Three-dimensional porous bowl-shaped carbon cages interspersed with carbon coated Ni–Sn alloy nanoparticles as anode materials for high-performance lithium-ion batteries†
Zhiyuan
Wang
*abc,
Dan
Wang
abc,
Shaohua
Luo
abc,
Shuo
Bao
a,
Yanguo
Liu
abc,
Xiwei
Qi
abc,
Chunnian
He
d,
Chunsheng
Shi
d and
Naiqin
Zhao
d
aSchool of Materials Science and Engineering, Northeastern University, Shenyang 110819, P. R. China. E-mail: zhiyuanwang@neuq.edu.cn
bSchool of Resources and Materials, Northeastern University at Qinhuangdao, Qinhuangdao 066004, China
cKey Laboratory of Dielectric and Electrolyte Functional Material Hebei Province, Qinhuangdao, China
dSchool of Materials Science and Engineering, Tianjin University, Tianjin 300072, P. R. China
First published on 24th November 2016
Abstract
The structural damage induced by huge volume change during lithiation/delithiation results in poor cycle stability of tin-based anode materials, which becomes the major obstacle to their practical application. In this work, we fabricated three-dimensional (3D) porous bowl-shaped carbon cages interspersed with carbon coated Ni–Sn alloy nanoparticles (Ni3Sn2 and Ni3Sn4; 10–30 nm) by a freeze-drying method with self-assembled NaCl as a template followed by annealing. Both Ni3Sn2/C and Ni3Sn4/C exhibit excellent electrochemical performance as anode materials for lithium-ion batteries. In particular, the Ni3Sn4/C nanocomposites exhibit superior rate capability (735, 661, 622, 577, 496, and 377 mA h g−1 at 0.1, 0.2, 0.5, 1, 2, and 5 A g−1, respectively) and excellent cycling stability (568 mA h g−1 at 0.5 A g−1 for the second cycle and gradually increased to 732 mA h g−1 after 200 cycles). The superior electrochemical performance is attributed to the synergetic effect of Ni–Sn alloy nanoparticles and 3D porous bowl-shaped carbon networks. The uniformly embedded Ni–Sn alloy nanoparticles can effectively alleviate the absolute stress/strain and shorten the Li+ diffusion path, and Ni in the Ni–Sn alloy acts as a buffer to suppress the volume expansion. Moreover, the 3D bowl-shaped carbon networks with high conductivity can provide abundant space for volume expansion, suppress the agglomeration of Ni–Sn nanoparticles, ensure the structural integrity, and facilitate lithium-ion diffusion as well as electron transportation.
Introduction
Nowadays, the development of portable electronic devices and electric vehicles or hybrid electric vehicles (EVs/HEVs) put forward higher demands on energy density and power density of lithium-ion batteries (LIBs). It is urgent to exploit new electrode materials with high capacity and excellent rate capability. Conventional graphite anode materials are limited to their insufficient theoretical capacity (LiC6, 372 mA h g−1) and poor rate capability (sluggish diffusion of Li+ due to its anisotropic structure).1 Thus, some alternative anode materials with high capacity such as Si, Ge, Sn, transition metal oxides, and metal sulphides/phosphides/nitrides, etc. are being investigated.2–4 Among these materials, metallic Sn has been considered as a good anode material due to its high theoretical capacity (Li4.4Sn, 991 mA h g−1) and applicable operating potential (0.05–1.0 V vs. Li+/Li).5,6 However, the high capacity cannot be well retained due to the huge volume change (>300%) during Li+ insertion/deinsertion which results in the cracking and pulverization of the electrode, and the loss of electrical contact with the current collector.7 To address these issues, various approaches have been proposed to suppress the volume change of Sn anodes, such as fabricating Sn nanostructures, constructing Sn/C composites, and combining Sn with other components to form an alloy. A variety of Sn-based nanostructures such as nanoparticles,8 nanospheres,9 nanorods,10 and nanowires11 have been widely synthesized, and an improved cycling stability is achieved. It is found that reducing Sn to the nanoscale can make the inevitable stress/strain small and shorten the diffusion path of Li+.12 Nevertheless, Sn-based nanostructures with a large surface area are unstable and tend to aggregate to reduce the large surface energy.4,12Correspondingly, dispersing the nano-sized Sn in a conductive carbon matrix can reduce the aggregation of nanoparticles, accommodate volume expansion for maintaining its structural stability, and enhance the conductivity of the electrode. So various nanostructured Sn/C composites have been synthesized to enhance the electrochemical performance.13–17 For instance, Bruno Scrosati reported a nanostructured Sn–C composite which delivered a stable capacity over more than 200 cycles.13 Recently, we reported a graphene network anchored with Sn@graphene synthesized by an in situ chemical vapor deposition (CVD) technique, and it demonstrated excellent electrochemical performance.14 However, the volume change of Sn cannot be completely eliminated, and the capacity decay still remains a problem.
Another effective approach to further improve the electrochemical performance of Sn anodes is the preparation of an Sn-based alloy, in which the inactive metal (such as Co, Ni, Fe, and Cu, etc.) acts as a buffer to suppress the volume expansion. In particular, Ni–Sn alloy/carbon composite anodes display better cycling stability than those of pure Sn because Ni and carbon can maintain the structural integrity and enhance the conductivity of the electrode.18–21 For instance, we reported carbon-coated Ni3Sn2 nanoparticles embedded in two-dimensional porous carbon nanosheets and a good electrochemical performance was demonstrated.18 In addition, Hou et al.19 reported Sn@Ni3Sn4 embedded in a nanocable-like carbon matrix, and the composite exhibited a long cycle life due to the synergistic effects of Ni3Sn4 and the nanocable carbon. Very recently, a Ni3Sn2@reduced graphene oxide composite was synthesized by a solvothermal method, and an excellent cycling performance is achieved.20 Thus, it is obvious that nano-sized Ni–Sn alloy compounded with carbon is highly beneficial for lithium storage capacity and cycling performance because of the synergistic effects between the nano-sized Ni–Sn alloy and the carbon matrix. However, fabricating uniform NiSn alloy nanoparticles (less than 20 nm) dispersed in a carbon matrix with high NiSn loading by a one-step method remains a challenge. In particular, the high-rate performance to meet practical applications is still needed.
It is reported that the three-dimensional (3D) carbon structure with porous morphology is generally considered as a superior matrix for hosting active species due to its advantages such as easy electrolyte penetration and fast Li+ diffusion. Moreover, 3D carbon network structures with their outstanding electrical conductivity and flexibility can act as an excellent buffer layer that mitigates volume changes of active materials during Li-ion insertion/extraction by absorption of stress, thereby enhancing the structural stability and cyclability of the overall electrode.14,22–25
In this work, we developed a Ni–Sn alloy/carbon composite electrode with a novel 3D nanostructure by a feasible freeze-drying method with self-assembled NaCl as a template followed by annealing. In the composite, carbon coated Ni–Sn intermetallic alloy (Ni3Sn4 and Ni3Sn2) nanoparticles (NPs: 10–30 nm) were uniformly interspersed in 3D interconnecting porous bowl-shaped carbon cages. Owing to the advantages of its unique Ni3Sn4/C nanostructure, including abundant space for volume expansion, stable surface to reduce aggregation, and high-speed transport pathway for electrons and ions, high charge capacity, excellent rate capability, and stable cycling performance were achieved, making it suitable for practical applications in lithium-ion batteries with high energy density and power density.
Experimental section
Materials preparation
2.5 g of citric acid (carbon source, also a guarantee preventing tetravalent tin from hydrolyzation) and 20.65 g of sodium chloride (template) of analytical reagent grade with a proportion of C/NaCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199 were dissolved in 75 ml of distilled water. Afterwards, metallic ions (SnCl2·H2O/Ni(NO3)2·6H2O = 2/3, 4/3) and carbon atoms in the ratio of 1/40 were dissolved in the solution, and stirred overnight to obtain a homogeneous solution. Then the solution was transferred to a Petri dish and kept in a refrigerator to freeze firmly. After that the frozen samples were placed in a vacuum freeze-dryer, and the water was allowed to sublimate completely by vacuum freeze-drying. The obtained powder was heated to 750 °C with a rate of 10 °C min−1 and kept for 2 h, and a flow of Ar/H2 = 1
199 were dissolved in 75 ml of distilled water. Afterwards, metallic ions (SnCl2·H2O/Ni(NO3)2·6H2O = 2/3, 4/3) and carbon atoms in the ratio of 1/40 were dissolved in the solution, and stirred overnight to obtain a homogeneous solution. Then the solution was transferred to a Petri dish and kept in a refrigerator to freeze firmly. After that the frozen samples were placed in a vacuum freeze-dryer, and the water was allowed to sublimate completely by vacuum freeze-drying. The obtained powder was heated to 750 °C with a rate of 10 °C min−1 and kept for 2 h, and a flow of Ar/H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is necessary to reduce metallic ions to Ni–Sn alloy. After that, the powder was cooled to ambient temperature, and then the NaCl was removed by repeated washing and filtering with water. The wet powder was dried at 80 °C and grinded to get the final products. Sn embedded in 3D porous carbon cages was prepared by the same method as a reference sample to compare with the designed samples.
2 is necessary to reduce metallic ions to Ni–Sn alloy. After that, the powder was cooled to ambient temperature, and then the NaCl was removed by repeated washing and filtering with water. The wet powder was dried at 80 °C and grinded to get the final products. Sn embedded in 3D porous carbon cages was prepared by the same method as a reference sample to compare with the designed samples.
Physical characterization
The phase composition of the obtained samples was characterized by an X-ray diffractometer (Rigaku SmartLab) with Cu Kα radiation (λ = 0.15406 nm), scanning from 10° to 80°. Raman spectra were used to define the crystallization degree of carbon with a micro-Raman spectrometer (Renishaw, inVia microscope, a 532 nm laser). Thermogravimetry and differential thermal analyses (Hengjiu Instrument Co., Ltd, HCT-2, Beijing, China) were performed in air to 1000 °C at a rate of 10 °C min−1 to quantify the ratio of Ni–Sn alloy and carbon. The microstructure was studied for a general view and a specific one, respectively, via a field emission scanning electron microscope (SEM, ZEISS SUPRA55) and a high-resolution transmission electron microscope (HRTEM, JEM 2100F). An Autosorb-iQ instrument (Quantachrome U.S.) was operated at 77 K, and the statistics of nitrogen absorption and desorption was then calculated using the Brunauer–Emmett–Teller (BET) method to obtain the surface area, and the Barrett–Joyner–Halenda (BJH) method to obtain the pore size distribution.Electrochemical measurements
The electrochemical measurements were evaluated via a CR2032 type coin cell with the Ni–Sn alloy/carbon composite as a working electrode and lithium wafer as the reference and auxiliary electrode. The working electrode was prepared by a copper foil coated with a slurry, which was a mixture of as-obtained material, carbon black and polyvinylidene fluoride (PVDF) at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 added to N-methyl-2-pyrrolidinone solvent, dried at 80 °C overnight in a vacuum oven to be pressed and cut into wafers and then dried at 120 °C for 8 h. Then the coin cells were assembled in an argon-filled glove box with Celgard 2400 polypropylene films as the separator and 1 M LiPF6 dissolved in ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/dimethyl carbonate (DEC) (1
10 added to N-methyl-2-pyrrolidinone solvent, dried at 80 °C overnight in a vacuum oven to be pressed and cut into wafers and then dried at 120 °C for 8 h. Then the coin cells were assembled in an argon-filled glove box with Celgard 2400 polypropylene films as the separator and 1 M LiPF6 dissolved in ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/dimethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as the electrolyte. After 12 hours, the cells were tested galvanostatically in a voltage between 0.005 V and 3 V at different current densities (0.1, 0.2, 0.5, 1, 2, and 5 Ag−1) with a CT2001A instrument (Wuhan LAND Electronics Co., Ltd, China). Cyclic voltammograms were measured using an electrochemical workstation (Solartron 1260 + 1287) in the voltage range of 0.01–3.0 V versus Li/Li+ at a scanning rate of 0.1 mV s−1 to analyze the intercalation/deintercalation behavior of lithium. Electrochemical impedance spectroscopy (EIS) was conducted with an amplitude voltage of 5 mV and a frequency range of 10 mHz–100 kHz using the same electrochemical station.
1 in volume) as the electrolyte. After 12 hours, the cells were tested galvanostatically in a voltage between 0.005 V and 3 V at different current densities (0.1, 0.2, 0.5, 1, 2, and 5 Ag−1) with a CT2001A instrument (Wuhan LAND Electronics Co., Ltd, China). Cyclic voltammograms were measured using an electrochemical workstation (Solartron 1260 + 1287) in the voltage range of 0.01–3.0 V versus Li/Li+ at a scanning rate of 0.1 mV s−1 to analyze the intercalation/deintercalation behavior of lithium. Electrochemical impedance spectroscopy (EIS) was conducted with an amplitude voltage of 5 mV and a frequency range of 10 mHz–100 kHz using the same electrochemical station.
Results and discussion
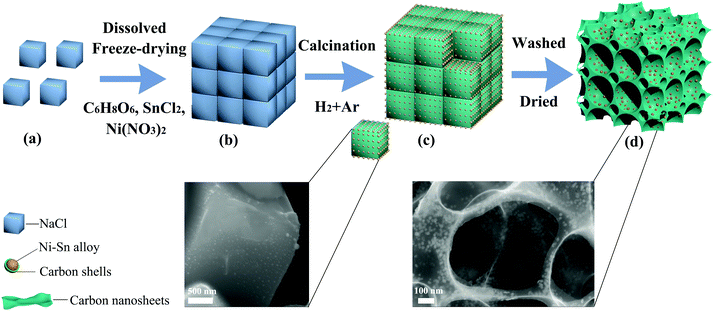
The synthesis process of Ni3Sn2/C and Ni3Sn4/C is schematically depicted in Fig. 1. At first, citric acid, NaCl, SnCl2, and Ni(NO3)2 were dissolved in water to form a clear solution, frozen to the solid state and then subjected to freeze-drying. During this step, the cubic NaCl particles were self-assembled into a 3D structure coated with SnCl2, Ni(NO3)2, and C6H8O6, and then the water was removed by sublimation under vacuum freeze-drying and the 3D structure of NaCl can be well preserved using the in situ formed SnCl2/citric acid/Ni(NO3)2 coated on (as shown in Fig. 1(b)). After calcination under a reducing atmosphere (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 1
Ar = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), citric acid was pyrolyzed and three-dimensional carbon networks were formed with the assistance of NaCl as a template, meanwhile, Ni3+ and Sn2+ were reduced and Ni–Sn alloy nanoparticles uniformly embedded in the carbon networks were formed (as illustrated in Fig. 1(c)). Then NaCl was removed by subsequent washing with deionized water, and the alloy nanoparticles still remain in their original position but the carbon networks collapse slightly exhibiting bowl-shaped carbon cages caused by the shrinkage of ultrathin carbon nanosheets during heat treatment (Fig. 1(d)), which indicates that NaCl plays the role of a template in the growth of 3D porous bowl-shaped carbon cages and Ni–Sn alloy nanoparticles. Moreover, the carbon produced from the pyrolysis of citric acid wraps the alloy homogeneously, which reduced the aggregation of Ni–Sn alloy nanoparticles.
3), citric acid was pyrolyzed and three-dimensional carbon networks were formed with the assistance of NaCl as a template, meanwhile, Ni3+ and Sn2+ were reduced and Ni–Sn alloy nanoparticles uniformly embedded in the carbon networks were formed (as illustrated in Fig. 1(c)). Then NaCl was removed by subsequent washing with deionized water, and the alloy nanoparticles still remain in their original position but the carbon networks collapse slightly exhibiting bowl-shaped carbon cages caused by the shrinkage of ultrathin carbon nanosheets during heat treatment (Fig. 1(d)), which indicates that NaCl plays the role of a template in the growth of 3D porous bowl-shaped carbon cages and Ni–Sn alloy nanoparticles. Moreover, the carbon produced from the pyrolysis of citric acid wraps the alloy homogeneously, which reduced the aggregation of Ni–Sn alloy nanoparticles.
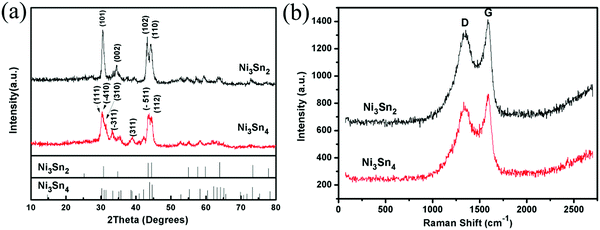
XRD patterns of Ni3Sn2/C and Ni3Sn4/C along with standard patterns are displayed in Fig. 2(a). The sample Ni3Sn2/C with strong diffraction peaks shows good crystallinity, which can be indexed to hexagonal Ni3Sn2 (PDF#06-0414). The sample Ni3Sn4/C is well-matched with monoclinic Ni3Sn4 of the C2/m space group (PDF#04-0845). The broadened peak around 30° in the patterns of Ni3Sn4/C indicates an inhomogeneous strain in the crystallites, which should be ascribed to the reduction in the crystallite size and the existence of an amorphous carbon layer. The average particle sizes of Ni3Sn2 and Ni3Sn4 calculated according to the Scherrer equation are about 18.8 nm and 17.9 nm, respectively. Nano-sized particles not only make the inevitable stress/strain smaller during lithiation/delithiation but also shorten the diffusion path of Li+ greatly. From the XRD patterns of both samples, no diffraction peaks assigned to carbon were detected, which may be owing to the disordered property of carbon and the high-intensity peaks of Ni–Sn alloy. TG-DTA was carried out in air to determine the content of Ni–Sn alloy in Ni3Sn2/C and Ni3Sn4/C composites (Fig. S1, ESI†). The sample was heated to 1000 °C so that the Ni–Sn alloy could be oxidized to NiO and SnO2, and carbon could be oxidized to CO2 completely. According to eqn (S1-1) and (S1-2) (ESI†), based on the remaining weight (NiO and SnO2) and the TG-DTA curves, the original contents of Ni3Sn2 and Ni3Sn4 are calculated to be 47.2 wt% and 57.7 wt%, respectively, which are in accordance with the designed values (Ni3Sn2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 1
C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, the mass fraction of Ni3Sn2 is 46.3%; Ni3Sn4
40, the mass fraction of Ni3Sn2 is 46.3%; Ni3Sn4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 1
C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, the mass fraction of Ni3Sn2 is 57.5%). In addition, Raman spectra were performed to evaluate the characteristic of carbon in Ni3Sn2/C and Ni3Sn4/C composites. As shown in Fig. 2(b), there are two sharp peaks located at around 1357 cm−1 and 1591 cm−1, corresponding to the D band and the G band, respectively. Generally, the D band corresponds to disordered carbon, edges and other defects, the G band originates from the ordered sp2 bonded carbon, and the intensity ratio (ID/IG) of the D band and the G band represents the disordered extent.26–28ID/IG ratios of Ni3Sn2/C and Ni3Sn4/C are 0.93 and 0.90, respectively. The disordered carbon can provide more active sites for Li+, which is beneficial for the capacity of an anode. No other peaks could be observed, which indicates that Ni–Sn alloy nanoparticles in the composite have not been oxidized during the synthesis process.
40, the mass fraction of Ni3Sn2 is 57.5%). In addition, Raman spectra were performed to evaluate the characteristic of carbon in Ni3Sn2/C and Ni3Sn4/C composites. As shown in Fig. 2(b), there are two sharp peaks located at around 1357 cm−1 and 1591 cm−1, corresponding to the D band and the G band, respectively. Generally, the D band corresponds to disordered carbon, edges and other defects, the G band originates from the ordered sp2 bonded carbon, and the intensity ratio (ID/IG) of the D band and the G band represents the disordered extent.26–28ID/IG ratios of Ni3Sn2/C and Ni3Sn4/C are 0.93 and 0.90, respectively. The disordered carbon can provide more active sites for Li+, which is beneficial for the capacity of an anode. No other peaks could be observed, which indicates that Ni–Sn alloy nanoparticles in the composite have not been oxidized during the synthesis process.
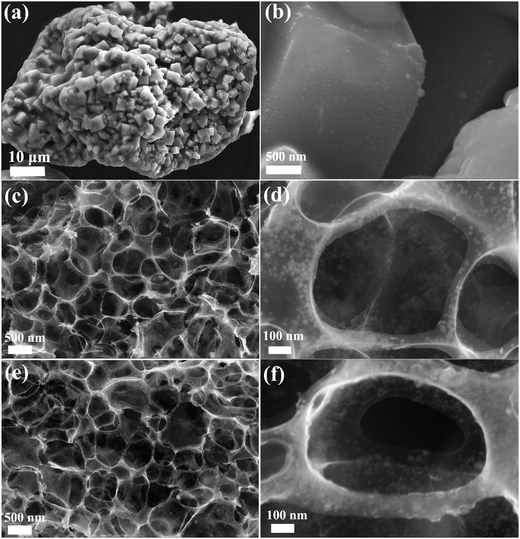
Fig. 3 shows the SEM images of Ni3Sn2/C before the removal of the NaCl template (a and b), and the final products Ni3Sn2/C (c and d) and Ni3Sn4/C (e and f). According to Fig. 3(a and b), it can be seen that the cubic NaCl (500 nm–2 μm) was self-assembled into a large 3D aggregate with nano-sized particles evenly dispersed on the surface after freeze-drying and annealing. After washing to remove the NaCl, as shown in Fig. 3(c–e), both Ni3Sn2/C and Ni3Sn4/C exhibit three-dimensional (3D) porous bowl-shaped carbon cages interspersed with nanoparticles, which were verified to be Ni–Sn alloy nanoparticles with carbon-coated shells by XRD and TEM. There were traces of Na and Cl residual in the final products, as shown in Fig. S2 (ESI†). The bowl-shaped carbon cages are submicrometer-sized macropores (300 nm–1 μm), which are roughly in agreement with the size of the cubic NaCl, which proves that the NaCl served as a template during the preparation. High magnification SEM images of Ni3Sn2/C (Fig. 3(d)) and Ni3Sn4/C (Fig. 3(f)) show that the sizes of Ni–Sn alloy nanoparticles were less than 20 nm. The nano-sized Ni–Sn alloy can shorten the diffusion path of Li+, reduce the mechanical stress and subsequently retard the crack of the electrode material, which are helpful to improve the structure stability of the Ni3Sn4/C composite. The unique three-dimensional porous bowl-shaped carbon cages can provide more space for buffering the volume variation, and the interconnected carbon cages are beneficial for the transportation of electrons and ions as well as the effective contact with the electrolyte.
TEM was performed to further investigate the microstructure of Ni3Sn2/C and Ni3Sn4/C, as displayed in Fig. 4. Fig. 4(a–c) show the images of Ni3Sn2/C at different magnifications; it exhibits well-defined 3D porous carbon networks interspersed with Ni–Sn alloy nanoparticles in a diameter range of 10–30 nm, in agreement with the XRD results, and their unique structure can provide elastic buffer spaces to accommodate the volume changes caused by Li+ insertion/extraction. What is more, after the preparation of the TEM sample even with a vigorous sonication for more than 2 h, it can be observed that most of the Ni–Sn alloy nanoparticles are still firmly embedded in the carbon nanosheets except for several escaped particles with the voids left (Fig. 4(b) and (e)), indicating the strong bonding force between the Ni–Sn alloy nanoparticles and the carbon nanosheets. Thus, the in situ growth of the Ni–Sn/carbon composite is different from the surface loading of Ni–Sn alloy nanoparticles on graphene in the aspect of interface bonding force. It is believed that the intimate interface bonding between Ni–Sn and carbon nanosheets can not only effectively impede the aggregation of Ni–Sn alloy nanoparticles to ensure a stable structure and good cycling stability, but also facilitate the transportation of electrons and diffusion of ions at the interface for improving the rate capacity.
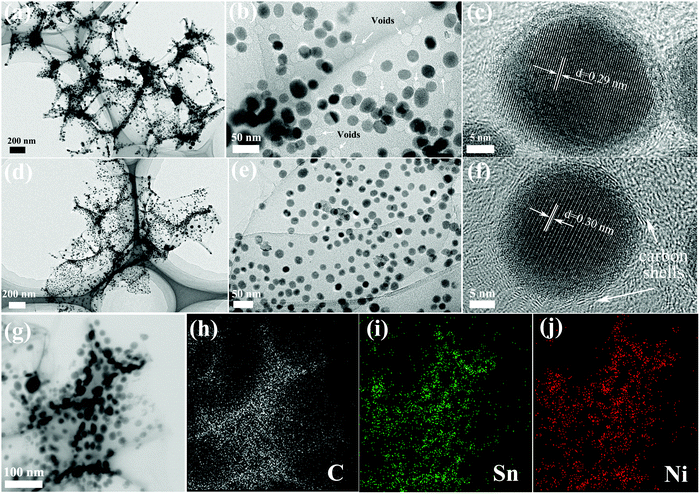 | ||
| Fig. 4 TEM images of Ni3Sn2/C (a–c) and Ni3Sn4/C (d–f), and STEM-EDS mapping images of Ni3Sn4/C (g–j). | ||
The HRTEM (Fig. 4(c)) image shows highly ordered lattice fringes with a spacing of 0.29 nm, which matches well with the d-spacing of the (101) plane of Ni3Sn2. When viewed in detail (Fig. 4(c and f)), there are graphitic carbon shells (d = 0.34 nm) coated around Ni–Sn alloy nanoparticles while the other carbon far from Ni–Sn nanoparticles is amorphous, which demonstrates the catalytic effect of Ni–Sn alloy nanoparticles on carbon. The Ni–Sn alloy nanoparticles without a carbon coating have a tendency to grow up and aggregate during the synthesis process and repeated lithiation/delithiation (Fig. S3(d), ESI†). Thus, the graphitic carbon shells can not only effectively restrain the growth of nano-sized Ni–Sn particles but also suppress the volume variation by segregating the alloy grain. Fig. 4(d–f) and Fig. S3 (ESI†) display the TEM images of Ni3Sn4/C, which exhibit 3D structures very similar to Ni3Sn2/carbon except for a lattice fringe with a spacing of 0.30 nm corresponding to the (111) plane of Ni3Sn4. The 3D carbon nanosheets exhibit a low contrast and the edge of the nanosheet is identified to be one-layer carbon (Fig. S3(c), ESI†), which demonstrates that the carbon nanosheets are ultrathin. Interestingly, some crystallized-amorphous core–shell structures of Ni3Sn4 nanoparticles were observed (Fig. S3(b), ESI†), which can be attributed to the rapid cooling process during the calcination step. Furthermore, the STEM-EDS mapping was performed to further verify the elemental composition and distribution of Ni3Sn4/C, as displayed in Fig. 4(g–j), and the result shows the homogeneous distribution of Sn and Ni in the carbon networks. The unique three-dimensional porous carbon structure, uniformly dispersed Ni–Sn nano-alloy, and homogeneous coating with carbon shells are beneficial for buffering the volume variation of active materials and fast electron transfer and rapid diffusion of Li+ during the cycling, resulting in improved electrochemical properties.
To evaluate the specific surface area (SSA) and pore size distribution of the composite, nitrogen adsorption–desorption measurements were executed at 77 K, as shown in Fig. 5. The specific surface areas of Ni3Sn2/C and Ni3Sn4/C according to BET are measured to be 460.635 m2 g−1 and 443.144 m2 g−1, respectively. The large surface area is good for rapid mass transport and surface reactions. The adsorption–desorption isotherm with a hysteresis loop in the P/P0 range of 0.5–0.95 suggests the existence of micropores and mesopores in the Ni–Sn/C composite. The pores presumably arise from the defects and the porous structure in the C layers induced by gases releasing during the carbonization process. The pore size distributions of the composites are analyzed by the BJH method (as shown in Fig. 5(b and d)), and the pore volumes of Ni3Sn2/C and Ni3Sn4/C are measured to be 0.933 cm3 g−1 and 0.889 cm3 g−1. This porous structure with a large surface area can tolerate the volume change of the Ni–Sn nanocrystals during charging/discharging.
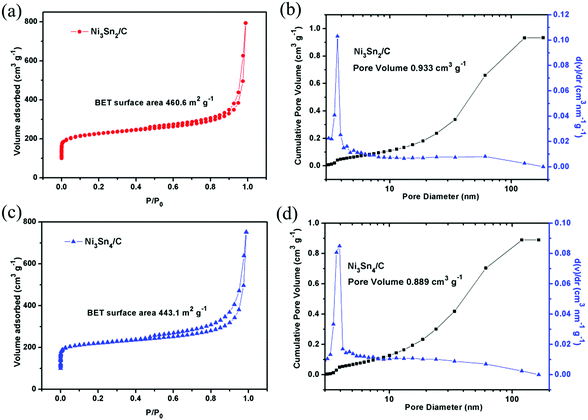 | ||
| Fig. 5 Nitrogen adsorption/desorption isotherms and pore distribution analysis of Ni3Sn2/C (a and b) and Ni3Sn4/C (c and d). | ||
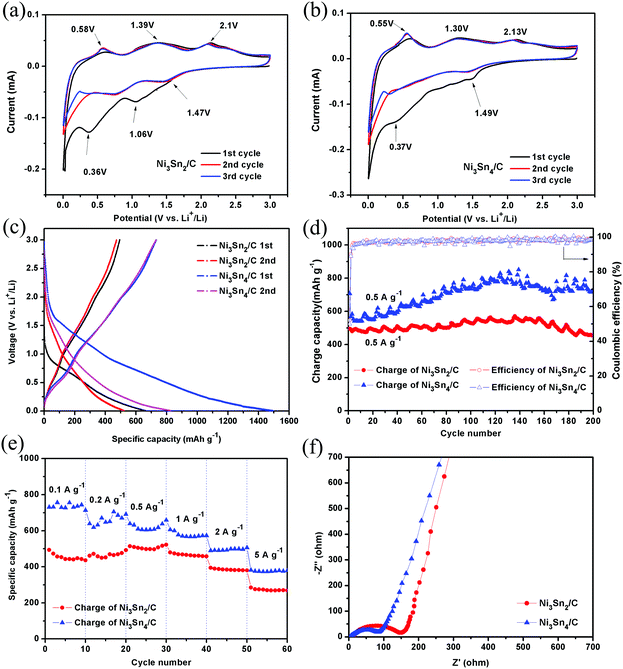
The electrochemical reactions of Ni3Sn2/C and Ni3Sn4/C electrodes were first investigated by cyclic voltammetry (CV). Fig. 6(a and b) show the CV curves of the initial three cycles of Ni3Sn2/C and Ni3Sn4/C at a scan rate of 0.1 mV s−1 between 0.005 V and 3 V (vs. Li/Li+). There is a large difference between the initial cycle and the following cycles in both Ni3Sn2/C and Ni3Sn4/C composites, the irreversible cathodic peaks between 0.5 and 1.5 V reveal the irreversible reactions related to the formation of solid electrolyte interphase (SEI) films because of solvent decomposition and the activation reaction, which result in irreversible capacity in the initial discharge process and match well with discharge curves in Fig. 6(c). The reduction peaks located at around 0.36 V in Ni3Sn2/C may be assigned to the formation of LixSn (x < 4.4) alloy. The corresponding oxidation peaks between 0.5 and 2.5 V may be assigned to the delithiation reaction of the LixSn alloy and the broad anodic peak at around 1.39 V represents lithium extraction from carbon.16 The CV curves of Ni3Sn2/C and Ni3Sn4/C match well with those of the Sn/C composite electrode (Fig. S4c, ESI†), indicating that the Ni in the Ni–Sn alloy is electrochemically inactive during the lithiation/delithiation process. The reaction process of Ni–Sn alloy is expected to be described by the following equations:13,29,30
| Ni3Sn4 + 17.6Li+ + 17.6e− → 4Li4.4Sn + 3Ni | (1) |
| Ni3Sn2 + 8.8Li+ + 8.8e− → 2Li4.4Sn + 3Ni | (2) |
| Li4.4Sn ⇔ Sn + 4.4Li+ + 4.4e− | (3) |
Reactions (1) and (2) only occur in the initial cycle and are irreversible. After the first cycle, Ni3Sn2 and Ni3Sn4 alloy nanoparticles are converted into a Sn/Ni composite, and the subsequent lithiation/delithiation reaction occurs according to eqn (3). The overlapped CV curves after the first cycle suggests the good reversibility and stability of the Ni–Sn/C electrode.
The electrochemical cycling stabilities of Ni3Sn2/C and Ni3Sn4/C electrodes were tested by the galvanostatic charge and discharge. Fig. 6(c) shows the initial two charge/discharge curves of Ni3Sn2/C and Ni3Sn4/C at a constant current density of 100 mA g−1. As anode materials, the discharge/charge process is in accordance with the insertion/extraction of Li+, according to the initial discharge curves (Fig. 6(c)), and it is evident that there is no obvious voltage platforms but smooth slashes appear; this is due to the successive insertion of Li+ and multi-step formation of intermetallic compounds (Li2Sn5, LiSn, Li7Sn3, Li5Sn2, Li13Sn5, Li7Sn2, and Li22Sn5) in different potential stages between 0.66 and 0.3 V (vs. Li/Li+), which are consistent with the multi-broad current peaks observed in CV scans.
Take the initial charge/discharge curves of Ni3Sn2/C and Ni3Sn4/C for comparison (Fig. 6(c)), where Ni3Sn4/C shows a higher initial charge/discharge capacity of 730.8 mA h g−1/1493 mA h g−1 compared with that of Ni3Sn2/C (494.1 mA h g−1/661.2 mA h g−1), which indicates that the excess Ni introduced in the Sn-based alloy results in a low total capacity because Ni in the Ni–Sn alloy is electrochemically inactive. Although high capacities are obtained for Ni–Sn/C, the initial Coulombic efficiency values of Ni3Sn2/C and Ni3Sn4/C are only 49% and 74.7%, respectively. Such low initial Coulombic efficiency was mainly attributed to the formation of solid electrolyte interphase (SEI) films at the surface of the electrode and the storage of Li+ in nano-voids which cannot be extracted in the subsequent cycles. In addition, the activating reaction of Ni3Sn2/C and Ni3Sn4/C (eqn (1) and (2)) accompanied by the formation of the Li4.4Sn with a separate Ni for the first cycle also produces irreversible capacity. Nonetheless, lithium ions could reversibly insert into the Ni–Sn composite in the subsequent cycling, which can be observed in Fig. 6(a and d) and is consistent with the CV curves. As shown in Fig. 6(c), the Ni3Sn4/C delivers a charge/discharge capacity of 731.3 mA h g−1/827.2 mA h g−1 with a Coulombic efficiency of 88.4% for the second cycle compared with that of Ni3Sn2/C (472.6 mA h g−1/521.8 mA h g−1, 90.6%). Moreover, Ni3Sn2/C and Ni3Sn4/C exhibit almost complete stable cycling performance even with gradually increased capacity after the second cycle and the Coulombic efficiency increases dramatically to almost 100% in subsequent cycles (Fig. 6(d)), indicating a perfect reversibility and stability of Ni–Sn/C alloy nanoparticles. In particular, the Ni3Sn4/C delivers a capacity of 568 mA h g−1 at 0.5 A g−1 for the second cycle and gradually increases to 732.7 mA h g−1 after 200 cycles. The similar capacity increasing behavior during cycling has also been observed in SnO2/graphene nano-composites,31 Sn/carbon composite anodes,32 and Sn alloy/carbon nanotube composite anode materials,33 which are normally attributed to a gradual activation process of the electrode, maybe the increase of active sites at the interfaces between the alloy and the carbon matrix.
To demonstrate the effect of Ni in the unique nanostructure, a reference sample of Sn embedded in 3D porous carbon cages was prepared by the same method and compared with the designed samples, as shown in Fig. S4 (ESI†). The capacity of Sn/C decreased rapidly with cycling, when the cells were disassembled after 100 cycles, it was found that the active material Sn/C has fallen off the current collector due to repeated volume changes. In contrast, both Ni3Sn4/C and Ni3Sn2/C exhibited a better cycling stability than that of Sn/C, and the active material was adhered firmly to the current collector after 100 cycles. This indicates that the Ni in Ni–Sn alloy plays a role in restraining the volume change and enhancing the stability of the composite anode. The morphology of the Ni3Sn4/C electrode after 200 cycles was explored by SEM and TEM, as shown in Fig. S5 (ESI†). It can be clearly seen that the three-dimensional porous bowl-shaped carbon cages were well kept after repeated cycling. However, the Ni3Sn4 nanoparticles cannot be observed because the surface was covered with an SEI film (Fig. S5(a and b), ESI†). Fig. S5(c and d) (ESI†) (TEM) displays that the monodisperse nanoparticles with a diameter of 10–30 nm have not aggregated and still firmly embedded in the three-dimensional porous bowl-shaped carbon cages, which is very similar to the morphology of the original sample, and this demonstrates the stability of their unique structure.
Rate performances were measured at a current density of 100 mA g−1, 200 mA g−1, 500 mA g−1, 1 A g−1, 2 A g−1, and 5 A g−1 10 times, respectively, as shown in Fig. 6(e). Although the capacity decreased slightly with rapidly increasing current density, both Ni3Sn2/C and Ni3Sn4/C exhibit excellent rate capability (Fig. 6(e)). In particular, Ni3Sn4/C can still deliver reversible capacities of 661, 622, 577, 496, and 377 mA h g−1 at 0.2, 0.5, 1, 2, and 5 A g−1, which are superior to those of most of the previously reported Ni–Sn alloy anode materials,11,13,18–20,34 as displayed in Table S2 (ESI†). The superior rate capability of Ni3Sn4/C is due to effective integration of Ni–Sn nanoparticles into a highly conductive three-dimensional porous carbon matrix. Nano-sized Ni–Sn particles provide a short path for lithium diffusion and the inactive Ni in Ni–Sn alloy nanoparticles can suppress the volume expansion of Sn during cycling. What is more, the unique 3D porous carbon structure could offer a large surface area to prevent the aggregation of alloy particles and provide more buffer space to restrain the strain induced by volume variation during cycling as well as provide high conductivity to facilitate electron transportation. To demonstrate the fast transmission of the lithium ion in the unique Ni3Sn4/C nanostructure, cyclic voltammetry of cells made of Ni3Sn4/C under different scanning rates (0.2 mV s−1, 0.4 mV s−1, 0.6 mV s−1, 0.8 mV s−1, 1 mV s−1) was performed, as shown in Fig. S6 (ESI†). The diffusion coefficient of the lithium ion was calculated to be 2.45 × 10−7 cm2 s−1via the CV plots according to the Randles–Sevcik equation at room temperature, which is higher than those of nano-sized Sn (8 × 10−8 cm2 s−1) and the Sn/Cu6Sn5 composite thin film electrode (1.91 × 10−7 cm2 s−1).35,36 Thus, the Ni3Sn4/C composite anode exhibits excellent rate capability and cycle performance.
To further understand the electrochemical behaviors of Ni3Sn2/C and Ni3Sn4/C, EIS of freshly coined cells and after 200 cycles was performed, as shown in Fig. 6(f) and Fig. S7 (ESI†). In accordance with the EIS of other Ni–Sn/carbon composite anodes,16–18 the Nyquist plots of both electrodes consist of a semicircle at high frequency and an inclined line at low frequency. The semicircle is associated with the SEI film impedance and the charge transfer resistance, while the inclined line represents the Warburg impedance (W) corresponding to the Li+ ion diffusion in the Ni–Sn/C composite electrode. As can be seen in Fig. 6(f), the diameter of the semicircle for both the Ni–Sn/carbon composites is smaller than that of some reported Sn and Ni–Sn alloy anodes,20,27,37 indicating that the unique 3D carbon architecture can provide high electrical conductivity and enhance the electrochemical reaction kinetics. Furthermore, the kinetic parameters (Rs and Rct) were calculated by modeling EIS on the basis of the modified equivalent circuit (Fig. S7, ESI†) and are displayed in Table S1 (ESI†). It is obvious that the diameter of the semicircle for the Ni3Sn4/C electrode is much smaller than that of Ni3Sn2/C; moreover, the SEI film resistance Rf and the charge-transfer resistance Rct of Ni3Sn4/C are lower than those of Ni3Sn2/C (Table S1, ESI†), and thus, Ni3Sn4/C displays better rate capability than that of Ni3Sn2/C. By comparing the kinetic parameters (Rs and Rct) of Ni–Sn/C before cycling with that of Ni–Sn/C after 200 cycles, it can be found that the Rf of both samples increased a little after 200 cycles due to the formation of SEI films. While the Rct of both samples decreased significantly after 200 cycles, for instance, the Rct of Ni3Sn4/C decreased from 64.8 Ω to 38.8 Ω, which could be attributable to the activation of interfaces between the alloy and the carbon matrix, contributing to better rate capability, as displayed in Fig. 6(e) and Table S2 (ESI†).
The above evidence clearly demonstrates that 3D porous bowl-shaped carbon cages interspersed with carbon coated Ni–Sn alloy nanoparticles (Ni3Sn2 and Ni3Sn4) possess high reversible capacity, excellent cycling capability, and superior rate capability, which can be ascribed to the synergetic effect of Ni–Sn alloy nanoparticles and unique 3D porous bowl-shaped carbon nanostructures. First, moderate Ni in Ni–Sn alloy can effectively buffer the volume variation of Sn during lithium-ion insertion/extraction. Second, the ultrathin 3D carbon nanosheets firmly embedded with Ni–Sn alloy nanoparticles can prevent the aggregation of Ni–Sn nanoparticles, accommodate their volume changes, and ensure the structural integrity of the electrode upon lithium-ion insertion/extraction. What is more, the small Ni–Sn alloy nanoparticles can shorten the diffusion path of Li+, reduce the mechanical stress and subsequently retard the crack of the electrode material. Last but not least, the 3D porous bowl-shaped carbon cage networks with excellent electrical conductivity, rich porous structure and large surface area facilitate the transportation of electrons and promote the diffusion of Li+ in the electrode, leading to high reversible capacity and excellent rate capability. Furthermore, abundant pores can provide more buffering space for the expansion of the Ni–Sn alloy upon lithiation, release the stress, and avoid the pulverization of the electrode after repeated cycling. All the above points are accountable for the superior electrochemical performance of the Ni–Sn alloy/carbon composite anode.
Conclusions
In summary, a novel Ni–Sn alloy/carbon composite with 3D nanostructures was successfully synthesized by a feasible freeze-drying method with self-assembled NaCl as a template following by annealing. In this composite, Ni3Sn2@C/Ni3Sn4@C nanoparticles (10–30 nm) were well dispersed and confined to the 3D porous bowl-shaped carbon cages. Owing to the shortened diffusion path of Li+, the good electronic conductivity, the buffer effect of inactive Ni, and the 3D structural integrity to accommodate volume variations, the Ni3Sn4/C nanocomposite demonstrates excellent cycling stability with high overall capacities (732 mA h g−1 after 200 cycles at 0.5 A g−1) and exceptional high rate capability (496 mA h g−1 for 2 A g−1 and 377 mA h g−1 for 5 A g−1), allowing its application in high energy and high power batteries. Moreover, the in situ salt-template-assisted synthesis strategy for constructing 3D ultrathin carbon networks interspersed with carbon coated Ni–Sn nanoparticles can be easily extended to other materials (metal, alloy, or metal oxides) used for photocatalysis, oxygen reducing catalysts, electrochemical energy storage, and so on.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51404055, 51374056, and 51571054), the Special Fund for Basic Scientific Research of Central Colleges, Northeastern University (No. N142304002, N100123003, and N120523001), the Natural Science Foundation of Hebei Province (No. B2015501020 and E2013501135), the Youth Foundation of Hebei Educational Committee (No. QN2014325), the Program for New Century Excellent Talents in University (No. NCET-10-0304), the Key Technologies R & D Program of Qinhuangdao of Hebei Province (No. 201402B005), and the Doctoral Scientific Research Foundation of Northeastern University at Qinhuangdao, China (No. XNB201511).References
- L. W. Ji, Z. Lin, M. Alcoutlabi and X. W. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- P. Roy and S. K. Srivastava, J. Mater. Chem. A, 2015, 3, 2454–2484 CAS.
- M. N. Obrovac and V. L. Chevrier, Chem. Rev., 2014, 114, 11444–11502 CrossRef CAS PubMed.
- H. J. Tian, F. X. Xin, X. L. Wang, W. He and W. Q. Han, J. Materiomics, 2015, 1, 153–169 CrossRef.
- X. L. Wu, Y. G. Guo and L. J. Wan, Chem. – Asian J., 2013, 8, 1948–1958 CrossRef CAS PubMed.
- M. G. Kim, S. J. Sim and J. Cho, Adv. Mater., 2010, 22, 5154–5158 CrossRef CAS PubMed.
- X. L. Wang, M. Feygenson, M. C. Aronson and W. Q. Han, J. Phys. Chem. C, 2010, 114, 14697–14703 CAS.
- D. D. Jiang, X. H. Ma and Y. B. Fu, J. Appl. Electrochem., 2012, 42, 555–559 CrossRef CAS.
- M. Tian, W. Wang, S. H. Lee, Y. C. Lee and R. G. Yang, J. Power Sources, 2011, 196, 10207–10212 CrossRef CAS.
- B. Wang, B. Luo, X. L. Li and L. J. Zhi, Mater. Today, 2012, 15, 544–552 CrossRef CAS.
- J. Hassoun, S. Panero, P. Simon, P. L. Taberna and B. Scrosati, Adv. Mater., 2007, 19, 1632–1635 CrossRef CAS.
- J. Qin, C. N. He, N. Q. Zhao, Z. Y. Wang, C. S. Shi, E. Z. Liu and J. J. Li, ACS Nano, 2014, 8, 1728–1738 CrossRef CAS PubMed.
- X. S. Zhou, L. Yu, X. Y. Yu and X. W. Lou, Adv. Energy Mater., 2016 DOI:10.1002/aenm.201601177.
- Y. H. Xu, Q. Liu, Y. J. Zhu, Y. H. Liu, A. Langrock, M. R. Zachariah and C. S. Wang, Nano Lett., 2013, 13, 470–474 CrossRef CAS PubMed.
- Z. Q. Zhu, S. W. Wang, J. Du, Q. Jin, T. R. Zhang, F. Y. Cheng and J. Chen, Nano Lett., 2014, 14, 153–157 CrossRef CAS PubMed.
- J. Qin, X. Zhang, N. Q. Zhao, C. S. Shi, E. Z. Liu, J. J. Li and C. N. He, RSC Adv., 2014, 4, 49247–49256 RSC.
- X. Y. Hou, Y. J. Hu, H. Jiang, Y. F. Li, X. F. Niu and C. Z. Li, Chem. Commun., 2015, 51, 16373–16376 RSC.
- Z. Yi, X. Tian, Q. G. Han, Y. Cheng, J. S. Lian, Y. M. Wu and L. M. Wang, Electrochim. Acta, 2016, 192, 188–195 CrossRef CAS.
- W. Wang and M. H. Cao, Mater. Chem. Phys., 2016, 177, 198–205 CrossRef CAS.
- C. N. He, S. Wu, N. Q. Zhao, C. S. Shi, E. Z. Liu and J. J. Li, ACS Nano, 2013, 5, 4459–4469 CrossRef PubMed.
- J. W. Zhou, J. Qin, X. Zhang, C. S. Shi, E. Z. Liu, J. J. Li, N. Q. Zhao and C. N. He, ACS Nano, 2015, 9, 3837–3848 CrossRef CAS PubMed.
- Z. Y. Wang, S. H. Luo, F. Chen, D. Wang, Y. G. Liu, X. W. Qi, C. S. Shi and N. Q. Zhao, RSC Adv., 2016, 6, 54718–54726 RSC.
- D. Y. Ren, Y. J. Hu, H. B. Jiang, Z. N. Deng, S. H. Petr, H. Jiang and C. Z. Li, ACS Sustainable Chem. Eng., 2016, 4, 1148–1153 CrossRef CAS.
- Z. Zhu, S. Wang, J. Du, Q. Jin, T. Zhang, F. Cheng and J. Chen, Nano Lett., 2014, 14, 153–157 CrossRef CAS PubMed.
- C. Botas, D. Carriazo, G. Singh and T. Rojo, J. Mater. Chem. A, 2015, 3, 13402–13410 CAS.
- Z. Y. Fan, J. Liang, W. Yu, S. J. Ding, S. D. Cheng, G. Yang, Y. L. Wang, Y. X. Xi, K. Xi and R. V. Kumar, Nano Energy, 2015, 16, 152–162 CrossRef CAS.
- J. Liu, Y. R. Wen, P. A. V. Aken, J. Maier and Y. Yu, Nano Lett., 2014, 14, 6387–6392 CrossRef CAS PubMed.
- B. D. Polat, A. Abouimrane, N. Sezgin, O. Keles and K. Amine, Electrochim. Acta, 2014, 135, 585–593 CrossRef CAS.
- P. C. Lian, X. F. Zhu, S. Z. Liang, Z. Li, W. S. Yang and H. H. Wang, Electrochim. Acta, 2011, 56, 4532–4539 CrossRef CAS.
- Y. H. Xu, J. C. Guo and C. S. Wang, J. Mater. Chem., 2012, 22, 9562–9567 RSC.
- X. F. Li, Y. Zhong, M. Cai, M. P. Balogh, D. N. Wang, Y. Zhang, R. Y. Li and X. L. Sun, Electrochim. Acta, 2013, 89, 387–393 CrossRef CAS.
- L. Huang, H. B. Wei, F. S. Ke, X. Y. Fan, J. T. Li and S. G. Sun, Electrochim. Acta, 2009, 54, 2693–2698 CrossRef CAS.
- T. Zhang, L. J. Fu, J. Gao, Y. P. Wu, R. Holze and H. Q. Wu, J. Power Sources, 2007, 174, 770–773 CrossRef CAS.
- R. Z. Hu, M. Q. Zeng and M. Zhu, Electrochim. Acta, 2009, 54, 2843–2850 CrossRef CAS.
- I. Amadei, S. Panero, B. Scrosati, G. Cocco and L. Schiffini, J. Power Sources, 2005, 143, 227–230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TG-DTA curves of Ni3Sn2/C and Ni3Sn4/C composites in air, HRTEM images of Ni3Sn4/C taken from different representative parts, the EIS curves of Ni3Sn2/C and Ni3Sn4/C composites and equivalent circuit, and electrochemical performances of Ni3Sn2/C and Ni3Sn4/C, and some representative Ni–Sn alloy anodes in the literature. See DOI: 10.1039/c6nj02458k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |