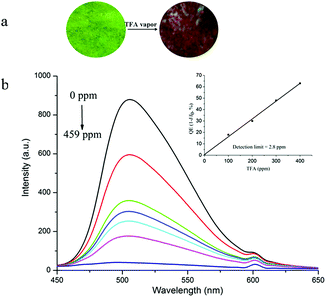
The above results proved that TVBT solution could respond to protons in terms of fluorescence and color. The response of the ground film of TVBT with strong emission to the volatile acid was expected.28 When a small amount of TFA vapor was added into the surface of the ground film of TVBT, its color changed from yellowish green into a dark red after a few seconds (Fig. 8a). Herein, the ground film of TVBT was expected to detect volatile acid vapors. As shown in Fig. 8b, the emission intensity at 505 nm for the ground film significantly decreased with increasing TFA vapor concentration. The fluorescence emission was completely quenched upon exposure of the ground film to TFA with a high concentration. For example, the quenched efficiency of 95.3% was observed upon exposure to TFA vapor (459 ppm). To demonstrate the sensitivity of the ground film in sensing gaseous TFA, the concentration-dependent fluorescence quenching efficiency (1 − I/I0) is shown in the inset of Fig. 8b. A good linear relationship between the quenching efficiencies and the TFA vapor concentration was observed when the concentration was below 400 ppm. Accordingly, the detection limit could be determined to be 2.8 ppm for the TFA vapor.29 Thus, the ground film of TVBT could be applied to detect TFA vapor based on fluorescence quenching. The UV-vis absorption spectra of the ground film of TVBT upon exposure to different amounts of TFA vapor were investigated (Fig. S7, ESI†). We found that the absorption band in the range of 370–460 nm for the ground film of TVBT decreased gradually and a new broad absorption band at ca. 520 nm, which was ascribed to protonated TVBT emerged upon exposure to TFA vapor.
Moreover, we also investigated the fluorescence response behaviors of TVBT in the ground film upon exposure to other volatile acid vapors (such as HCl and HNO3). As shown in Fig. S8 and S9 (ESI†), upon exposure of the ground film of TVBT to saturated HCl or HNO3 vapors, the emission intensity of the film was completely quenched. When the film was treated with saturated TEA vapor, the fluorescence and color could be recovered to its original state. Such a process can be repeated many times, indicating excellent reversibility. Therefore, the ground film of TVBT could be used as fluorescence chemosensing materials to detect volatile acid vapors selectively by the naked eye with high performance.
Conclusions
In summary, a new D–π–A type triphenylamine functionalized benzothiazole derivative TVBT was designed and synthesized. It was found that TVBT showed intense fluorescence emission in solution and in the solid state. A strong ICT process could be detected in the donor–acceptor π-conjugated system. In particular, the solid state emission color of TVBT was different before and after grinding, exhibiting PFC properties. The XRD patterns demonstrated that the reversible piezofluorochromism observed by repeating the grinding–fuming/heating process was due to the phase transformation between the crystalline and amorphous states. The single crystal of TVBT revealed that the intermolecular interactions of C–H⋯N, C–H⋯S and C–H⋯π would lock the molecular conformation. When external force caused the crystalline lattice to collapse, the emitters might adjust to a more planar conformation, leading to a red-shifted emission. TVBT also displayed reversible acidochromism in response to protonation and deprotonation in the solution. Interestingly, when the ground film of TVBT was exposed to saturated TFA vapor, we found that its emission was obviously quenched, and its color immediately transformed from yellowish green into dark red. For instance, the fluorescence emission intensity of the ground film of TVBT was obviously decreased with increasing TFA concentration, and the quenching efficiency achieved was 95.3% when the concentration of TFA vapor was 459 ppm after a few seconds. The detection limit of the ground film for gaseous TFA was ca. 2.8 ppm. We can consider that the ground film of TVBT showed high performance in probing TFA vapor. It should be noted that the ground film of TVBT could also detect other volatile acid vapors of HCl and HNO3 at room temperature. This work will be helpful in the design of novel PFC materials for application as fluorescent chemosensors showing high performance for detecting volatile acid vapors.Experimental section
Measurement and characterization
1H-NMR spectra and 13C-NMR spectra were measured on a Bruker AMX-400 NMR spectrometer at 400 MHz and 100 MHz in CDCl3 as the solvent at room temperature. FT-IR spectra were measured using a Nicolet-360 FT-IR spectrometer by the incorporation of samples in KBr disks. Mass spectra were recorded on Agilent 1100 MS series and AXIMA CFR MALDI/TOF (Compact) mass spectrometers. C, H and N elemental analyses were performed using a Perkin-Elmer 240C elemental analyzer. The UV-vis absorption spectra were recorded on a Beijing Purkinje TU-1810 spectrophotometer. Fluorescence emission spectra were obtained on a Shimadzu RF-5301 PC spectrofluorophotometer. The fluorescence quantum yields in solvents were estimated by comparing with a standard (9,10-diphenyl anthracene in benzene, ΦF = 0.85). The absolute fluorescence quantum yields were measured on an Edinburgh FLS920 steady state spectrometer using an integrating sphere. Cyclic voltammetry was performed using a CHI 830D electrochemical workstation and measurements were carried out in CH2Cl2 with a scan rate at 100 mV s−1. A three-electrode configuration was used for the measurement: a platinum button was used as the working electrode, a platinum wire as the counter electrode, and Ag/AgCl as the reference electrode. The solution of Bu4NPF6 in DCM (0.1 M) was used as the supporting electrolyte. XRD patterns were obtained on an Empyrean X-ray diffraction instrument. The DSC curve was recorded using a Netzsch DSC 204F1 at a heating rate of 10 °C min−1. A single crystal was obtained by slow solvent evaporation from the solution in DCM/methanol at room temperature. A single crystal of TVBT was selected for X-ray diffraction studies performed using a Rigaku RAXIS-RAPID diffractometer using graphite-monochromated MoKα radiation (λ = 0.71073 Å). The single crystal was kept at room temperature during the data collection. The structure was solved by direct methods and refined on F2 by full-matrix least-squares using the SHELXTL-97 program. The frontier orbital plots of the HOMO and LUMO of TVBT were obtained by density functional theory (DFT) calculations at the B3LYP/6-31G level using the Gaussian 09W program package.Preparation of the ground film
The ground film of TVBT used as a sensor was prepared as follows. Firstly, the as-prepared crystal was uniformly coated on the quartz substrate, and then shear stress was applied using a spatula to form a thin film.Investigation of the fluorescence sensing properties
The sensing investigation for the ground film of TVBT was carried out at room temperature. Firstly, the acid vapors at a certain concentration were obtained by diluting the saturated vapors with nitrogen, and then injecting them into a quartz cell (1 cm in width) containing the film. The changes in the emission intensity and wavelength were recorded after injection of the acid vapors for 30 s.Materials
DMF was dried over phosphorus pentoxide. The other chemicals and reagents were used as received without further purification. Compounds 1 and 2 were synthesized according to the literature.Synthetic procedures and characterization
Acknowledgements
This work is financially supported by the Scientific Research Fund of Liaoning Provincial Education Department of China (No. L2015528), and the Program for Innovative Research Team of the Ministry of Education and Program for Liaoning Innovative Research Team in University.Notes and references
- H. Y. Wang, H. Cui, X. L. Liu, L. L. Li, Y. Cao, T. H. Liu and Y. Fang, Chem. – Asian J., 2013, 8, 101 CrossRef CAS PubMed.
- R. Z. Tang, X. Y. Wang, W. Z. Zhang, X. D. Zhuang, S. Bi, W. B. Zhang and F. Zhang, J. Mater. Chem. C, 2016, 4, 7640 RSC.
- (a) C. W. Zhao, J. P. Ma, Q. K. Liu, X. R. Wang, Y. Liu, J. Yang, J. S. Yang and Y. B. Dong, Chem. Commun., 2016, 52, 5238 RSC; (b) Z. C. Zeng, J. Wen, H. Yang, Z. L. Liu, Y. Q. Xu, H. J. Li, C. M. Zhong, F. Y. Liu and S. G. Sun, RSC Adv., 2016, 6, 37385 RSC.
- J. Larreta, K. A. Saila and E. H. Unibertsitatea, J. Sep. Sci., 2007, 30, 2293 CrossRef CAS PubMed.
- O. Lahav, B. E. Morgan and R. E. Loewenthal, Environ. Sci. Technol., 2002, 36, 2736 CrossRef CAS PubMed.
- A. J. Ward, E. Bruni, M. K. Lykkegaard, A. Feilberg, A. P. S. Adamsen, A. P. Jensen and A. K. Poulsen, Bioresour. Technol., 2011, 102, 4098 CrossRef CAS PubMed.
- (a) P. C. Xue, R. Lu, P. Zhang, J. H. Jia, Q. X. Xu, T. R. Zhang, M. Takafuji and H. Ihara, Langmuir, 2013, 29, 417 CrossRef CAS PubMed; (b) P. F. Robert, J. L. Boudenne and B. Coulomb, Talanta, 2013, 115, 737 CrossRef PubMed; (c) E. Cariati, C. Dragonetti, E. Lucenti, F. Nisic, S. Righetto, D. Roberto and E. Tordin, Chem. Commun., 2014, 50, 1608 RSC.
- D. D. Li, Y. P. Zhang, Z. Y. Fan and J. H. Yu, Chem. Commun., 2015, 51, 13830 RSC.
- L. Zhang, Y. T. Chen and J. Z. Jiang, Dyes Pigm., 2016, 124, 110 CrossRef CAS.
- P. C. Xue, J. B. Sun, B. Q. Yao, P. Gong, Z. Q. Zhang, C. Qian, Y. Zhang and R. Lu, Chem. – Eur. J., 2015, 21, 4712 CrossRef CAS PubMed.
- J. B. Sun, P. C. Xue, J. B. Sun, P. Gong, P. P. Wang and R. Lu, J. Mater. Chem. C, 2015, 3, 8888 RSC.
- Z. Mao, Z. Y. Yang, Y. X. Mu, Y. Zhang, Y. F. Wang, Z. G. Chi, C. C. Lo, S. W. Liu, A. Lien and J. R. Xu, Angew. Chem., Int. Ed., 2015, 54, 6270 CrossRef CAS PubMed.
- M. Y. Liu, L. Zhai, J. B. Sun, P. C. Xue, P. Gong, Z. Q. Zhang, J. B. Sun and R. Lu, Dyes Pigm., 2016, 128, 271 CrossRef CAS.
- P. C. Xue, J. P. Ding, P. P. Wang and R. Lu, J. Mater. Chem. C, 2016, 4, 6688 RSC.
- (a) Z. Q. Zhang, P. C. Xue, P. Gong, G. H. Zhang, J. Peng and R. Lu, J. Mater. Chem. C, 2014, 2, 9543 RSC; (b) X. Q. Zhang, Z. G. Chi, Y. Zhang, S. W. Liu and J. R. Xu, J. Mater. Chem. C, 2013, 1, 3376 RSC; (c) T. Jadhav, B. Dhikale and R. Misra, J. Mater. Chem. C, 2015, 3, 9063 RSC; (d) X. Q. Zhang, Z. G. Chi, H. Y. Li, B. J. Xu, X. F. Li, W. Zhou, S. W. Liu, Y. Zhang and J. R. Xu, Chem. – Asian J., 2011, 6, 808–811 CrossRef CAS PubMed; (e) B. J. Xu, Z. G. Chi, X. Q. Zhang, H. Y. Li, C. J. Chen, S. W. Liu, Y. Zhang and J. R. Xu, Chem. Commun., 2011, 47, 11080–11082 RSC; (f) Y. Zhan, Y. N. Xu, P. Yang, H. Zhang, Y. Li and J. Y. Liu, Tetrahedron Lett., 2016, 57, 5385–5389 CrossRef CAS.
- H. W. Sun, Y. Zhang, W. Yan, W. X. Chen, Q. Lan, S. W. Liu, L. Jiang, Z. G. Chi, X. D. Chen and J. R. Xu, J. Mater. Chem. C, 2014, 2, 5812 RSC.
- (a) P. C. Xue, P. Chen, J. H. Jia, Q. X. Xu, J. B. Sun, B. Q. Yao, Z. Q. Zhang and R. Lu, Chem. Commun., 2014, 50, 2569 RSC; (b) X. Zhao, P. C. Xue, K. Wang, P. Chen, P. Zhang and R. Lu, New J. Chem., 2014, 38, 1045 RSC.
- (a) P. S. Hariharan, N. S. Venkataramanan, D. Moon and S. P. Anthony, J. Phys. Chem. C, 2015, 119, 9460 CrossRef CAS; (b) J. B. Wei, B. Y. Liang, X. Cheng, Z. L. Zhang, H. Y. Zhang and Y. Wang, RSC Adv., 2015, 5, 71903 RSC; (c) C. Y. K. Chan, J. W. Y. Lam, S. M. Chen, P. Lu, H. H. Y. Sung, H. S. Kwok, Y. G. Ma, I. D. Williams and B. Z. Tang, J. Mater. Chem. C, 2014, 2, 4320 RSC.
- T. Jadhav, B. Dhokale, S. M. Mobin and R. Misra, RSC Adv., 2015, 5, 29878 RSC.
- Y. Zhan, J. Peng, K. Q. Ye, P. C. Xue and R. Lu, Org. Biomol. Chem., 2013, 11, 6814 CAS.
- E. B. Shi, J. H. He, H. Zhuang, H. Z. Liu, Y. F. Zheng, H. Li, Q. F. Xu, J. W. Zheng and J. M. Lu, J. Mater. Chem. C, 2016, 4, 2579 RSC.
- Z. Q. Zhang, Z. Wu, J. B. Sun, B. Q. Yao, P. C. Xue and R. Lu, J. Mater. Chem. C, 2016, 4, 2854 RSC.
- (a) G. H. Zhang, J. B. Sun, P. C. Xue, Z. Q. Zhang, P. Gong, J. Peng and R. Lu, J. Mater. Chem. C, 2015, 3, 2925 RSC; (b) Y. Zhan, P. Gong, P. Yang, Z. Jin, Y. Bao, Y. Li and Y. N. Xu, RSC Adv., 2016, 6, 32697 RSC.
- (a) P. C. Xue, B. Q. Yao, J. B. Sun, Z. Q. Zhang, K. C. Li, B. J. Liu and R. Lu, Dyes Pigm., 2015, 112, 255 CAS; (b) P. C. Xue, B. Q. Yao, P. P. Wang, J. B. Sun, Z. Q. Zhang and R. Lu, RSC Adv., 2014, 4, 58732 RSC; (c) Y. Zhan, J. Y. Zhao, P. Yang and W. J. Ye, RSC Adv., 2016, 6, 92144 RSC.
- K. C. Naeem, A. Subhakuman, S. Varughese and V. C. Nair, J. Mater. Chem. C, 2015, 3, 10225 RSC.
- C. P. Ma, B. J. Xu, G. Y. Xie, J. J. He, X. Zhou, B. Y. Peng, L. Jiang, B. Xu, W. J. Tian, Z. G. Chi, S. W. Liu, Y. Zhang and J. R. Xu, Chem. Commun., 2014, 50, 7374 RSC.
- Y. X. Lei, Y. Z. Liu, Y. Guo, J. X. Chen, X. B. Huang, W. X. Gao, L. B. Qian, H. Y. Wu, M. C. Liu and Y. X. Cheng, J. Phys. Chem. C, 2015, 119, 23138 CAS.
- M. S. Kwon, J. Gierschner, J. Seo and S. Y. Park, J. Mater. Chem. C, 2014, 2, 2552 RSC.
- P. C. Xue, B. Q. Yao, P. P. Wang, P. Gong, Z. Q. Zhang and R. Lu, Chem. – Eur. J., 2015, 21, 17508 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR, 13C-NMR and MALDI-TOF MS spectra; photophysical data; DSC curve; cyclic voltammetry diagrams; optimal molecular conformations; reversibility of PFC processes and fluorescence spectral changes upon exposure to volatile acid vapors. CCDC 1515093. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6nj02735k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |