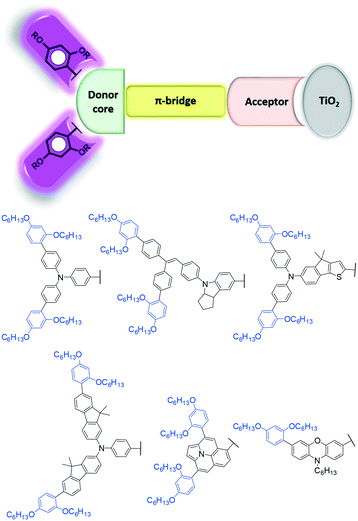
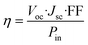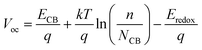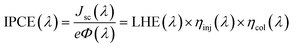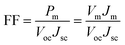Molecular engineering of organic sensitizers with o,p-dialkoxyphenyl-based bulky donors for highly efficient dye-sensitized solar cells
Xing
Li
,
Xiaoyu
Zhang
,
Jianli
Hua
 and
He
Tian
*
and
He
Tian
*
Key Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, Shanghai 200237, People's Republic of China. E-mail: tianhe@ecust.edu.cn; Fax: (+)86 21 64252758; Tel: (+)86 21 64252756
First published on 20th January 2017
Abstract
Charge recombination has always been the main factor restricting the performance improvement of dye-sensitized solar cells (DSSCs). From the view of the dye structure, the introduction of substituents like alkoxy/alkyl chains with a large steric hindrance is one of the commonly used strategies to enhance the blocking effect of dye molecules at the interface between TiO2 and the electrolyte/hole conductor. As a result, the charge recombination can be effectively hampered to improve the solar cells' performance. Here, we review the recent progress and molecular designs in the field of organic sensitizers with o,p-dialkoxyphenyl (DAP)-based bulky donor groups. The advantages and disadvantages of the bulky donor groups for the absorption spectrum, charge recombination and dye regeneration are discussed. At the end, feasible design strategies of organic sensitizers for achieving high open-circuit voltage (Voc), short circuit photocurrent density (Jsc) and stability in both liquid-electrolyte DSSCs and all-solid-state DSSCs are proposed.
1 Introduction
In order to relieve the global energy crisis and the environmental pollution caused by the consumption of fossil fuels, the development of effectively-utilized solar energy has been regarded by many researchers as a promising and practical solution. Therefore, exploration of high-performance and low-cost photoelectric conversion materials is of great significance for both fundamental research and practical applications. As early as 1988, Tian et al. systematically investigated the structure–property relationship of organic dyes and proved the sensitizers' substituents effect on the photovoltaic performance of organic solar cells.1In 1991, Grätzel and his colleague introduced mesoporous TiO2 nanocrystal layers into a dye-sensitized solar cell (DSSC) system, which dramatically increased the dye loading amount, leading to a ground breaking photovoltaic technology with significant improvement of the photoelectric conversion efficiency (PCE).2 DSSC has become one of the most promising technologies to harness solar power because of its easy preparation process, high performance-price ratio and low toxicity in comparison to other types of solar cells.3 The PECs of DSSCs were gradually and steadily enhanced with various sensitizers during the last 25 years, as shown in Fig. 1. Ruthenium-based dye N719 maintains the highest certified PCE record of 11.9% in conjunction with an iodide-based electrolyte,4,5 and an 11.5% efficiency was achieved by porphyrin dye XW11 co-sensitized with the metal-free organic dye WS-5 using an iodide-based electrolyte, recently.6 However, regeneration of oxidized dyes with the iodide-based electrolyte has a severe potential loss of 300–600 mV due to larger gaps between the redox potential of the electrolyte couples and the highest occupied molecular orbital (HOMO) level of the sensitizers.7 Moreover, the corrosiveness of the iodide/triiodide redox couple toward most metals and sealing materials is also the main hindrance to the long-term stability of DSSCs.8 To date, the most successful alternative redox mediator is a cobalt-based redox couple, which has resulted in a more promising PCE compared to the iodide-based electrolyte.9 Then, a new efficiency record of 14.5% was reported through collaborative sensitization with an alkoxysilyl-anchor dye ADEKA-1 and a carboxy-anchor dye LEG4,10 while the porphyrin dye SM315 exhibited a 13% PCE without using co-sensitizers with the cobalt-based electrolyte.11 The higher PCEs of DSSCs with cobalt-based electrolytes benefit from the more positive redox potentials, which lead to the higher Voc. All-solid-state DSSCs (ssDSSCs) using solid hole conductors instead of a liquid electrolyte are also capable of obtaining high Voc.12,13 Interestingly, LEG4-sensitized ssDSSCs achieved a PCE of 7.7% (ref. 14) and 8.2% (ref. 15) with 2,2′,7,7′-tetrakis (N,N-di-p-methoxy phenylamine)-9,9′-spirobifluorene (spiro-MeOTAD) and copper(I/II) phenanthroline as hole conductors, respectively, which are the state of the art in the field of ssDSSCs. It is noteworthy that problems like the leakage and corrosion of liquid electrolytes can be avoided in ssDSSCs, which opens up a new promising path for the industrialization of DSSCs.
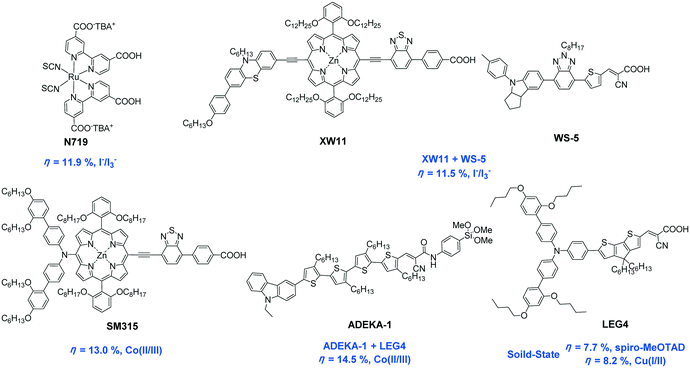 | ||
| Fig. 1 The highest efficiencies with various sensitizers reported to date (PCEs and redox couples employed are given in blue). | ||
1.1 Device structure and operational principles
The conventional n-type DSSCs can be simply classified as liquid-electrolyte and solid-state DSSCs based on the reductive media in the dye-regeneration and hole-transporting processes. A typical device structure of a liquid-electrolyte DSSC has five main components: (I) transparent conducting oxide (TCO) substrates, fluorine doped tin oxide (FTO) conductive glass has usually been used as the substrate because of its high chemical inertness and thermal stability; (II) a mesoporous semiconductor film (mainly TiO2); (III) dye sensitizers for light harvesting and electron generation; (IV) a redox electrolyte for the regeneration of the oxidize sensitizers and hole transportation; (V) a counter electrode (CE), platinum and carbon materials are two primary catalysts applied on CEs.16 In addition, a schematic representation and electron transfer processes occurring in a typical liquid-electrolyte DSSC device are depicted in Fig. 2a. When a DSSC is exposed to light, a dye molecular anchored on the TiO2 surface is excited from the ground state dye (D) to the excited state (D*). The electron is injected into the conduction band (CB) of TiO2 and through the external circuit to reach the counter electrode; the oxidized dye (D+) is immediately regenerated by a reductive mediator in the electrolyte, while the corresponding oxidizing mediator diffuses to the counter electrode to gain an electron and regenerate. Consequently, a complete photo-electric circuit system is achieved for generating an electric current.16 However, some charge transfer processes present in a DSSC system are detrimental to the performance as the dashed-line arrows show. These are comprised of the decay of the excited states and intrinsic relaxation process of the exciton, as well as the electron recombination processes from TiO2 to the oxidized dye/the oxidizing agent present in the electrolyte.2,15 To understand the kinetics and dynamics of the charge movement, the different time scales of the relevant processes are depicted in Fig. 2b.17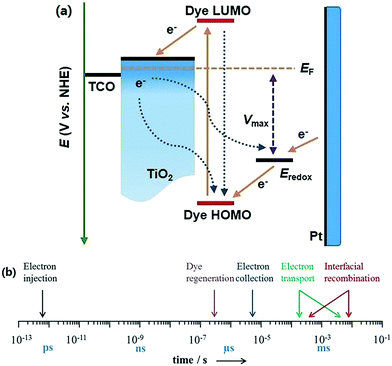 | ||
| Fig. 2 (a) Energy level diagram and electron transfer processes and (b) the different time scales of kinetic processes in a typical DSSC device. | ||
Taking the requirements of long-term stability and industrial production processes into account, the problems of volatile solvent leakage and corrosion of metal contacts associated with liquid electrolytes should be avoided, and therefore, solid-state hole-transport materials (HTM) have been investigated and developed to replace the liquid electrolyte.18–20 The basic structure of a ssDSSC with an organic small molecular HTM is shown in Fig. 3a, which shows the following differences compared to the liquid-electrolyte DSSCs: 1) all-solid-state DSSCs are normally monolithic in structure, which entirely differs from the sandwich structure of liquid-electrolyte DSSCs; 2) partial conductive layer of FTO should be etched to avoid the short circuit caused by direct contact between the two electrodes; 3) solid-state HTM replaces the liquid electrolyte; 4) a compact TiO2 layer should be deposited on the FTO surface, which can inhibit the charge recombination at the interface between the HTM and FTO; and 5) a metal back contact has been adopted in all solid-state DSSCs (typically gold or silver).21
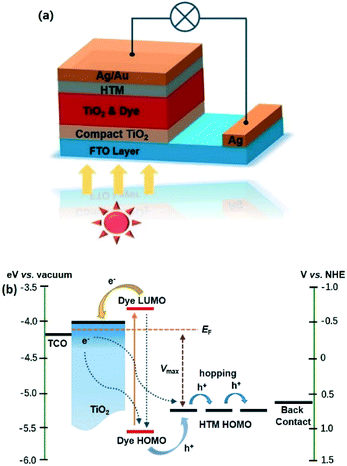 | ||
| Fig. 3 (a) Device architecture and (b) energy level diagram and charge processes in a typical ssDSSC. | ||
As shown in Fig. 3b, the ssDSSCs system shares almost the same charge transfer processes as the liquid-electrolyte DSSCs, except that the regeneration process of an oxidized dye in an ssDSSC is through hole injection from the oxidized dye to HTM, and then, the holes transfer to the back electrode through a hopping mechanism. The hole injection efficiency for dye regeneration is mainly governed by the potential difference between the HOMO levels of the oxidized dye and the HTM and the charge recombination.21,22 In addition, effective and compact interface contact is also favorable for the charge transfers between the dye and the HTM. Spiro-MeOTAD, the most successful HTM so far, was first introduced into ssDSSCs by Bach et al. in 1998. The following studies rapidly increased the PCE from less than 1% to over 5%.18 This impressive advance for ssDSSCs comes from the high glass-transition temperature, good solubility, and moderate molecule size of spiro-OMeTAD. However, the recombination rate at the spiro-OMeTAD/TiO2 interface is much higher in ssDSSCs with respect to that of liquid-electrolyte DSSCs.23,24 Therefore, the inhibition of charge recombination at the spiro-OMeTAD/TiO2 interface is of great importance for obtaining highly efficient ssDSSCs.
1.2 Photovoltaic parameters of DSSCs
In order to evaluate the performance of the solar cells, the current–voltage (J–V) characteristic of the device is usually measured (Fig. 4). An external bias voltage is applied stepwise, and the photocurrent value of the device is recorded simultaneously. The interval change of the bias voltage is usually 10 mV, which can be swept from the short circuit to the open circuit (forward) and can be reverse swept. However, a delay time before each step of the bias voltage is needed to keep the device in a static state. Han et al. proved that an average delay time of at least 40 ms is needed in DSSCs.25 There are four main parameters of solar cell performance obtained by analyzing the J–V curves, including Voc, Jsc, fill factor (FF), and photoelectric conversion efficiency (PCE, η). Their relationship is shown in the following formula: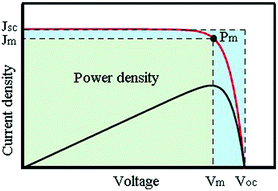 | ||
| Fig. 4 Typical current–voltage curve (red line) and the power as a function of voltage (black line). | ||
V oc is the measured value under open circuit conditions, which depend on the difference between the quasi-Fermi level of TiO2 and the redox level:
J
sc is measured under the short circuit condition, which is mainly subject to the light-harvesting capability (absorption spectral range and molar extinction coefficient) of the dye and energy alignment. The light trapping capability can be improved by reducing the band gap between the HOMO and lowest unoccupied molecular orbital (LUMO) levels of the dye and increasing the molar extinction coefficient. Furthermore, in order to maintain a proper energy level alignment, the HOMO level of the dye should remain sufficiently below the Eredox to ensure thermodynamically favorable regeneration of the oxidized dyes, and the LUMO level of the dye should not be lowered too much to guarantee the electron injection into the CB of TiO2. In addition, the incident photon-to-current conversion efficiency (IPCE) spectrum is also a commonly used measurement for analyzing Jsc comprehensively. The IPCE can be calculated from the photocurrent density produced in the external circuit of the cell under monochromatic illumination divided by the incident photon flux. IPCE is often expressed as the product of the efficiency of different charge transfer processes inside the device for better understanding and analysis:
FF is defined as the ratio of the maximum output power (Pm) and the multiplication value of Voc and Jsc:
1.3 General background and intentions
In order to improve the performance of DSSCs, a lot of researchers are interested in how to overcome the prominent problem of charge recombination that existed in both liquid-electrolyte and solid-state DSSCs, especially at the interface of TiO2, sensitizers and the oxidized redox mediator/HTM. Nonetheless, organic sensitizers are fairly easy to synthesize with molecular modifications to enhance the blocking effect, which has been a key and effective method to reduce charge recombination. Since 2008, our group synthesized a series of organic sensitizers based on starburst triarylamine, which can form a more compact sensitizer layer on the TiO2 surface, reducing the charge recombination with regard to that of single triphenylamine (TPA).28–32 Besides, adding long alkyl chains onto the sensitizer is also an important strategy to suppress the charge recombination arising from impeding the approach of the oxidized redox mediator/HTM to the TiO2 surface, and it has been widely demonstrated that the Voc and efficiency would be improved effectively compared to sensitizers without the alkyl chains.33–36 In 2010, Hagfeldt and Sun et al. demonstrated a significant enhancement in the PCE of cobalt-based cells by adding insulating o,p-dibutoxylphenyl units to an organic dye, owing to the reduced charge recombination as well as the effective diffusion coefficients of the redox couples.37 With many years of development, the o,p-dialkoxyphenyl (DAP) unit has been successfully used as an effective blocking unit in the donor group of those dyes for high efficiency in DSSCs, particularly in the cobalt-based DSSCs and ssDSSCs, as shown in Fig. 1.Accordingly, the structure characteristics of bulky donor groups can be categorized into two groups: one is increasing the size by expanding the conjugation, which is propitious to form a compact sensitizer layer on the TiO2 surface; the other is enhancing the steric hindrance by introducing long alkyl or alkoxy chains, which help block the approach of the oxidized redox mediator/HTM to the TiO2 surface. In other words, a larger donor group is more favorable for obtaining a high efficiency in systems with a severe charge recombination problem like the cobalt-based DSSCs and ssDSSCs, in which the charge recombination between the Co(III)-complex/HTM and TiO2 surface as well as the molecular aggregation can be effectively suppressed. So far, many DAP-based bulky donors, based on different cores like TPA, indoline, fluoren-substituted aniline, and phenoxazine have been developed in organic dyes for DSSCs. Generally, a typical structure of an organic dye is the donor (D)–π-bridge(π)–acceptor (A) configuration because of the good intramolecular charge transfer (ICT) property.16,38 In this review, we focused on the sensitizers with DAP-based bulky donor groups and the significant progress in reducing the charge recombination and their molecular design strategies to enhance the light-harvesting capability in both liquid-electrolyte DSSCs and ssDSSCs (Fig. 5).
2 Liquid-electrolyte DSSCs
2.1 TPA based donor
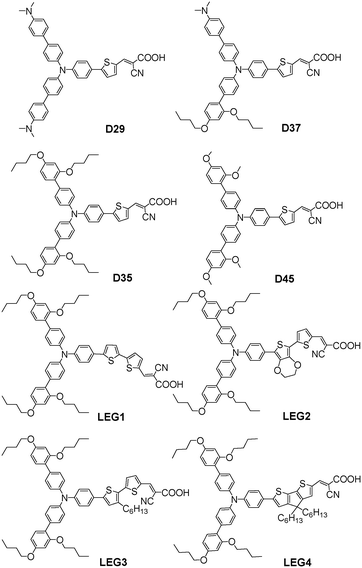
In 2009, dye D35 first applied the DAP unit in DSSCs and was systematically studied with respect to dyes D29 and D37 through the functionalized TPA donor units with electron-donating groups and bulky substituents, respectively, by Hagfeldt and Sun et al.39 The spectral response of D35 was not red-shifted as much as D29 and D37 due to the lower electron-donating ability of the DAP unit compared to the p-dimethylamine group. The calculated absorption spectra show that the main absorption band is predicted to broaden at around 450 nm for the unsymmetrical dye D37, which is essentially forbidden in D29 and D35. This difference is mainly due to a larger contribution from the HOMO-1/LUMO transition arising from a better overlap between the HOMO-1 and LUMO in D37 (Fig. 6). However, D35-based cells in combination with iodine-based electrolytes showed the highest efficiency because the insulating effect of the bulky DAP groups decreased the electron recombination, resulting in a remarkably high Voc of 0.75 V.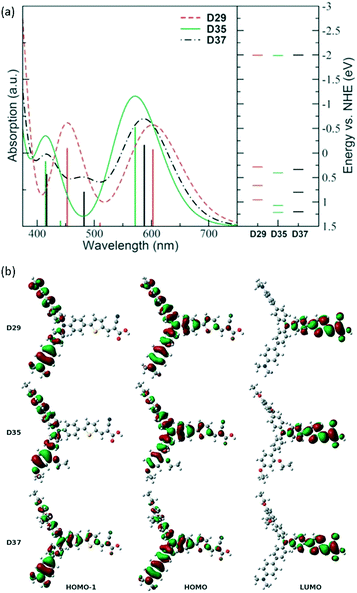 | ||
| Fig. 6 (a) Calculated gas-phase absorption spectra of the senzitisers together with the relative energy levels of the three dyes, (b) the electron distribution of the frontier molecular orbitals of D29, D35 and D37. Reprinted with permission from ref. 39. Copyright © 2009 Royal Society of Chemistry. | ||
Furthermore, a comparative study of D29 and D35 was conducted by employing cobalt-based redox mediators with various polypyridine ligands. As a result, when the insulating bulky groups were added to the donor group of the organic dyes, the recombination in the system was reduced by at least one order of magnitude. The insulating groups were removed from the polypyridine ligands, allowing the use of cobalt redox couples with smaller steric bulk, which efficiently improved the diffusion of the cobalt-based redox couples. Thus, the device based on D35 achieved a high PCE of 6.7% with a high Voc of 0.92 V and Jsc of 10.7 mA cm−2 using the [Co(bpy)3]2+/3+-based electrolyte under simulated AM 1.5G conditions, which was higher than the PCE of the counterpart with the iodide-based electrolyte (PCE = 5.5%). The best efficiency for the DSSCs sensitized with D29 was only 2.6% using the [Co(dmb)3]2+/3+-based electrolyte. It was found that introducing the bulky DAP units on the donor groups can retard effective electron recombination between TiO2 and cobalt(III)-complexes.37 Then, the bulky DAP group is considered as a promising alternative constitutional unit for the design and synthesis of new organic dyes, especially in the cobalt electrolyte-based DSSCs.
Recently, an organic dye D45 was introduced by replacing the butoxy chains in D35 with methoxy chains, and the groups also possess similar energy levels and HOMO–LUMO differences. The influence of the length of the alkoxy chains on the kinetics of the dye regeneration and recombination were investigated. D45 with methoxy chains showed faster recombination and regeneration than D35 in DSSCs using the [Co(bpy)3]2+/3+ electrolyte as a result of the shorter distance of the redox species approaching the donor group. However, the D45-sensitized device yielded a significantly decreased PCE compared to that of D35 for both DSSCs with Pt and poly(3,4-ethylenedioxythiophene) (PEDOT) as counter electrodes, owing to the lower Voc and faster charge recombination rate for D45.40 This shows that the longer dibutoxyphenyl groups can prevent the oxidized redox couple from reaching the dye surface and reducing the recombination.
The relatively narrow absorption spectrum for D35 limits the available photocurrent and the PCE of the devices. Since DSSCs with cobalt-based electrolytes typically use very thin TiO2 films, it is especially important that the dyes have high extinction coefficients for efficient light harvesting. In order to enhance the spectral response of the D35 dye in the red wavelength region, Hagfeldt and Sun et al. further synthesized four DAP-based TPA dyes (LEG1–LEG4), and the π-bridge of thiophene in D35 was replaced with bithiophene in LEG1, 3,4-ethylenedioxythiophene (EDOT) in LEG2, bithiophene substituted with a hexyl chain in LEG3 and CPDT in LEG4. The red-shifts of the absorption occurred, and the extinction coefficient increased.41 At the same time, another specific work was also carried out by them, and only LEG3 and LEG4 exhibited improved PCEs in DSSCs with the cobalt-based electrolyte compared to the reference D35 dye. It should be noted that LEG3 showed a significantly higher Voc compared to the other dyes, owing to the more efficient electron recombination inhibition, which reflects that the π-linker containing alkyl chains plays a crucial role in achieving a high Voc. However, LEG4 together with LEG2 showed a relatively low Voc compared to the other sensitizers, which came from the lower dye coverage for LEG4 and serious dye aggregation for LEG2. Interestingly, LEG4 showed the best PCE of 6.78% with the [Co(bpy)3]2+/3+-based electrolyte due to the strong light-harvesting ability and steric effect arising from a CPDT linker (Fig. 7).42
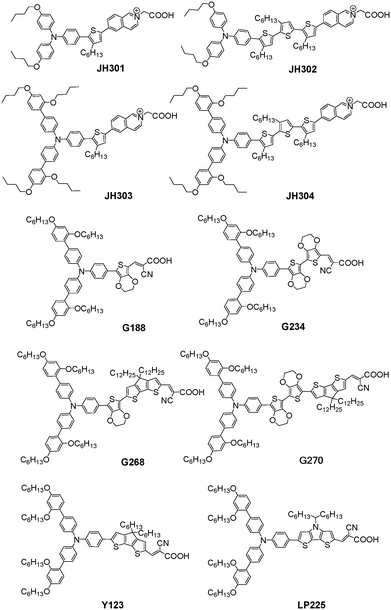
Then, Sun et al. also successfully synthesized four iso-quinoline cationic organic dyes (JH301, JH302, JH303, and JH304) without a vinyl group for DSSCs.43 JH302 and JH304, with three 3-hexylthiophene units, were significantly red-shifted (10–15 nm) in comparison with those of JH301 and JH303 due to the longer π-conjugation bridges. However, according to the density functional theory (DFT) calculations (Fig. 8), the dihedral angles between the triphenylamine and the hexylthiophene ring were 29.14°, 36.62°, 29.13°, and 34.69° for JH301–JH304, respectively, indicating that a longer π-conjugation bridge can increase the torsion angle near the donor group, leading to a lower molar extinction coefficient. DSSCs based on JH303 and JH304 with DAP-based TAP donor groups resulted in a higher Voc than that of JH301 and JH302, whereas little difference was observed in the Jsc values stemming from the various substituent groups in the TAP moiety. The recombination resistance values at the dye/TiO2/electrolyte interface were measured by electrochemical impedance spectroscopy (EIS) for JH301, JH302, JH303 and JH304 were 30 Ω cm2, 31 Ω cm2, 38 Ω cm2 and 40 Ω cm2, respectively. This suggested that the DAP substituent unit can enhance the steric hindrance effect and improve the Voc by suppressing electron recombination. As a result, the JH304-based cell exhibited the highest PCE of 7.3% with a Jsc of 14.4 mA cm−2, (Voc) of 684 mV and FF of 0.74 with the I−/I3−-based electrolyte under standard AM 1.5G illumination compared to the PCEs of 6.1%, 7.0%, and 6.5% for JH301–JH303, respectively.
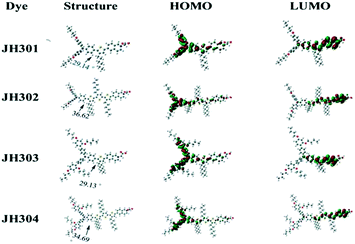 | ||
| Fig. 8 The optimized structures and electron distribution in the HOMO and LUMO levels of the JH301–JH304 dyes. Reprinted with permission from ref. 43. Copyright © 2012 Royal Society of Chemistry. | ||
However, the longer π-bridge and the red-shift in the absorption spectrum are not necessarily favorable for a better performance of DSSCs, which was demonstrated by Gao et al. via elongating the conjugated bridges with EDOT and CPDT. Four organic dyes (G188, G234, G268, and G270) were synthesized by changing the length of the π-bridges (EDOT, bisEDOT, EDOT-CPDT, and bisEDOT-CPDT, respectively). The absorption spectra of these four dyes gradually red-shifted with the further extended π-bridges, and their corresponding cell performance was investigated using the [Co(bpy)3]2+/3+-based electrolyte. Their Jsc values gradually increased from 12.02 to 16.27 mA cm−2 with the extension of the π-conjugated bridges, while the Jsc of G270 was only 5.1 mA cm−2 due to poor dye regeneration. As a result, G268 showed the best PCE of 9.24%. This work also showed that the introduction of the CPDT unit was beneficial for the enhancement of the molar extinction coefficient and red shift in absorption, which can result in a much higher Jsc compared to the thiophene and EDOT units.44
Actually, a CPDT bridging unit was first introduced in the D–π–A structure for dye Y123 by Grätzel et al. in 2011, which exhibited a Jsc of 14.6 mA cm−2, Voc of 855 mV and FF of 0.70, corresponding to a high PCE of 8.8% under full sunlight with the [Co(bpy)3]2+/3+ redox couple. The efficiency of the champion DSSCs could reach even 9.6%.45 However, the synthesis of the CPDT bridge is very costly, whereas the dithieno[3,2-b:2′,3′-d]pyrrole (DTP) bridge is more accessible according to the report by Rasmussen et al. Compared to the Y123, Grätzel et al. introduced the N-substituted DTP bridge in a D–π–A dye (LP225) incorporated with the DAP-based TPA donor group to further investigate the performance of DSSCs. Although LP225 showed a slightly blue-shifted absorption spectrum, the decrease in Jsc was only around 1 mA cm−2 compared to that of Y123. In addition, a faster oxidized dye regeneration was obtained due to the favorable charge-transfer kinetics of the DTP bridge, leading to a comparable PCE of 8.86% with a larger Voc of 901 mV, Jsc of 13.4 mA cm−2 and FF of 0.74 using the [Co(bpy)3]2+/3+ redox couple under simulated AM 1.5G conditions (Fig. 9).46
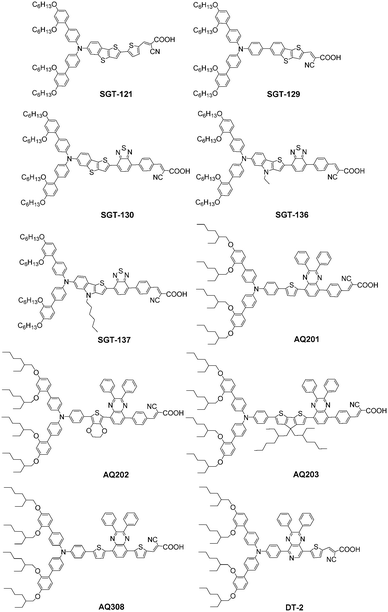
Thieno[3,2-b][1]benzothiophene (TBT) with an asymmetric structure has a good planarity and has been widely used in ferroelectric liquid crystal and organic thin film transistors for good electrical performance, strong fluorescence, and high ambient stability. Kim et al. introduced TBT as a π-bridge in a D–π–A structure and synthesized three organic dyes with DAP-based bulky donor groups (SGT-121, SGT-129, and SGT-130).47 The maximum absorptions of SGT-121, SGT-129, and SGT-130 were at 470.5, 426, and 514.5 nm, and the corresponding extinction coefficients were 2.8 × 104, 1.4 × 104, and 2.9 × 104 M−1 cm−1, respectively, which were much lower than that of Y123 (5.2 × 104 M−1 cm−1 at 506 nm), indicating that the TBT unit as a π-bridge has a weaker absorption ability than the CPDT unit in Y123. According to the theoretical calculation, the HOMO level of the CPDT unit was more negative compared to the TBT unit, and the corresponding HOMO–LUMO band gaps were more narrow because of upshifting the HOMO and lowering the LUMO levels in comparison to TBT due to the introduction of thiophene and BTD-phenyl in SGT-121 and SGT-130, respectively (Fig. 10). Finally, the DSSCs based on SGT-130 showed the highest PCE of 10.47% with a Jsc of 16.77 mA cm−2, Voc of 851 mV, and FF of 0.73 using the [Co(bpy)3]2+/3+ electrolyte under simulated AM 1.5G conditions, outperforming the PCE of 9.5% for Y123. Moreover, the DSSCs based on SGT-130 displayed a broader IPCE spectrum compared to the other three dyes, and the Jsc values increased in the order of SGT-129 < SGT-121 < Y123 < SGT-130. The highest Voc was also achieved by the SGT-130-based DSSC and was in the order of SGT-130 > Y123 > SGT-121 > SGT-129, which could be explained by the EIS and intensity modulated photovoltage spectroscopy (IMVS) measurements. Compared to SGT-130, the low PCEs of 8.24% and 5.21% for SGT-129 and SGT-121 were obtained under the same conditions, respectively. As a result, the BTD-phenyl unit is beneficial to obtain organic sensitizers for highly efficient DSSCs.
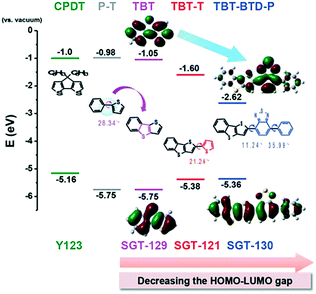 | ||
| Fig. 10 Theoretical HOMO and LUMO energy levels of the main chromophores of relevant sensitizers. Reprinted with permission from ref. 47. Copyright © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Kim and co-workers further introduced the thieno[3,2-b]indole (TI) moiety as the π-bridge instead of the TBT of SGT-130, such as SGT-136 and SGT-137. The TI moiety is substituted with the nitrogen (N) as the bridging atom in the sulphur (S) core atom of TBT. The λmax values for SGT-136 and SGT-137 are significantly red-shifted by 17 and 28 nm, respectively, compared to that of the SGT-130 due to the strong electron-donating ability and coplanarity of the indole-based structure. The DSSCs based on SGT-136 (18.42 mA cm−2) and SGT-137 (=18.83 mA cm−2) show a significant improvement in the Jsc with respect to that of SGT-130 (16.07 mA cm−2), suggesting that the substitution of the π-bridge unit from the TBT to the TI unit is a rational strategy for the enhancement of the photocurrent density. The increased Jsc and Voc of SGT-137 can be attributed to its long alkyl chain effect using a [Co(bpy)3]2+/3+ redox couple, which originates from the inhibition of dye aggregation and charge recombination by hexyl chains on the TI π-bridge. This presents an innovative architecture for parallel tandem-DSSC to compensate for the absorption ability of SGT-137 in the NIR region, which is composed of an SGT-137-based top cell and an SGT-021-based bottom cell. The tandem-DSSC showed a significantly improved PCE of 14.64% with Jsc = 22.06 mA cm−2, Voc = 878.4 mV, and FF = 0.756 (Fig. 11).48
In order to further understand the influence of the π-bridge on the dye regeneration and photovoltaic performance, our group designed and synthesized three quinoxaline-based organic dyes (AQ201, AQ202, and AQ203) containing thiophene, EDOT, and CPDT in the π-system to fine tune the HOMO energy levels of the dyes, respectively.49 Gradually red-shifted absorption spectra were obtained by altering the π-bridge between the DAP-based TPA donor and quinoxaline group due to a negative shift of the HOMO levels and similar LUMO levels of these dyes. In the end, the AQ202 performed best with a PCE of 8.37% and Jsc of 14.61 mA cm−2 because of the wider absorption spectrum as well as the effective driving force for promoting dye regeneration based on the [Co(bpy)3]2+/3+ electrolyte. Whereas, in DSSCs using the I−/I3− electrolyte, the AQ201-based device obtained the highest PCE of 6.57%. It is worth noting that CPDT unit had more contributions to broaden the absorption spectrum with a high molar extinction coefficient, while the HOMO energy level of the dye would be increased too much due to the strong electron-donating quality of the CPDT unit. As a result, the AQ203 performed no better due to the worse dye regeneration and the fast electron recombination with a disappointing energy level alignment compared to the two other dyes. Then, we further synthesized the AQ308 based on the DAP-based TPA donor by introducing thiophene groups on both sides of the quinoxaline moiety. Compared to the AQ201, a distinct red-shift appeared in the absorption spectrum of the AQ308 after replacing the benzene ring with a thiophene unit near the cyanoacetic acid moiety. Most importantly, the dye regeneration process presented in the AQ308-based cell was no trouble because it had almost the same oxidation potential as AQ201, ensuring the charge-transfer kinetics. Meanwhile, a small amount of stacked graphene platelet nanofibers (SGNF) was dispersed in the [Co(bpy)3]2+/3+ electrolyte to improve the charge transport and reduce the diffusion limitation of the [Co(bpy)3]2+/3+ redox couple. Lastly, the AQ308-based cell achieved the highest PCE of 9.81% with Jsc of 16.75 mA cm−2, Voc of 830 mV and FF of 70.56% with SGNF (0.2 mg mL−1) dispersed in the [Co(bpy)3]2+/3+ electrolyte under standard AM 1.5G simulated sunlight.50
Generally, extending the π-bridge length and strengthening the electron-donating properties will enhance the light harvesting capacity with the reduced optical band gap. However, a too narrow optical band gap easily leads to the mismatch of the energy levels and a corresponding decrease in the dye regeneration or electron injection efficiency. Therefore, the trade-off between the light harvesting capacity and energy level matching by replacing with π-bridge units needs to be carefully handled in rational designs of metal-free organic dyes.
2.2 Indoline based donor
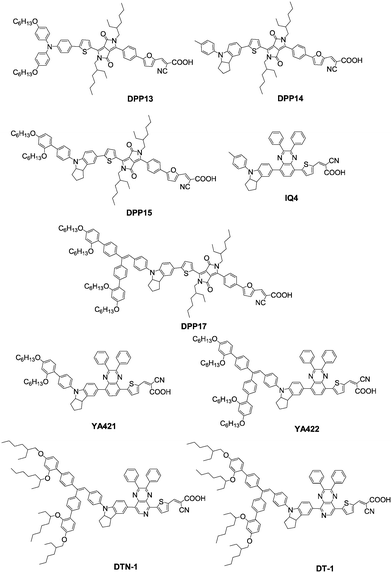
Compared to the TAP donor group, indoline shows a stronger electron-donating ability that contributes to the red-shift in the absorption spectrum.51,52 In 2013, Grätzel et al. designed and synthesized a series of asymmetric diketopyrrolopyrrole (DPP) blue-colored dyes (DPP13, DPP14, DPP15, and DPP17) with different donor sizes. Red-shift absorption responses were achieved by employing indoline groups in DPP14, DPP15, and DPP17 compared to DPP13 with the TPA donor group. In particular, the Voc values increased with the size of the indoline-based donor in both DSSCs with cobalt- and iodine-based electrolytes. Accordingly, the DPP17 achieved the highest PCE of 10.1%, followed by DPP15 (PCE = 9.81%), DPP13 (PCE = 8.97%) and DPP14 (PCE = 8.23%) using the [Co(bpy)3]2+/3+-based electrolyte under standard AM 1.5G simulated sunlight. However, in DSSCs with the iodine electrolyte, a reversed sequence of photovoltaic performance was observed, and the dye DPP14, which did not incorporate the DAP steric bulk in the indoline donors, provided the best PCE of 7.73%.53Therefore, a specified study was further carried out in our group and Grätzel's group on the donor size and its effects no an N-substituted indoline-based system with quinoxaline as the internal electron-withdrawing unit. The trends of donor size are the same as dye DPP14, DPP15, and DPP17 in the order of IQ4 < YA421 < YA422. Compared to the IQ4 dye bearing the smallest donor, the bulky indoline donor dyes of YA421 and YA422 acquired larger molar extinction coefficients as well as a red-shift absorption response of over 10 nm for YA422. Meanwhile, the influence of the donor size on the photovoltaic performance in DSSCs with I−/I3− and [Co(bpy)3]2+/3+-based electrolytes also were compared with Pt as the counter electrode. It was found that the PCEs of the three dyes exhibited the opposite trend in the I−/I3− electrolyte (IQ4 > YA421 > YA422) with respect to the [Co(bpy)3]2+/3+ electrolyte (YA422 > YA421 > IQ4). According to EIS analysis, the cobalt electrolyte-based devices exhibited gradient increased electron lifetimes (τn) with the extended donor sizes, whereas the τn values were comparable in the case of the iodide redox electrolyte-based devices, indicating the recombination could be more efficiently retarded because of the blocking effect of the DAP units in the cobalt electrolyte. Furthermore, on the basis that carbon materials and gold at the counter electrode of DSSCs with the cobalt-based electrolyte outperform Pt, a high PCE of 10.65% was obtained by introducing Au + graphene nanoplatelets (GNP) as the counter electrode in AM 1.5 simulated sunlight.54
It was concluded that the utility of the bulky DAP unit in the indoline donor was effective towards realizing low electron recombination and high PCE with the cobalt-based electrolyte. Besides, both DPP and quinoxaline as internal electron-withdrawing units were used to construct D–A–π–A organic dyes for better photovoltaic performance and tuning of energy levels, spectral response, electron transfer as well as photostability.55,56 Based on this strategy, our group further investigated two D–A–π–A organic dyes with bulky DAP-based indoline donor groups, DTN-1 and DT-1, incorporating pyrido[3,4-b]pyrazine (PP) as an additional electron-withdrawing unit,57,58 and the effect of the pyridyl N atom position in PP on the photophysical properties and the performance in DSSCs with [Co(bpy)3]2+/3+ electrolyte was examined. The pyridyl N atom was adjacent to the anchoring group in DTN-1, and the opposite pyridyl N atom position approaching the indoline donor group in DT-1. Although the maximum absorption wavelength of DTN-1 was red-shifted around 15 nm compared to dye DT-1, the molar extinction coefficient was reduced greatly after changing the position of the N atom from the left side to the right side. In contrast, the DTN-1-based device only showed a PCE of 6.10% with a Voc of 789 mV, Jsc of 10.67 mA cm−2 and FF of 0.66 using the [Co(bpy)3]2+/3+-based electrolyte under simulated AM 1.5G, and a higher PCE of 8.57% was gained by DT-1 under the same conditions. This might be attributed to the decreased dihedral angle between the thiophene moiety and the pyrido[3,4-b]pyrazine unit, accelerating the electron recombination process in DTN-1 (Fig. 12).59
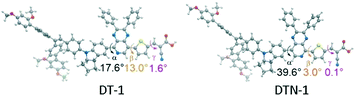 | ||
| Fig. 12 The optimized structures and dihedral angles of the DT-1 and DTN-1 dyes. Reprinted with permission from ref. 59. Copyright © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Therefore, our group further synthesized an organic dye (DT-2) by replacing the indoline with the TPA donor group for a comparative study of the influences of different cores in bulky DAP-based donors on the photophysical and DSSCs performance. As expected, DT-2 showed a significant blue-shifted absorption compared to DT-1 due to the weaker electron-donating capability of the TAP unit than the indoline moiety. Under simulated AM 1.5G irradiation, the DT-2-based cell obtained a low Jsc and Voc as well as a high dark current compared to those of DT-1, leading to a lower PCE of 7.74% with the [Co(bpy)3]2+/3+-based electrolyte, which was attributed to the larger electron recombination processes at the TiO2/DT-2/electrolyte interface according to the EIS measurements. We also carried out density function theory calculations that showed the biphenyl branch at the cis-position of DT-1 could effectively inhibit the dye aggregation and charge recombination. While the HOMO distribution of DT-2 was evenly located on the large TPA-based donor segment and was extended to the DAP moieties, and more electronic communication at the interface of TiO2/dye/electrolyte was expected (Fig. 13). It can be seen clearly that the indoline unit can optimize the energy levels more efficiently and reduce the electron recombination more than the TPA unit, which is beneficial for light-harvesting and the whole photoelectric conversion efficiency.60
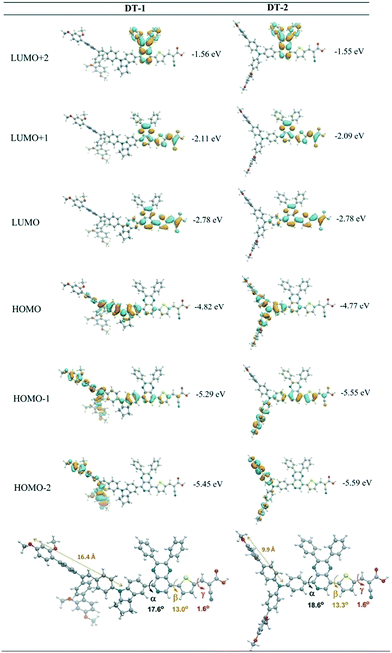 | ||
| Fig. 13 The optimized structures and spatial distribution of the frontier molecular orbitals of DT-1 and DT-2. Reprinted with permission from ref. 60. Copyright © 2015 American Chemical Society. | ||
All in all, the minor changes in the donor size and additional electron-withdrawing units can give rise to a great difference in the photophysical properties and photovoltaic performance of a dye. The research has demonstrated that bulky DAP-based indoline donor groups can effectively suppress charge recombination, especially in DSSCs with the cobalt-based electrolyte. Besides, the indoline group can become more stable after it is connected with an additional electron-withdrawing unit (Fig. 14).
2.3 Indeno[1,2-b]thiophene based arylamine donor
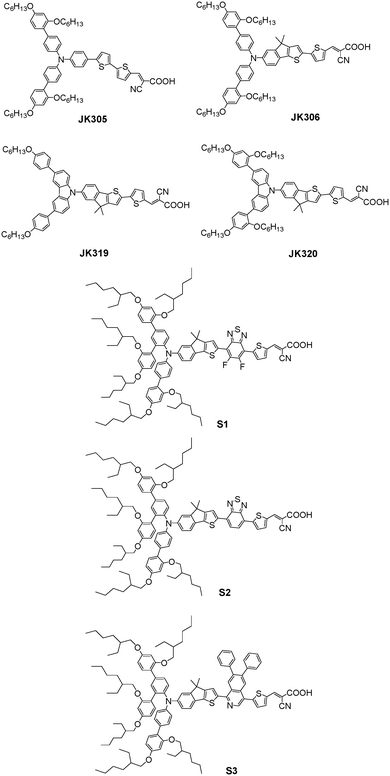
Indeno[1,2-b]thiophene, a planar unit just like CPDT and fluorine units, has been successfully developed in organic dyes to obtain broad absorption.61,62 In this case, Ko et al. reported an organic dye JK-306 by incorporating the indeno[1,2-b]thiophene-based arylamine donor group, and the counterpart JK-305 was also prepared by introducing a dithiophene π-bridge to evaluate the advantage of a planar bridging unit. Thus, a distinct red-shifted absorption peak of JK-306 with a high molar extinction coefficient with respect to JK-305 were achieved due to the increased planarity of the conjugated system. However, the introduction of the planar units as a spacer in the dye molecule always leads to more π–π stacking or dye aggregation, which can impede a serious charge recombination process. Accordingly, they further studied chenodeoxycholic acid (CDCA) and 4-[bis(9,9-dimethyl-9H-fluoren-2-yl)amino]benzoic acid (HC-A)63,64 as co-adsorbents to retard π–π stacking or dye aggregation, which retarded electron recombination with the cobalt(III)-complex. Under standard AM 1.5G conditions, the JK-306-based cell in the presence of CDCA provided a higher PCE of 8.37% compared to that of JK-305 of 7.29% with I−/I3− electrolyte, whereas the PCE of the JK-306-based cell increased to 8.52% with HC-A as co-adsorbents. Furthermore, the cell using JK-306 with HC-A showed a notably enhanced PCE of 10.02% with a Jsc of 14.33 mA cm−2, Voc of 0.95 V, and FF of 0.71 using the [Co(bpy)3]2+/3+-based electrolyte under standard AM 1.5G conditions.65 A higher Voc with HC-A should come from the negative shift of the quasi-Fermi level of its electrode calculated from the density of states (DOS) (Fig. 15).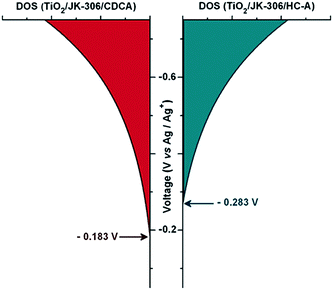 | ||
| Fig. 15 The exponential distribution of DOS. Reprinted with permission from ref. 65. Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Ko et al. further synthesized two organic dyes, JK-319 and JK-320, incorporating indeno[1,2-b]thiophene as the π-bridge to investigate the influence of the number/position of the alkoxy substituent chains in the donor segment upon electron recombination. Two long hexyloxy chains were located in the para-position in JK-319 and four long hexyloxy chains were located in the para-position and ortho-position in JK-320. Although there was a little difference between JK-319 and JK-320 in absorption, a higher Jsc and Voc were achieved by the JK-320-based cells than the JK-319 in both the I−/I3− and [Co(bpy)3]2+/3+-based electrolytes. As a result, the JK-320 dye with two DAP substituents in the donor segment showed a higher PCE of 5.88% and 6.66% compared to the PCE of 5.41% and 6.38% for JK-319 in the I−/I3− and [Co(bpy)3]2+/3+-based electrolytes under standard AM 1.5G condition.66
In this case, our group introduced three DAP units with six long 2-ethylhexyloxy chains into the indeno[1,2-b]thiophene based arylamine donor segment to further suppress dye aggregation and electron recombination, which was then used to construct three near-infrared D–A–π–A dyes, S1, S2, and S3, containing 5,6-difluorobenzothiadiazole (DFBT), benzothiadiazole (BT), and PP as the additional electron-withdrawing units, respectively.67 Amazingly, the maximum absorption wavelength of S3 had reached 628 nm, resulting in a notable red-shift of over 10 nm with respect to both S1 and S2 in the absorption spectra. This was attributed to the strong electron-withdrawing property of the PP unit with the narrow HOMO–LUMO gap compared to that of the DFBT and BT units in S1 and S2, respectively. However, the HOMO levels were gradually more positive in the order of S1 < S2 < S3, and [Co(Me2bpy)3]2+/3+ with a lower redox potential compared with [Co(bpy)3]2+/3+ was introduced into the electrolyte system to meet the energy level alignment for facilitating dye regeneration effectively.68 In the end, the S3-based cell in the presence of I−/I3− and [Co(Me2bpy)3]2+/3+ electrolytes showed the best PCE of 6.29% and 7.23% under standard AM 1.5G condition, respectively.
It was concluded that the indeno[1,2-b]thiophene unit plays a significant role in the conjugating spacer to enhance the planarity and broaden the absorption range to achieve highly efficient DSSCs. In addition, the introduction of multiple long alkoxy chains for building a bulky donor group could be successfully used to overcome dye aggregation and charge recombination from the incorporated planar bridging units (Fig. 16).
2.4 Bisfluorenylamine based donor
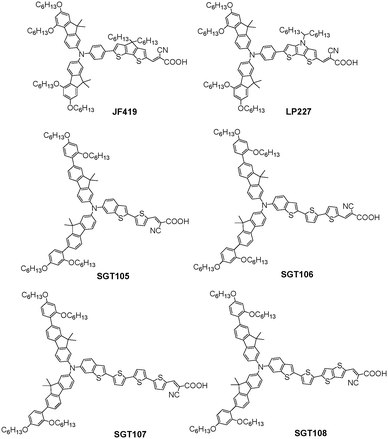
Variation of the electron donor is an easy method to modulate the HOMO and LUMO levels of the dyes as well as absorption spectra and influence the DSSCs performance. As stated previously, a lot of donor groups with steric units have been obtained and successfully adopted to synthesize organic dyes for DSSCs over the past few years. Y123 has been demonstrated to have a favorable electron-donating ability and steric properties of the DAP-based TPA bulky donor. Grätzel et al. also designed an analogous dye, JF419, incorporating a fluorene-based bulky donor to improve the light-harvesting ability and prevent electron recombination. In contrast, the maximum absorption of JF419 was significantly red-shifted over 10 nm with a similar molar extinction coefficient, which indicated that the fluorene could effectively control the electron transfer processes. Although the strong electron-donating ability induced a negative shift of the HOMO level of JF419 and led to a smaller regeneration driving force, JF419 possessed a nearly identical regeneration efficiency in the [Co(bpy)3]2+/3+ electrolyte compared to Y123 according to the transient absorption spectroscopy measurements. In addition, comparable electron lifetimes were yielded between those two dyes, indicating that the bulky fluorene donor displayed a similar surface blocking capability as Y123’s donor group. Hence, the JF419-based cell reached the highest PCE of 10.3% with a high Jsc of 16.2 mA cm−2, Voc of 840 mV and FF of 0.76 with the [Co(bpy)3]2+/3+ electrolyte under simulated AM 1.5G condition, while the Y123-based device showed a lower PCE of 9.8% under the same operation.69 On the basis of the LP225 dye, they also investigated another organic dye (LP227) containing a bulky fluorene donor just like the JF419 dye. However, DSSCs based on LP225 did not show any increases compared to that of LP227, resulting in a lower PCE of 8.72% due to the smallest Voc arising from the shorter electron lifetime (Fig. 17).46Previous studies of Kim et al. indicated that bisfluorenylamine and its derivatives were able to obtain relatively high molar extinction coefficients as well as efficient charge injection and electron lifetimes in DSSCs.70 Thereafter, they further developed four organic dyes (SGT105–SGT108) containing DAP-based bisfluorenylamine donor groups through connections with various thiophene or oligothiophene units to investigate the effect of π-bridging length on the relevant properties.71 The absorption spectra of those four SGT organic dyes showed little difference with the extended π-conjugation. SGT-107 and SGT-108 were slightly blue-shifted compared to the other dyes, demonstrating that the longer the π-bridge was, the stronger the π–π stacking was. The lengths of the π-bridges were also calculated as 2.52, 6.54, 10.47, and 8.54 Å for SGT-105, SGT-106, SGT-107, and SGT-108, respectively (Fig. 18). They found that a long π-bridging length over 8 Å could easily increase the dye aggregation and charge recombination. Under standard AM 1.5G condition, the SGT-106-based cell gave a best PCE of 7.22% with a higher Jsc of 12.4 mA cm−2, Voc of 883 mV and FF of 0.64 with the [Co(bpy)3]2+/3+-based electrolyte, which was attributed to the strong light-harvesting ability with respect to SGT-105 and the weak π–π stacking on the TiO2 surface compared to SGT-107 and SGT-108.
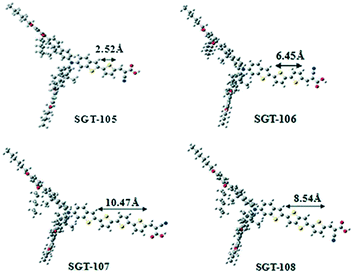 | ||
| Fig. 18 The optimized structures and π-bridge lengths of the SGT105-SGT108 dyes. Reprinted with permission from ref. 71. Copyright © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Based on previous work, they also tried to further improve the DSSCs performance based on sensitizers resembling SGT-106 with a π-bridging length of approximately 6 Å. Therefore, three organic dyes, SGT-109, SGT-110 and SGT-111, were designed and synthesized by introducing CPDT and BT units into the π-conjugation, and the corresponding π-bridging lengths were calculated as 6.38, 6.87, and 7.27 Å, respectively (Fig. 19). The advantages of the CPDT and BT units have been depicted as effective electronic communications for CPDT, optimization of energy levels and increase of the photostability, especially for BT unit. The absorption spectra of SGT-110 and SGT-111 were significantly red-shifted compared to SGT-109, owing to the introduction of the BT unit. However, the molar extinction coefficient of SGT-109 was much higher than that of SGT-110 and SGT-111, indicating that the CPDT unit was very beneficial for better light-harvesting ability. Hence, the SGT-109-based cell exhibited the highest IPCE values, while the SGT-110-based cell showed the worst IPCE values because of the low LUMO level, which resulted in insufficient electron injection from the excited dyes into the TiO2 layer. As a result, the SGT-109-based cell gave a best PCE of 8.61% with a high Jsc of 14.1 mA cm−2, Voc of 846 mV and FF of 0.72, while the SGT-110-based cell showed lower Jsc and Voc, leading to a much lower efficiency of 4.64% with the [Co(bpy)3]2+/3+-based electrolyte under standard AM 1.5G condition. In contrast, the Jsc of the SGT-111-based cell decreased to 11.3 mA cm−2 due to the reduced molar absorption coefficient compared to that of SGT-109, and a lower PCE of 7.01% was obtained.
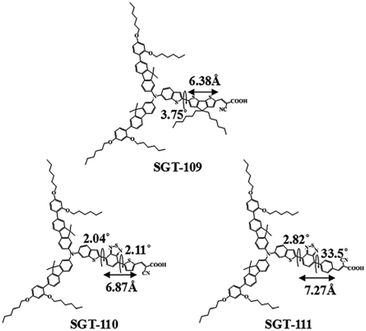 | ||
| Fig. 19 Dihedral angles and π-bridge lengths of the SGT109-SGT111 dyes. Reprinted with permission from ref. 71. Copyright © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Since DAP-based bisfluorenylamine donors have been proven efficient in the enhancement of the light-harvesting ability and inhibition of charge recombination, Kim et al. further developed three dyes (SGT-101, SGT-102, and SGT-103), containing triphenylamine, bisfluorenylamine and bisphenothiazinylamine as the electron donors, respectively. The maximum absorption of the SGT-103 dye showed a slight red-shift as well as a higher molar absorption coefficient compared to that of SGT-101 and SGT-102, indicating that the SGT-103 dye had a better light-harvesting ability. However, the SGT-103-based cell exhibited a lower Jsc and Voc, leading to a lower efficiency of 5.16% with the [Co(bpy)3]2+/3+-based electrolyte under simulated AM 1.5G condition. This was due to the less negative HOMO level of SGT-103 than the two other dyes, which resulted in the insufficient driving force for dye regeneration. Under the same operation, SGT-102-based cells provided a better PCE of 7.22% with a higher Jsc of 12.1 mA cm−2, Voc of 865 mV, and FF of 0.69, while the SGT-101-based cell gave a comparable PCE of 7.11%. As a result, DAP-based bisfluorenylamine donors were more applicable to the cobalt-based electrolyte than other DAP-based donors in this system.72
It could be seen that sensitizers with the DAP-based bisfluorenylamine donor are able to achieve a relatively high light-harvesting ability as well as a good photovoltaic performance, while the DSSCs based on dyes with π-bridging lengths over 8 Å exhibited lower PCEs because of the increased dye aggregation and charge recombination. In addition, a π-bridging length of about 6 Å was found to provide a nice trade-off for the energy levels alignment and light-harvesting ability (Fig. 20).
2.5 Phenoxazine and phenothiazine based donors
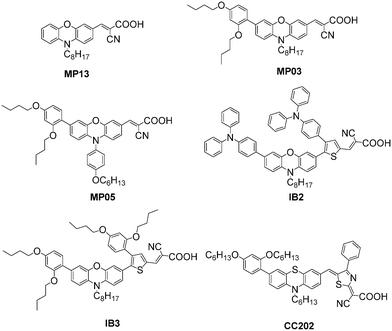
Phenoxazine (POZ) as a donor group had been studied in DSSCs by Sun et al. and gave promising efficiencies in DSSCs. It should be noted that POZ displays a stronger electron-donating ability with respect to the TPA donor group and could enhance the light-harvesting capability of the sensitizers. Based on MP13, an additional DAP unit at the 7-position of the POZ was introduced to develop two new organic dyes (MP03 and MP05), resulting in a red-shifted absorption peak due to the stronger electron-donating properties of the DAP unit. Moreover, the molar extinction coefficients were also enhanced, leading to the higher Jsc values of the DSSCs based on both MP03 and MP05 compared to that of MP13. The Voc values of both MP03 and MP05 based DSSCs were also higher than that based on MP13 because of the enhanced blocking effect caused by the presence of the steric DAP substituent in the donor group. In addition, the absorption spectrum of MP05 (λmax = 500 nm) was slightly blue-shifted compared to MP03 (λmax = 506 nm), owing to the poor orbital overlap and inefficient conjugation of, according to the DFT study. Moreover, the presence of the relatively bulky hexyloxy-phenyl unit at the N(10) decreased the dye-loading amounts. Those unfavorable factors contributed to a lower Jsc (13.09 mA cm−2) in the MP05-based device. However, a higher PCE of 7.4% was achieved for MP05 because of its higher Voc of 800 mV and FF of 0.70 under the AM 1.5G condition compared to the PCE of 7.17% for MP03, which suggested that a bulky substituent at the N(10) of POZ was beneficial for decreasing molecular aggregation and charge recombination. Additionally, photoelectron spectroscopy (PES) and X-ray absorption spectroscopy (XAS) were used to investigate the surface structure of MP05, and the results indicated that the cyano (CN) group was closer to the TiO2 surface than the POZ group. Therefore, the MP05 molecules with well-distributed structure were aligned on the TiO2 surface (Fig. 21).73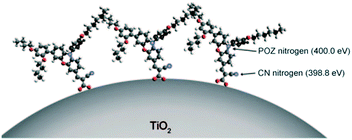 | ||
| Fig. 21 Description of MP05 standing on the TiO2 surface. Reprinted with permission from ref. 73. Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Afterwards, the other two organic sensitizers with the POZ donor group, IB2 and IB3, were also developed containing TPA and DAP units as steric groups, respectively, by Hagfeldt's group and Sun's group. The influence of the steric properties of TPA and DAP units on the correlative performance was investigated. The absorption spectrum of the IB2 dye displayed a lower extinction coefficient compared to IB3, which might be attributed to the relatively weak π-conjugated system. Incorporating the TPA groups in the POZ unit, the IB2 dye rendered a higher Voc of 0.76 V. However, by replacing the TPA groups with DAP units for the IB3 dye, the Voc value was increased to 0.8 V using the I−/I3− electrolyte, yielding an ideal efficiency of just under 7.0%. Furthermore, an almost identical Jsc of about 10.3 mA cm−2 was achieved for both IB2- and IB3-based DSSCs with the [Co(bpy)3]2+/3+ electrolyte, as well as two very similar PCEs of 6.3% and 6.4%, respectively. Meanwhile, the dye LEG4 was measured as a reference to further assess the performance of the bulky POZ donor group. A higher PCE of 7.0% was achieved for LEG4 under the same conditions, while the Voc decreased by about 30 mV compared to that of IB2 and IB3. This indicates that the POZ containing bulky DAP units is a promising alternative electron-donor group for an efficient blocking effect, especially for cobalt-based electrolyte DSSCs.74,75
Phenothiazine is an analogue of phenoxazine, which is also widely applied as a donor part in organic dyes and shows unique electronic and optical properties. Sun et al. employed phenothiazine with a DAP-based bulky unit as a donor and introduced a distinguished dihydrothazole derivative into the dyes as a π-bridge to fabricate the organic dye CC202.76 The device based on CC202 showed an efficiency of 5.4%, with a Jsc of 13.9 mA cm−2, Voc of 540 mV, and FF of 0.72 using the I−/I3− electrolyte. When the cobalt electrolyte was employed, the efficiency of the DSSCs based on CC202 improved to 6.1% (Voc = 615 mV, Jsc = 14.5 mA cm−2, FF = 0.67) under standard AM 1.5G. These results indicate that the phenothiazine donor group containing the DAP unit is more applicable to the cobalt-based DSSCs compared to the iodide-based electrolyte, demonstrating the good blocking effect of the DAP unit can effectively reduce charge recombination (Fig. 22).
2.6 Other dyes
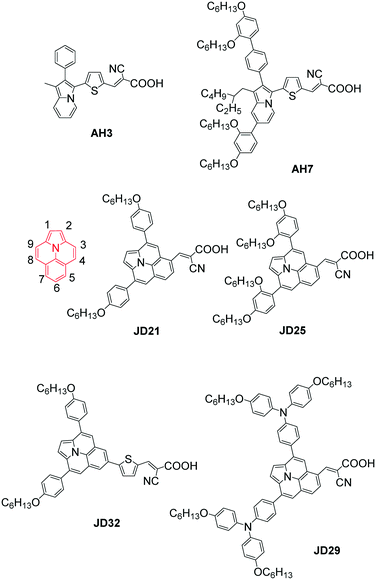
Indolizine, a fully conjugated planar nitrogen-containing donor, was first examined by Delcamp' group and Grätzel's group and displayed a very strong electron-donating ability compared to the arylamine donors.77 They further synthesized a indolizine-based organic dye, AH7, containing bulky DAP units and an alkyl substituent on the donor and compared to that of the reported AH3. As a result, the absorption peak of AH7 was red-shifted by about 50 nm, and the molar extinction coefficient increased compared to that of AH3 with the non-alkylated indolizine donor. The known D35 was introduced as a comparative dye because of the low charge recombination rate. Accordingly, the highest Jsc values were gained by the AH7-based cell in both I−/I3− and [Co(bpy)3]2+/3+ electrolytes with respect to that of D35 and AH3, which was attributed to the broader IPCE spectrum of AH7, leading to the highest PCEs of 7.99% and 8.10%, respectively. It is remarkable that the Voc values of devices based on AH3 were dramatically decreased by over 100 mV in both I−/I3− and [Co(bpy)3]2+/3+-based electrolytes compared to that of AH7 and D35 owing to the significantly reduced electron recombination. Therefore, the indolizine donor functionalized with bulky groups is suitable for DSSCs based on either iodide or cobalt based redox shuttles because of the excellent light absorption and lower charge recombination rates.78The ullazine core is a 16 π-electron nitrogen-containing heterocycle that is isoelectronic with pyrene, displaying both electron donating and accepting properties, and offers a number of active sites for functionalized-ullazine materials. Based on the DFT studies, electrophilic aromatic substitution would functionalize at the 5- and 6-positions of the ullazine core, and it was found that the sensitizer with a substitution at the 5-position gave a strong ICT process.79 Therefore, those dyes with acceptors at the 5-position of the ullazine core (JD21, JD25 and JD29) showed a broad absorption range. While the maximum absorption of JD32 with the substitutes at the 6-position of ullazine was blue-shifted by nearly 200 nm, and the molar extinction coefficient was also dramatically decreased to only 1600 M−1 cm−1, giving a very poor PCE of 1.7% for JD32-based DSSCs in conjunction with the I−/I3− electrolyte under simulated AM 1.5G condition. In the same condition, the JD21-based device achieved a high PCE of 8.4% with Jsc = 15.4 mA cm−2, Voc = 730 mV and FF = 0.75. The same PCE of 6.7% was provided by the JD25- and JD29-based DSSCs, and the JD25-sensitized cell showed a best Voc of 807 mV due to the better blocking effect of DAP units with respect to those of other steric substituents at the 3- and 9-positions of ullazine (Fig. 23).80
2.7 DAP-based porphyrin dyes
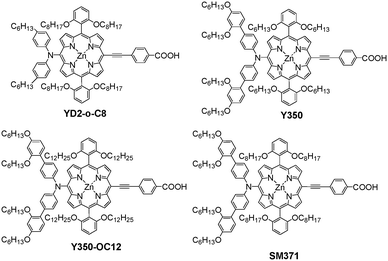
Porphyrin dyes previously exhibited a relatively low efficiency in DSSCs on account of the following two aspects: (1) serious dye aggregation arising from good molecular planarity; (2) poor electron injection as a result of excited electrons without fixing the flow direction.81 Since Grätzel et al. reported a porphyrin dye D2-o-C8-based cell showed a PCE up to 12.3% with co-sensitization of the Y123 dye in the [Co(bpy)3]2+/3+ electrolyte, porphyrin-based D–π–A dyes have been widely highlighted in the DSSCs field.82 On the basis of this, they further synthesized two porphyrin dyes comprised of the bulky DAP-based donors (SM371 and SM315) to boost adaptability with the cobalt-electrolyte and introduced the BTD unit in SM315 to improve the light-harvesting capability. Furthermore, the absorption of SM315 between the Soret and Q-bands showed a significant improvement with respect to SM371, resulting in the panchromatic property of the BTD-functionalized dye together with a shift towards lower energy and higher oscillator strength (Fig. 24). Most importantly, the SM315-based cell achieved an excellent improved PCE of 13.0% with a high Jsc of 18.1 mA cm−2, Voc of 0.91 V, and FF of 0.78 compared to that of SM371, which achieved a slightly enhanced PCE of 12.0% with a high Voc of 0.96 V, Jsc of 15.9 mA cm−2 and FF of 0.79 with respect to that of YD2-o-C8 (11.9%) under AM 1.5G illumination. It should be noted that BT as an electron-withdrawing unit in SM315 significantly reduced the electron lifetime compared to the SM371, leading to a decrease in Voc from 0.96 to 0.91 V.11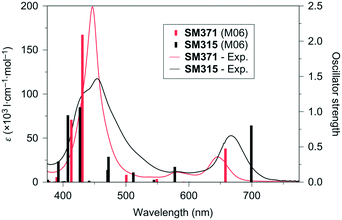 | ||
| Fig. 24 The experimental spectra and theoretical electronic transitions for both SM371 (red) and SM315 (black). Reprinted with permission from ref. 11. Copyright © 2014 Nature Publishing Group. | ||
Meanwhile, Grätzel et al. also investigated the effect of the length of the alkoxy chains at the meso-position of the porphyrin on the photophysical properties and the DSSCs performance in combination with a cobalt-based electrolyte. Therefore, the two 2,6-dihexoxyphenyl groups of Y350 in the meso-position were replaced by two 2,6-didodecoxyphenyl groups in Y350-OC12 to further reduce the dye aggregation and charge recombination by shielding the porphyrin chromophore with longer alkoxy chains. The absorption spectra of Y350 and Y350-OC12 were almost the same, indicating that the alkoxy chain length had little effect on the photophysical properties. Nonetheless, Y350-OC12 with two 2,6-didodecoxyphenyl groups showed a higher Voc and a longer electron lifetime than that of Y350 with two 2,6-dihexoxyphenyl groups, which reflected that the length of the alkoxy chains in the meso-position of the porphyrin had a greater influence on dye aggregation and charge recombination. However, the Y350-OC12-based cell showed a lower PCE of 11.5% compared to that of Y350 with a PCE of 12.0% under simulated AM 1.5G sunlight, which was ascribed to the lower Jsc for Y350-OC12 due to diffusion problems of the redox shuttle through the dyed porous layer.83
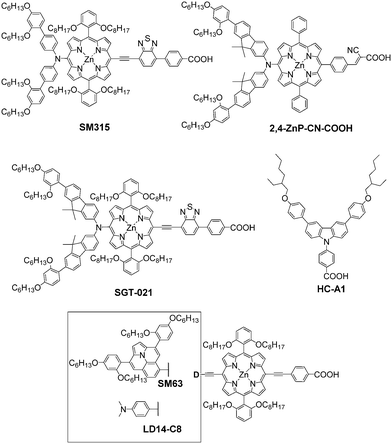
Kim et al. demonstrated that introducing a cyanoacrylic acid as an acceptor linked through a phenylene bridge to the porphyrin dye eventually increased the DSSC performance.84 Therefore, they further designed a porphyrin dye 2,4-ZnP-CNCOOH with the bulky bisfluorenylamine based donor group in order to effectively suppress charge recombination. As mentioned above, co-sensitization is an effective way to improve the DSSCs performance through combination with another dye to form a complementary absorption in the visible and NIR regions. The 2,4-ZnP-CNCOOH-based cell only showed a PCE of 4.9% in the absence of a co-sensitizer, whereas a significantly improved PCE of 8.4% was obtained with HC-A1 as the co-sensitizer. This was also confirmed by the fact that the IPCE spectrum of the 2,4-ZnP-CN-COOH-based cell with the co-adsorbent HC-A1 showed an onset at around 750 nm and sharply reached a plateau value of over 70% from around 520 to 410 nm, indicating the efficient light harvesting in the visible region. It was noteworthy that the introduction of HC-A1 as a co-sensitizer could also efficiently retard the charge recombination at the TiO2/dye/electrolyte interface according to the EIS measurements, thus leading to a higher Voc (Fig. 25).85
Recently, they further designed and synthesized a new porphyrin sensitizer (SGT-021) by structurally integrating 2,4-ZnP-CN-COOH with SM315 through donor structural engineering to enhance the light harvesting ability and retard the charge recombination. The DSSC utilizing the SGT-021 exhibited a higher average photovoltaic performance (Jsc = 17.5 mA cm−2, Voc = 0.910 V, FF = 75.4%, and PCE = 12.05%) employing the [Co(bpy)3]2+/3+ redox couples, and the PCE was much higher than the 11.67% of SM315 under simulated one sun illumination. On the basis of SGT-021 based DSSC, the tandem solar cells with parallel and series configurations have been established to achieve the high photo-current and -voltage performance of SGT-021. The parallel tandem-DSSCs with SGT-021 (bottom) and SGT-121 (top) showed an extremely high PCE of 14.0% with a remarkably high current density (19.7 mA cm−2) (Fig. 26). Furthermore, the series tandem-DSSCs with an increased Voc value of >1.825 V were externally connected to a typical hydrogen evolution reaction (HER) Pt electrocatalyst for solar water splitting. The applied bias photon-to-current efficiency of 7.4% was achieved with an applied bias of 0.248 V.86
 | ||
| Fig. 26 (a) Device structure of the tandem DSSCs, (b) absorption spectra of SGT-121 and SGT-021 in the THF. Reprinted with permission from ref. 86. Copyright © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In addition, Grätzel et al. also developed a new porphyrin dye SM63 employing ullazine with DAP units as a bulky donor group, and it displayed a panchromatic absorption in the long and visible wavelengths due to the introduction of the ullazine donor group, giving a maximum absorption approaching 750 nm. Interestingly, SM63 was a brown/black solid instead of green because of the broader absorption in the range of 500–620 nm. In contrast, a reference dye LD14-C8 that incorporated a simple 4-(N,N-dimethylamino)phenyl unit as a donor group had an absorption onset at around 700 nm. On the basis of computational studies, the pendant DAP groups on the ullazine gave rise to a bent donor group structure of the ground state geometry for SM63, while the excited state of SM63 revealed an enhancement of this donor bending causing the larger Stokes shift of SM63. Finally, the SM63-based cell afforded a lower PCE of 7.35% than that of LD14-C8 (PCE = 8.45%) using the I−/I3− electrolyte under AM 1.5G illumination because SM63 suffered a significant aggregation originating from the large aromatic structure of the ullazine unit.87
Although the porphyrin chromophore has intrinsically strong light absorption in the Soret and Q-bands, there is a lack of significant absorption in the spectral region between these two features. It is an effective way to use co-sensitization and tandem technique to make up the absorption deficiency in the visible and NIR regions and increase the Jsc. Meanwhile, dye aggregation can be inhibited by co-sensitization to enhance the Voc as well. Besides, the longer length of the alkoxy chains in the meso-position of the porphyrin is conducive to inhibiting the dye aggregation and charge recombination, but it is detrimental to the diffusion of the redox mediator as well as dye regeneration.88 Also, further optimization of the length of the alkoxy chains in the dye structure should be conducted after careful consideration (Fig. 27).
3 All-solid-state DSSCs
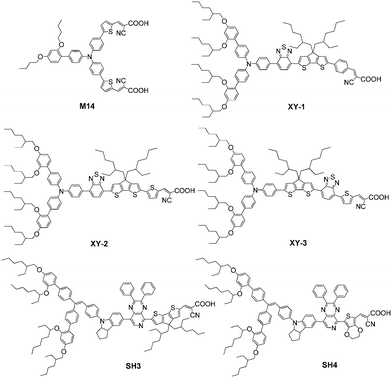
Many investigations suggest that the short electron diffusion length and poor filling of HTM are the main bottlenecks in the development of highly efficient ssDSSCs.20 Furthermore, the optimal mesoporous-layer thickness was found to be around 2.5 μm for the ssDSSCs with spiro-OMeTAD as the HTM, which was far less than the optimized film thickness in liquid electrolyte-based DSSCs (8–15 μm).89,90 As discussed in the liquid-electrolyte DSSCs, the bulky DAP-substituted TPA donor groups showed an excellent surface insulation, which can slow down the charge recombination in the liquid-electrolyte DSSCs. Therefore, there have been multiple attempts in molecular engineering of dyes to solve those problems presenting in ssDSSCs. Hagfeldt et al. introduced D35 in ssDSSCs with spiro-OMeTAD as HTM and showed an excellent PCE of 4.5%. In order to overcome the low dye loading derived from the thin film, the dye with a high molar extinction coefficient of M14 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1 cm−1) was developed and compared to that of D35 (31
100 M−1 cm−1) was developed and compared to that of D35 (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1). In comparison with D35, M14 was constructed with one DAP unit and two cyanoacetic acid groups connected by the central TPA unit, which can block HTM approaching the TiO2 surface like D35 but without significant losses in Voc. Consequently, the M14-based ssDSSCs displayed a comparative PCE of 4.37% and a slightly higher Jsc of 8.08 mA cm−2 with respect to D35.91
300 M−1 cm−1). In comparison with D35, M14 was constructed with one DAP unit and two cyanoacetic acid groups connected by the central TPA unit, which can block HTM approaching the TiO2 surface like D35 but without significant losses in Voc. Consequently, the M14-based ssDSSCs displayed a comparative PCE of 4.37% and a slightly higher Jsc of 8.08 mA cm−2 with respect to D35.91
These studies indicated that the structures of the donor groups greatly affected the rates of the charge recombination as well as the optical properties of the dyes. In 2012, Grätzel et al. carried out related research by developing a series of ullazine dyes with various 3,9-disubstituted derivatives, and the position of the anchoring group (cyanoacrylic acid) was functionalized at the 4- and 5-positions. The anchoring group at the 5-position was preferential to promote a strong ICT and a broad absorption range, and the maximum absorption of JD25 was more red-shifted with the highest molar extinction coefficient at 572 nm (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 M−1 cm−1), followed by JD21 (15
260 M−1 cm−1), followed by JD21 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 M−1 cm−1 at 555 nm) and JD29 (12
070 M−1 cm−1 at 555 nm) and JD29 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 M−1 cm−1 at 537 nm). However, the molar extinction coefficients for the substitution at 4-position of JD26 (4700 M−1 cm−1 at 516 nm) and JD27 (5670 M−1 cm−1 at 525 nm) were considerably lower due to bad electron communication from the donor to the acceptor group, and the IPCE values did not reach a maximum above 40%, accounting for the low current densities of JD26 and JD27. It is interesting to note that the JD29-based device showed a highest PCE of 4.95% with a higher Voc of 797 mV, Jsc of 9.7 mA cm−2 and FF of 0.64, while the JD25-sensitized cell displayed a lower Voc of 752 mV, a similar Jsc of 9.7 mA cm−2, leading to a comparative PCE of 4.88%. Their difference in the Voc was demonstrated by the dye coverage measurements, which indicated that JD29 with the largest donor size loaded the highest amount of dye, and JD25 shows only 45% of the dye coverage compared to JD29. Subsequently, a direct relationship between the Voc and the dye coverage was observed, and JD29 increased the blocking effect close to the TiO2 surface, resulting in the decreased charge recombination. Additionally, D35 was used as a co-sensitizer to increase the light harvesting abilities of the JD29-based devices in the 400–500 nm range as evident from the IPCE, and the Jsc of the co-sensitization-based ssDSSCs was further improved to 10.6 mA cm−2 as well as an increased Voc of 801 mV, leading to an optimal PCE of 5.40%.92
110 M−1 cm−1 at 537 nm). However, the molar extinction coefficients for the substitution at 4-position of JD26 (4700 M−1 cm−1 at 516 nm) and JD27 (5670 M−1 cm−1 at 525 nm) were considerably lower due to bad electron communication from the donor to the acceptor group, and the IPCE values did not reach a maximum above 40%, accounting for the low current densities of JD26 and JD27. It is interesting to note that the JD29-based device showed a highest PCE of 4.95% with a higher Voc of 797 mV, Jsc of 9.7 mA cm−2 and FF of 0.64, while the JD25-sensitized cell displayed a lower Voc of 752 mV, a similar Jsc of 9.7 mA cm−2, leading to a comparative PCE of 4.88%. Their difference in the Voc was demonstrated by the dye coverage measurements, which indicated that JD29 with the largest donor size loaded the highest amount of dye, and JD25 shows only 45% of the dye coverage compared to JD29. Subsequently, a direct relationship between the Voc and the dye coverage was observed, and JD29 increased the blocking effect close to the TiO2 surface, resulting in the decreased charge recombination. Additionally, D35 was used as a co-sensitizer to increase the light harvesting abilities of the JD29-based devices in the 400–500 nm range as evident from the IPCE, and the Jsc of the co-sensitization-based ssDSSCs was further improved to 10.6 mA cm−2 as well as an increased Voc of 801 mV, leading to an optimal PCE of 5.40%.92
In section 2.7, an encouraging efficiency of 12% was achieved by a porphyrin dye Y350-based cell using the [Co(bpy)3]2+/3+ electrolyte. Also, they further investigated the ssDSSCs based on Y350 using spiro-OMeTAD as the HTM and achieved a PCE of 4.8% under simulated AM 1.5G conditions. A sequential optimization also was carried out by co-sensitization of Y350 with Y123, yielding a best PCE of 6.4% with a Jsc of 10.8 mA cm−2, Voc of 887 mV and FF of 0.66.83
Our previous work on DSSCs showed that organic sensitizers with the D–A–π–A configuration have great performances compared to their D–π–A analogues (Y123) because an auxiliary acceptor is helpful to tune the energy levels and extend the spectral response towards the red as well as improving the photostability. Hence, our group designed and synthesized three BTZ-based D–A–π–A sensitizers (XY1, XY2 and XY3) by introducing various π-bridges together with the CPDT unit. A high molar extinction coefficient of XY2 was gained by replacing the benzene ring in XY1 with a thiophene unit in XY2, resulting in an extension of the absorption response. Exchanging the position of BT and CPDT in XY2 yielded a dye of XY3 with a near-infrared absorption region due to a more negative shift of the HOMO level. Most importantly, a champion PCE of 7.51% was achieved by the XY2-sensitized ssDSSCs with a very thin mesoporous TiO2 layer of about 1.3 μm. Compared with the general optimal thickness of the TiO2 layers (about 2.0 μm), the thin film of about 1.3 μm did not discount the device performance owing to the highest molar extinction coefficient of XY2. Therefore, molecular engineering of organic dyes with a high extinction coefficient as well as suitable blocking effect can obtain efficient sensitizers for ssDSSCs (Fig. 28).93
Inspired by our previous works, our group further designed and synthesized two organic D–A–π–A sensitizers employing a PP unit as the auxiliary acceptor, SH3 and SH4, containing CPDT and EDOT as the π-bridge, respectively, for ssDSSCs. In order to inhibit the fast charge recombination at the interface of the TiO2/dye/spiro-OMeTAD, the bulky DAP-based indoline donor group was introduced. Although the absorption ranges seemed much like that of the two dyes, the molar extinction coefficient reduced dramatically from 5.65 × 104 M−1 cm−1 for SH3 to 1.80 × 104 M−1 cm−1 for SH4. This might be ascribed to the larger twist between the EDOT unit and carboxylic acid in the molecular configuration according to the DFT calculations. Finally, the SH3 sensitized-ssDSSCs achieved a higher PCE of 5.07% with an extremely thin TiO2 layer of 0.6 μm under simulated AM 1.5G conditions, while the ssDSSC based on SH4 obtained an inferior PCE of 1.69% under the same operation conditions. This was due to the increased charge recombination rates according to EIS measurements, resulting in poor charge collection efficiency as well as the low Jsc and Voc for SH4.94
Recently, our group also designed and synthesized two new organic blue colored dyes, S4 and S5, with indeno[1,2-b]thiophene functionalized triphenylamine as the donor, PP and quinoxaline as the auxiliary acceptor for ssDSSCs, respectively. It is noteworthy that S5 containing the quinoxaline unit exhibits a high molar extinction coefficient of 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 600 nm. Most importantly, the S5-based ssDSCs shows record PCEs of 7.81% and 8.25% under one sun and 0.5 sun light intensities, respectively, exceeding the PCE of LEG4-based solar cells (7.34%). These results clearly show that molecular engineering is a viable way to further develop blue-colored dyes with high molar extinction coefficients for application in ssDSSCs, which also opens up new co-sensitization routes in combination with traditional organic dyes with yellow-red colors.95 Meanwhile, two new quinoxaline-based D–A–π–A type organic dyes AQ309 and AQ310 were further synthesized by employing EDOT and CPDT, respectively, as the π-linker units based on the structure of the D–π–A dye LEG4. An impressive record PCE of 8.0% for AQ310-based ssDSSCs using Spiro-OMeTAD as the HTM was recorded under standard AM 1.5 (100 mW cm−2) solar intensity, which clearly outperforms the PCE of the state-of-the-art organic D–π–A dye LEG4-based devices with PCE of 7.3% under the same conditions. Moreover, an excellent high PCE of 8.6% was also achieved for AQ310-based devices under 0.5 sun light intensity. The results clearly identify that molecular engineering is a promising strategy to develop D–A–π–A organic sensitizers for highly efficient ssDSSCs.96
000 M−1 cm−1 at 600 nm. Most importantly, the S5-based ssDSCs shows record PCEs of 7.81% and 8.25% under one sun and 0.5 sun light intensities, respectively, exceeding the PCE of LEG4-based solar cells (7.34%). These results clearly show that molecular engineering is a viable way to further develop blue-colored dyes with high molar extinction coefficients for application in ssDSSCs, which also opens up new co-sensitization routes in combination with traditional organic dyes with yellow-red colors.95 Meanwhile, two new quinoxaline-based D–A–π–A type organic dyes AQ309 and AQ310 were further synthesized by employing EDOT and CPDT, respectively, as the π-linker units based on the structure of the D–π–A dye LEG4. An impressive record PCE of 8.0% for AQ310-based ssDSSCs using Spiro-OMeTAD as the HTM was recorded under standard AM 1.5 (100 mW cm−2) solar intensity, which clearly outperforms the PCE of the state-of-the-art organic D–π–A dye LEG4-based devices with PCE of 7.3% under the same conditions. Moreover, an excellent high PCE of 8.6% was also achieved for AQ310-based devices under 0.5 sun light intensity. The results clearly identify that molecular engineering is a promising strategy to develop D–A–π–A organic sensitizers for highly efficient ssDSSCs.96
In addition, chemical doping is an important method that has been extensively used in p-type organic HTMs, such as Spiro-OMeTAD in ssDSSCs, to improve the charge transport and the overall electrical conductivity. In 2011, Grätzel et al. reported a Co(III) complex of FK102 as a p-type dopant for spiro-MeOTAD and the influence of doping levels on the photovoltaic performance of the Y123 based-device. A champion PCE of 7.2% was achieved by a device containing 1.6% FK102 dopant under simulated AM 1.5G conditions and the corresponding Voc, Jsc, and FF of 986 mV, 9.5 mA cm−2, and 0.76, respectively.97 As interesting as the FK102 dopant, a low-cost, chlorinated, organic solvent, 1,1,2,2-tetrachloroethane (TeCA) was used as a co-solvent as well as an effective additive for Spiro-OMeTAD, and the influence of the doping levels on the photovoltaic performance of LEG4-based ssDSSCs was investigated by Sun et al. in 2015. The formed organic matrix of Spiro-OMeTAD+Cl− was likely to increase the density of the mobile holes of the HTM, and the conductivity was increased by two orders of magnitude upon addition of 20% TeCA (volume ratio relative to HTM solution). Therefore, the device based on LEG4 using 4% TeCA dopant exhibited a best PCE of 7.7% under simulated AM 1.5G conditions, which was considerably higher than the efficiencies of 5.8% and 6.8% yielded for the devices employing oxygen and FK209 as p-type dopants, respectively (Fig. 29).14
4 Summary and outlook
In summary, we have surveyed the investigation and achievements of organic dyes with o,p-dialkoxyphenyl (DAP)-based bulky donor groups for DSSCs. The effects of the molecular structures on the photophysical properties and device performance of these dyes have been highlighted, including the electron-donating ability of donor groups, the length of π-bridges, and steric chains (such as numbers, lengths and position). The detailed cell parameters are summarized in Table 1 (liquid-electrolytes DSSCs) and Table 2 (ssDSSCs) for a comprehensive comparison. These results proved that the DAP-based bulky donor groups can make a significant contribution to further development of highly efficient DSSCs, particularly in cobalt electrolyte-based and ssDSSCs. Compared to the traditional iodine-based electrolytes, the fast charge recombination is accelerated by the mass transport limitation presenting in cobalt-based electrolytes and the poor filling of HTMs in mesoporous TiO2. The blocking effect of this donor group can effectively suppress the charge recombination, which allows for a long electron lifetime and fast charge collection. All in all, the rational design of organic dyes with bulky donor groups would be useful for the development of highly efficient DSSCs.| Dye | λ max | J sc/mA cm−2 | V oc/mV | FF | PCE/% | Redox couples | Ref. |
|---|---|---|---|---|---|---|---|
| a Using Au + GNP as counter electrode. b Co-adsorption with CDCA. c Co-adsorption with HC-A. d Co-sensitization with HC-A1. | |||||||
| D29 | 482 | 12.00 | 670 | 0.60 | 4.83 | I−/I3− | 39 |
| D35 | 445 | 12.96 | 750 | 0.61 | 6.00 | I−/I3− | 39 |
| D37 | 459 | 12.50 | 710 | 0.59 | 5.24 | I−/I3− | 39 |
| D35 | 445 | 10.7 | 920 | 0.68 | 6.7 | [Co(bpy)3]2+/3+ | 37 |
| 9.38 | 910 | 0.65 | 5.5 | I−/I3− | 37 | ||
| D35 | 421 | 9.47 | 860 | 0.72 | 5.9 | [Co(bpy)3]2+/3+ | 40 |
| D45 | 420 | 9.50 | 800 | 0.71 | 5.4 | [Co(bpy)3]2+/3+ | 40 |
| JH301 | 479 | 12.5 | 670 | 0.73 | 6.1 | I−/I3− | 43 |
| JH302 | 493 | 14.3 | 671 | 0.73 | 7.0 | I−/I3− | 43 |
| JH303 | 474 | 12.8 | 680 | 0.75 | 6.5 | I−/I3− | 43 |
| JH304 | 485 | 14.4 | 684 | 0.74 | 7.3 | I−/I3− | 43 |
| D35 | 500 | 8.43 | 910 | 0.67 | 5.10 | [Co(bpy)3]2+/3+ | 42 |
| LEG1 | 515 | 7.77 | 870 | 0.63 | 4.26 | [Co(bpy)3]2+/3+ | 42 |
| LEG2 | 537 | 8.44 | 800 | 0.66 | 4.46 | [Co(bpy)3]2+/3+ | 42 |
| LEG3 | 514 | 8.99 | 920 | 0.71 | 5.83 | [Co(bpy)3]2+/3+ | 42 |
| LEG4 | 541 | 10.9 | 850 | 0.74 | 6.78 | [Co(bpy)3]2+/3+ | 42 |
| G188 | 518 | 12.02 | 878 | 0.73 | 7.68 | [Co(bpy)3]2+/3+ | 44 |
| G234 | 533 | 12.93 | 763 | 0.69 | 6.82 | [Co(bpy)3]2+/3+ | 44 |
| G268 | 581 | 16.27 | 827 | 0.69 | 9.24 | [Co(bpy)3]2+/3+ | 44 |
| G270 | 612 | 5.1 | 615 | 0.68 | 2.12 | [Co(bpy)3]2+/3+ | 44 |
| Y123 | 542 | 14.1 | 876 | 0.78 | 9.77 | [Co(bpy)3]2+/3+ | 46 |
| JF419 | 554 | 16.2 | 840 | 0.76 | 10.3 | [Co(bpy)3]2+/3+ | 68 |
| LP225 | 526 | 13.4 | 901 | 0.74 | 8.86 | [Co(bpy)3]2+/3+ | 46 |
| LP227 | 541 | 14.1 | 811 | 0.77 | 8.72 | [Co(bpy)3]2+/3+ | 46 |
| SGT-121 | 470.5 | 14.0 | 809 | 0.73 | 8.24 | [Co(bpy)3]2+/3+ | 47 |
| SGT-129 | 426 | 9.78 | 760 | 0.70 | 5.21 | [Co(bpy)3]2+/3+ | 47 |
| SGT-130 | 514.5 | 16.77 | 851 | 0.73 | 10.47 | [Co(bpy)3]2+/3+ | 47 |
| 16.84 | 810 | 0.72 | 9.83 | [Co(bpy)3]2+/3+ | 48 | ||
| SGT-136 | 531 | 18.35 | 804 | 0.75 | 11.04 | [Co(bpy)3]2+/3+ | 48 |
| SGT-137 | 542.5 | 19.39 | 825 | 0.74 | 11.84 | [Co(bpy)3]2+/3+ | 48 |
| 18.37 | 884 | 0.77 | 12.45d | [Co(bpy)3]2+/3+ | 48 | ||
| AQ201 | 491 | 12.52 | 838 | 0.68 | 7.10 | [Co(bpy)3]2+/3+ | 49 |
| 12.75 | 756 | 0.68 | 6.57 | I−/I3− | 49 | ||
| AQ202 | 515 | 14.61 | 830 | 0.69 | 8.37 | [Co(bpy)3]2+/3+ | 49 |
| 13.21 | 720 | 0.68 | 6.48 | I−/I3− | 49 | ||
| AQ203 | 545 | 14.39 | 797 | 0.70 | 8.06 | [Co(bpy)3]2+/3+ | 49 |
| 13.56 | 692 | 0.68 | 6.39 | I−/I3− | 49 | ||
| AQ308 | 548 | 16.75 | 830 | 0.71 | 9.81 | [Co(bpy)3]2+/3+ | 50 |
| DPP13 | 587 | 16.2 | 705 | 0.67 | 7.60 | I−/I3− | 53 |
| 15.6 | 743 | 0.78 | 8.97 | [Co(bpy)3]2+/3+ | 53 | ||
| DPP14 | 596 | 16.6 | 680 | 0.68 | 7.73 | I−/I3− | 53 |
| 15.2 | 716 | 0.76 | 8.23 | [Co(bpy)3]2+/3+ | 53 | ||
| DPP15 | 600 | 16.9 | 684 | 0.65 | 7.44 | I−/I3− | 53 |
| 17.6 | 745 | 0.75 | 9.81 | [Co(bpy)3]2+/3+ | 53 | ||
| DPP17 | 602 | 16.3 | 700 | 0.63 | 7.13 | I−/I3− | 53 |
| 17.9 | 761 | 0.74 | 10.1 | [Co(bpy)3]2+/3+ | 53 | ||
| IQ4 | 526 | 15.33 | 737 | 0.76 | 8.53 | I−/I3− | 54 |
| 14.69 | 771 | 0.69 | 7.79 | [Co(bpy)3]2+/3+ | 54 | ||
| YA421 | 522 | 15.41 | 741 | 0.71 | 8.12 | I−/I3− | 54 |
| 15.76 | 803 | 0.71 | 9.00 | [Co(bpy)3]2+/3+ | 54 | ||
| YA422 | 534 | 14.40 | 741 | 0.68 | 7.28 | I−/I3− | 54 |
| 15.26 | 876 | 0.69 | 9.22 | [Co(bpy)3]2+/3+ | 54 | ||
| 16.25 | 890 | 0.74 | 10.65a | [Co(bpy)3]2+/3+ | 54 | ||
| DTN-1 | 571 | 10.67 | 789 | 0.73 | 6.10 | [Co(bpy)3]2+/3+ | 59 |
| DT-1 | 557 | 16.08 | 802 | 0.66 | 8.57 | [Co(bpy)3]2+/3+ | 60 |
| DT-2 | 541 | 15.35 | 790 | 0.64 | 7.74 | [Co(bpy)3]2+/3+ | 60 |
| JK-305 | 454 | 13.12 | 760 | 0.73 | 7.29b | I−/I3− | 65 |
| JK-306 | 475 | 16.10 | 720 | 0.70 | 8.37b | I−/I3− | 65 |
| 16.14 | 750 | 0.71 | 8.52c | I−/I3− | 65 | ||
| 14.67 | 870 | 0.73 | 9.40b | [Co(bpy)3]2+/3+ | 65 | ||
| 14.83 | 950 | 0.71 | 10.02c | [Co(bpy)3]2+/3+ | 65 | ||
| JK-319 | 438 | 8.90 | 791 | 0.77 | 5.41 | I−/I3− | 66 |
| 9.33 | 930 | 0.74 | 6.38 | [Co(bpy)3]2+/3+ | 66 | ||
| JK-320 | 441 | 9.16 | 826 | 0.78 | 5.88 | I−/I3− | 66 |
| 9.85 | 938 | 0.72 | 6.66 | [Co(bpy)3]2+/3+ | 66 | ||
| S1 | 611 | 9.49 | 648 | 0.66 | 4.03 | [Co(Me2bpy)3]2+/3+ | 67 |
| 8.37 | 604 | 0.72 | 3.65 | I−/I3− | 67 | ||
| S2 | 616 | 12.97 | 651 | 0.65 | 5.49 | [Co(Me2bpy)3]2+/3+ | 67 |
| 12.26 | 621 | 0.70 | 5.32 | I−/I3− | 67 | ||
| S3 | 628 | 16.73 | 667 | 0.65 | 7.23 | [Co(Me2bpy)3]2+/3+ | 67 |
| 14.19 | 647 | 0.69 | 6.29 | I−/I3− | 67 | ||
| SGT-101 | 479 | 11.9 | 862 | 0.69 | 7.11 | [Co(bpy)3]2+/3+ | 72 |
| SGT-102 | 479 | 12.1 | 865 | 0.69 | 7.22 | [Co(bpy)3]2+/3+ | 72 |
| SGT-103 | 484 | 8.95 | 793 | 0.73 | 5.16 | [Co(bpy)3]2+/3+ | 72 |
| SGT-105 | 486 | 10.6 | 889 | 0.67 | 6.87 | [Co(bpy)3]2+/3+ | 71 |
| SGT-106 | 490 | 12.4 | 883 | 0.64 | 7.22 | [Co(bpy)3]2+/3+ | 71 |
| SGT-107 | 480 | 10.3 | 882 | 0.72 | 6.55 | [Co(bpy)3]2+/3+ | 71 |
| SGT-108 | 483 | 11.1 | 798 | 0.75 | 6.67 | [Co(bpy)3]2+/3+ | 71 |
| SGT-109 | 507 | 14.1 | 846 | 0.72 | 8.61 | [Co(bpy)3]2+/3+ | 71 |
| SGT-110 | 561 | 8.08 | 801 | 0.72 | 4.64 | [Co(bpy)3]2+/3+ | 71 |
| SGT-111 | 518 | 11.3 | 858 | 0.74 | 7.01 | [Co(bpy)3]2+/3+ | 71 |
| MP03 | 506 | 14.38 | 780 | 0.64 | 7.17 | I−/I3− | 73 |
| MP05 | 500 | 13.09 | 800 | 0.70 | 7.40 | I−/I3− | 73 |
| MP13 | 491 | 12.25 | 760 | 0.72 | 6.70 | I−/I3− | 73 |
| IB2 | 483 | 12.8 | 760 | 0.65 | 6.3 | I−/I3− | 74 |
| 480 | 10.25 | 880 | 0.70 | 6.3 | [Co(bpy)3]2+/3+ | 74 | |
| IB3 | 482 | 13.7 | 800 | 0.63 | 7.0 | I−/I3− | 74 |
| 496 | 10.36 | 890 | 0.69 | 6.4 | [Co(bpy)3]2+/3+ | 74 | |
| CC202 | 638 | 13.9 | 540 | 0.72 | 5.4 | I−/I3− | 76 |
| 14.5 | 615 | 0.67 | 6.1 | [Co(bpy)3]2+/3+ | 76 | ||
| JD21 | 582 | 15.4 | 730 | 0.75 | 8.4 | I−/I3− | 80 |
| JD25 | 598 | 11.5 | 807 | 0.72 | 6.7 | I−/I3− | 80 |
| JD29 | 598 | 13.3 | 716 | 0.70 | 6.7 | I−/I3− | 80 |
| JD32 | 393 | 3.7 | 553 | 0.78 | 1.7 | I−/I3− | 80 |
| AH3 | 558 | 9.0 | 545 | 0.75 | 3.74 | I−/I3− | 78 |
| 6.0 | 630 | 0.78 | 3.04 | [Co(bpy)3]2+/3+ | 78 | ||
| AH7 | 605 | 14.7 | 696 | 0.76 | 7.99 | I−/I3− | 78 |
| 13.5 | 757 | 0.78 | 8.10 | [Co(bpy)3]2+/3+ | 78 | ||
| YD-o-C8 | 448, 581, 645 | 17.3 | 965 | 0.71 | 11.9 | [Co(bpy)3]2+/3+ | 82 |
| SM371 | 447, 580, 646 | 15.9 | 960 | 0.79 | 12.0 | [Co(bpy)3]2+/3+ | 10 |
| SM315 | 440, 454, 581, 668 | 18.1 | 910 | 0.78 | 13.0 | [Co(bpy)3]2+/3+ | 10 |
| 16.9 | 819 | 0.75 | 10.4 | [Co(bpy)3]2+/3+ | 86 | ||
| Y350 | 446, 581, 645 | 17.2 | 942 | 0.74 | 12.0 | [Co(bpy)3]2+/3+ | 83 |
| Y350-OC12 | 447, 581, 645 | 16.0 | 968 | 0.74 | 11.5 | [Co(bpy)3]2+/3+ | 83 |
| 2,4-ZnP-CNCOOH | 422, 571, 630 | 10.92 | 629 | 0.71 | 4.88 | I−/I3− | 85 |
| 15.39 | 739 | 0.74 | 8.46d | I−/I3− | 85 | ||
| SGT-021 | 455, 674 | 17.9 | 819 | 0.75 | 11.1 | [Co(bpy)3]2+/3+ | 86 |
| SM63 | 450, 474, 530, 693 | 14.43 | 700 | 0.73 | 7.35 | I−/I3− | 87 |
| LD14-C8 | 459, 667 | 15.72 | 730 | 0.74 | 8.45 | I−/I3− | 87 |
| Dye | λ max/nm | J sc/mA cm−2 | V oc/mV | FF | PCE/% | Ref. |
|---|---|---|---|---|---|---|
| a The champion efficiency of XY2-based ssDSSC. b Under 0.5 sun light intensity. | ||||||
| M14 | 504 | 8.08 | 815 | 0.67 | 4.37 | 91 |
| D35 | 500 | 7.21 | 855 | 0.73 | 4.50 | 91 |
| JD21 | 555 | 8.5 | 743 | 0.66 | 4.19 | 92 |
| JD25 | 572 | 9.7 | 752 | 0.66 | 4.88 | 92 |
| JD26 | 516 | 4.7 | 723 | 0.62 | 2.11 | 92 |
| JD27 | 525 | 5.2 | 694 | 0.61 | 2.19 | 92 |
| JD29 | 537 | 9.7 | 797 | 0.64 | 4.95 | 92 |
| JD29 + D35 | 10.6 | 801 | 0.64 | 5.40 | 92 | |
| Y350 | 446, 581, 645 | 9.0 | 800 | 0.64 | 4.8 | 83 |
| Y350 + Y123 | 10.8 | 887 | 0.66 | 6.4 | 83 | |
| Y123 | 543 | 8.45 | 904 | 0.73 | 5.77 | 93 |
| XY1 | 552 | 10.02 | 942 | 0.67 | 6.69 | 93 |
| XY2 | 578 | 10.14 | 929 | 0.70 | 6.89 | 93 |
| XY2 | 10.96 | 902 | 0.76 | 7.51a | 93 | |
| XY3 | 618 | 11.06 | 798 | 0.60 | 5.50 | 93 |
| SH3 | 562 | 10.42 | 705 | 0.74 | 5.54 | 94 |
| SH4 | 561 | 7.71 | 579 | 0.76 | 3.43 | 94 |
| Y123 | 543 | 9.5 | 986 | 0.76 | 7.2 | 97 |
| LEG4 | 541 | 11.65 | 900 | 0.73 | 7.7 | 14 |
| 11.37 | 880 | 0.73 | 7.34 | 95 | ||
| S4 | 612 | 9.06 | 800 | 0.65 | 4.71 | 95 |
| S5 | 600 | 12.91 | 830 | 0.73 | 7.81 | 95 |
| 6.67 | 810 | 0.76 | 8.25b | 95 | ||
| AQ309 | 553 | 11.5 | 820 | 0.72 | 6.8 | 96 |
| AQ310 | 569 | 12.3 | 870 | 0.75 | 8.0 | 96 |
| 6.5 | 850 | 0.77 | 8.6b | 96 | ||
It is thus that the strategies of introducing bulky donor groups is through increasing the size and enhancing the steric hindrance for preventing charge recombination and dye aggregation to achieve a high Voc.98 It is necessary to point out that the molecular design of increasing the size and enhancing the steric hindrance should be considered from two perspectives. One is through expending the conjugation to increase the donor size, which is beneficial to form a compact sensitizer layer on the TiO2 surface; the other is through introducing long alkyl or alkoxy chains to enhance the steric hindrance, which helps to block the approach of the oxidized redox mediator/HTM to the TiO2 surface.27
In addition, a good light-harvesting capacity of dyes is obtained through broadening the spectral response, and increasing the extinction coefficient is also indispensable for obtaining a high Jsc. D–π–A configuration dyes are favorable for intramolecular charge transfer, and the introduction of strong electron-donating substituents on the donor system, elongation of the π-conjugated linker and an anchoring group with strong electron-withdrawing ability could be the fundamental approaches to induce a red-shifted absorption. However, we should bear in mind that a red-shift in the absorption stems from the narrowed band gap between the HOMO and LUMO levels of the dyes, which usually causes energy level mismatch, leading to an inadequate driving force for either dye regeneration or electron injection.99,100 Therefore, a rational design of dyes should depend on not only experience but also computational chemistry for a comprehensive understanding of the essential structure properties of dyes.
Last but not least, photostability of dyes has been a major problem constricting the development of commercialized DSSCs and applications. Fortunately, the emergence of the D–A–π–A structure by introducing an additional electron-withdrawing group between the donor and π-bridge brings hope to scientific researchers because it can not only increase the ICT process and spectral response but also significantly improve the photostability and electrochemical stability.55,101 Many kinds of additional electron-withdrawing groups have been applied in organic dyes based on the D–A–π–A configuration, such as benzothiadiazole,11,52,71,93,94,102 benzotriazole,103–105 quinoxaline,50,51,54,56,58 pyrido[3,4-b]pyrazine,57,59,60 diketopyrrolopyrrole,53,106–109 isoindigo110–112 units and so on.
Despite the many challenges involved, DSSCs have attracted attention as promising next-generation photovoltaic devices for indoor applications. Accordingly, a thin mesoporous TiO2 electrode would make DSSCs more competitive in the photovoltaic industry because a thin TiO2 electrode could increase the transparency and reduce the production costs. Moreover, the limitations of the short diffusion length and poor filling of HTMs in mesoporous TiO2 also can be overcome by a thin electrode, resulting in low charge recombination. The development of a high molar extinction coefficient is required to achieve good absorption under the circumstance of incapacity of loading more dyes. Meanwhile, a variety of colours can be presented in the devices for beautification and decoration, which cannot be matched by any other next-generation photovoltaic devices. Therefore, we believe DSSCs are expected to be further developed, particularly tandem DSSCs fabricated by several single junction cells with compensation of the light absorption spectrum using a series or parallel configuration.48,86 It is considered a promising candidate as a photoelectrochemical cell applied as a photocatalyst for light driven hydrogen production.113–115 Compared to the traditional photoelectrochemical cell, it is expected to be commercialized with low-cost materials and a simple process. In addition, abundant kinds of sensitizers covering the panchromatic spectrum can satisfy the ideal wavelength cutoffs to realize maximum efficiencies. Although present research in this scope has been restricted to two dye-sensitized photoelectrode architectures, the combination of DSSCs with various thin film solar cells, such as perovskite-DSSC, quantum dot-DSSC and organic photovoltaic-DSSC tandem solar cells, has bright prospects.
Acknowledgements
For financial support of this work, we thank NSFC/China (21572062, 21421004, 21372082, and 91233207), the National Basic Research 973 Program (2013CB733700) and the Programme of Introducing Talents of Discipline to Universities (B16017).Notes and references
- H. Tian, S. Huang and C. Zhou, Acta Phys.-Chim. Sin., 1988, 4, 314–319 CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- R. Komiya, A. Fukui, N. Murofushi, N. Koide, R. Yamanaka and H. Katayama, Technical Digest, 21st International Photovoltaic Science and Engineering Conference, Fukuoka, November 2011, 2 C-50-08 Search PubMed.
- L. Y. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. F. Zhang, X. D. Yang and M. Yanagida, Energy Environ. Sci., 2012, 5, 6057–6060 CAS.
- Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055–14058 CrossRef CAS PubMed.
- B. E. Hardin, H. J. Snaith and M. D. McGehee, Nat. Photonics, 2012, 6, 162–169 CrossRef CAS.
- T. W. Hamann, Dalton Trans., 2012, 41, 3111–3115 RSC.
- Z. Sun, M. Liang and J. Chen, Acc. Chem. Res., 2015, 48, 1541–1550 CrossRef CAS PubMed.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J.-I. Fujisawab and M. Hanaya, Dalton Trans., 2015, 51, 15894–15897 CAS.
- S. Mathew, A. Yella, P. Gao, R. H.-Baker, B. F. E. Curchod, N. A.-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- T.-Y. Kim, D. Song, E. M. Barea, J. H. Lee, Y. R. Kim, W. Cho, S. Lee, M. M. Rahman, J. Bisquert and Y. S. Kang, Nano Energy, 2016, 28, 455–461 CrossRef CAS.
- P. Chen, J. H. Yum, F. D. Angelis, E. Mosconi, S. Fantacci, S. J. Moon, R. H. Baker, J. Ko, M. K. Nazeeruddin and M. Grätzel, Nano Lett., 2009, 6, 2487–2492 CrossRef PubMed.
- B. Xu, E. Gabrielsson, M. Safdari, M. Cheng, Y. Hua, H. Tian, J. M. Gardner, L. Kloo and L. Sun, Adv. Energy Mater., 2015, 5, 1402340 CrossRef.
- M. Freitag, Q. Daniel, M. Pazoki, K. Sveinbjörnsson, J. Zhang, L. Sun, A. Hagfeldt and G. Boschloo, Energy Environ. Sci., 2015, 8, 2634–2637 CAS.
- Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032–4080 CrossRef CAS PubMed.
- A. B. F. Martinson, T. W. Hamann, M. J. Pellin and J. T. Hupp, Chem. – Eur. J., 2008, 14, 4458–4467 CrossRef CAS PubMed.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissörtel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583–585 CrossRef CAS.
- P. Docampo, S. Guldin, T. Leijtens, N. K. Noel, U. Steiner and H. J. Snaith, Adv. Mater., 2014, 26, 4013–4030 CrossRef CAS PubMed.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629–6634 CrossRef CAS PubMed.
- C. Y. Hsu, Y. C. Chen, R. Y. Y. Lin, K. C. Ho and J. T. Lin, Phys. Chem. Chem. Phys., 2012, 14, 14099–14109 RSC.
- U. Bach, Y. Tachibana, J. E. Moser, S. A. Haque, J. R. Durrant, M. Grätzel and D. R. Klug, J. Am. Chem. Soc., 1999, 121, 7445–7446 CrossRef CAS.
- H. J. Snaith, R. Humphry-Baker, P. Chen, I. Cesar, S. M. Zakeeruddin and M. Grätzel, Nanotechnology, 2008, 19, 424003 CrossRef PubMed.
- I.-K. Ding, N. Tétreault, J. Brillet, B. E. Hardin, E. H. Smith, S. J. Rosenthal, F. Sauvage, M. Grätzel and M. D. McGehee, Adv. Funct. Mater., 2009, 19, 2431–2436 CrossRef CAS.
- X. Yang, M. Yanagida and L. Han, Energy Environ. Sci., 2013, 6, 54–66 CAS.
- S. Zhang, X. Yang, Y. Numata and L. Han, Energy Environ. Sci., 2013, 6, 1443–1464 CAS.
- Z. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170–1181 CAS.
- Z. Ning, Q. Zhang, W. Wu, H. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3791–3797 CrossRef CAS PubMed.
- Z. Ning and H. Tian, Chem. Commun., 2009, 5483–5495 RSC.
- J. Tang, W. Wu, J. Hua, J. Li, X. Li and H. Tian, Energy Environ. Sci., 2009, 2, 982–990 CAS.
- J. Tang, J. Hua, W. Wu, J. Li, Z. Jin, Y. Long and H. Tian, Energy Environ. Sci., 2010, 3, 1736–1745 CAS.
- S. Qu, J. Hua and H. Tian, Sci. China: Chem., 2012, 55, 677–697 CrossRef CAS.
- N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256–14257 CrossRef CAS PubMed.
- Z. Ning, Q. Zhang, H. Pei, J. Luan, C. Lu, Y. Cui and H. Tian, J. Phys. Chem. C, 2009, 113, 10307–10313 CAS.
- Z.-S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993–4003 CrossRef CAS.
- N. Koumura, Z. Wang, M. Miyashita, Y. Uemura, H. Sekiguchi, Y. Cui, A. Mori, S. Mori and K. Hara, J. Mater. Chem., 2009, 19, 4829–4836 RSC.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, J. Am. Chem. Soc., 2010, 132, 16714–16724 CrossRef CAS PubMed.
- M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453–3488 RSC.
- D. P. Hagberg, X. Jiang, E. Gabrielsson, M. Linder, T. Marinado, T. Brinck, A. Hagfeldt and L. Sun, J. Mater. Chem., 2009, 19, 7232–7238 RSC.
- H. Ellis, I. Schmidt, A. Hagfeldt, G. Wittstock and G. Boschloo, J. Phys. Chem. C, 2015, 119, 21775–21783 CAS.
- E. Gabrielsson, H. Ellis, S. Feldt, H. Tian, G. Boschloo, A. Hagfeldt and L. Sun, Adv. Energy Mater., 2013, 3, 1647–1656 CrossRef CAS.
- H. Ellis, S. K. Eriksson, S. M. Feldt, E. Gabrielsson, P. W. Lohse, R. Lindblad, L. Sun, H. Rensmo, G. Boschloo and A. Hagfeldt, J. Phys. Chem. C, 2013, 117, 21029–21036 CAS.
- J. Zhao, X. Yang, M. Cheng, S. Li, X. Wang and L. Sun, J. Mater. Chem. A, 2013, 1, 2441–2446 CAS.
- P. Gao, H. N. Tsao, C. Yi, M. Grätzel and M. K. Nazeeruddin, Adv. Energy Mater., 2014, 4, 1301485 CrossRef.
- H. N. Tsao, C. Yi, T. Moehl, J.-H. Yum, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, ChemSusChem, 2011, 4, 591–594 CrossRef CAS PubMed.
- L. E. Polander, A. Yella, J. Teuscher, R. H.-Baker, B. F. E. Curchod, N. A. Astani, P. Gao, J.-E. Moser, I. Tavernelli, U. Rothlisberger, M. Grätzel, M. K. Nazeeruddin and J. Frey, Chem. Mater., 2013, 25, 2642–2648 CrossRef CAS.
- Y. K. Eom, I. T. Choi, S. H. Kang, J. Lee, J. Kim, M. J. Ju and H. K. Kim, Adv. Energy Mater., 2015, 5, 1500300 CrossRef.
- Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim and H. K. Kim, J. Mater. Chem. A, 2017, 5, 2297–2308 CAS.
- X. Li, Y. Hu, I. Sanchez-Molina, Y. Zhou, F. Yu, S. A. Haque, W. Wu, J. Hua, H. Tian and N. Robertson, J. Mater. Chem. A, 2015, 3, 21733–21743 CAS.
- X. Li, Y. Zhou, J. Chen, J. Yang, Z. Zheng, W. Wu, J. Hua and H. Tian, Chem. Commun., 2015, 51, 10349–10352 RSC.
- K. Pei, Y. Wu, W. Wu, Q. Zhang, B. Chen, H. Tian and W. Zhu, Chem. – Eur. J., 2012, 18, 8190–8200 CrossRef CAS PubMed.
- W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z.-S. Wang and H. Tian, Adv. Funct. Mater., 2011, 21, 756–763 CrossRef CAS.
- J.-H. Yum, T. W. Holcombe, Y. Kim, K. Rakstys, T. Moehl, J. Teuscher, J. H. Delcamp, M. K. Nazeeruddin and M. Grätzel, Sci. Rep., 2013, 3, 2446 Search PubMed.
- J. Yang, P. Ganesan, J. Teuscher, T. Moehl, Y. J. Kim, C. Yi, P. Comte, K. Pei, T. W. Holcombe, M. K. Nazeeruddin, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, J. Am. Chem. Soc., 2014, 136, 5722–5730 CrossRef CAS PubMed.
- Y. Wu, W.-H. Zhu, S. M. Zakeeruddin and M. Grätzel, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CAS.
- K. Pei, Y. Wu, A. Islam, Q. Zhang, L. Han, H. Tian and W. Zhu, ACS Appl. Mater. Interfaces, 2013, 5, 4986–4995 CAS.
- W. Ying, J. Yang, M. Wielopolski, T. Moehl, J.-E. Moser, P. Comte, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, Chem. Sci., 2014, 5, 206–214 RSC.
- X. Li, S. Cui, D. Wang, Y. Zhou, H. Zhou, Y. Hu, J. Liu, Y. Long, W. Wu, J. Hua and H. Tian, ChemSusChem, 2014, 7, 2879–2888 CrossRef CAS PubMed.
- Y. Zhou, X. Li, X. Li, J. Chen, F. Yu and J. Hua, Asian J. Org. Chem., 2016, 5, 293–300 CrossRef CAS.
- X. Zhang, J. Mao, D. Wang, X. Li, J. Yang, Z. Shen, W. Wu, J. Li, H. Ågren and J. Hua, ACS Appl. Mater. Interfaces, 2015, 7, 2760–2771 CAS.
- H. Choi, S. Paek, K. Lim, C. Kim, M.-S. Kang, K. Song and J. Ko, J. Mater. Chem. A, 2013, 1, 8226–8233 CAS.
- H. Choi, M. Shin, K. Song, M.-S. Kang, Y. Kang and J. Ko, J. Mater. Chem. A, 2014, 2, 12931–12939 CAS.
- M. S. Kang, S. H. Kang, S. K. Kim, I. T. Choi, J. H. Ryu, M. J. Ju, D. Cho, J. Y. Lee and H. K. Kim, Chem. Commun., 2012, 48, 9349–9351 RSC.
- S. H. Kang, I. T. Choi, M. S. Kang, Y. K. Eom, M. J. Ju, J. Y. Hong, H. S. Kang and H. K. Kim, J. Mater. Chem. A, 2013, 1, 3977–3982 CAS.
- K. Lim, M. J. Ju, J. Song, I. T. Choi, K. Do, H. Choi, K. Song, H. K. Kim and J. Ko, ChemSusChem, 2013, 6, 1425–1431 CrossRef CAS PubMed.
- K. Lim, K. Song, Y. Kang and J. Ko, Dyes Pigm., 2015, 119, 41–48 CrossRef CAS.
- Z. Shen, J. Chen, X. Li, X. Li, Y. Zhou, Y. Yu, H. Ding, J. Li, L. Zhu and J. Hua, ACS Sustainable Chem. Eng., 2016, 4, 3518–3525 CrossRef CAS.
- B. M. Klahr and T. W. Hamann, J. Phys. Chem. C, 2009, 113, 14040–14045 CAS.
- A. Yella, R. H.-Baker, B. F. E. Curchod, N. A. Astani, J. Teuscher, L. E. Polander, S. Mathew, J.-E. Moser, I. Tavernelli, U. Rothlisberger, M. Grätzel, M. K. Nazeeruddin and J. Frey, Chem. Mater., 2013, 25, 2733–2739 CrossRef CAS.
- M. S. Kang, S. H. Kang, S. G. Kim, I. T. Choi, J. H. Ryu, M. J. Ju, D. Cho, J. Y. Lee and H. K. Kim, Chem. Commun., 2012, 48, 9349–9351 RSC.
- K. D. Seo, I. T. Choi and H. K. Kim, Chem. – Eur. J., 2015, 21, 14804–14811 CrossRef CAS PubMed.
- K. D. Seo, I. T. Choi and H. K. Kim, Org. Electron., 2015, 25, 1–5 CrossRef CAS.
- K. M. Karlsson, X. Jiang, S. K. Eriksson, E. Gabrielsson, H. Rensmo, A. Hagfeldt and L. Sun, Chem. – Eur. J., 2011, 17, 6415–6424 CrossRef CAS PubMed.
- H. Tian, I. Bora, X. Jiang, E. Gabrielsson, K. M. Karlsson, A. Hagfeldt and L. Sun, J. Mater. Chem., 2011, 21, 12462–12472 RSC.
- Y. Hao, H. Tian, J. Cong, W. Yang, I. Bora, L. Sun, G. Boschloo and A. Hagfeldt, ChemPhysChem, 2014, 15, 3476–3483 CrossRef CAS PubMed.
- C. Chen, X. Yang, M. Cheng, F. Zhang, J. Zhao and L. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 10960–10965 CAS.
- A. J. Huckaba, F. Giordano, L. E. McNamara, K. M. Dreux, N. I. Hammer, G. S. Tschumper, S. M. Zakeeruddin, M. Grätzel, M. K. Nazeeruddin and J. H. Delcamp, Adv. Energy Mater., 2015, 5, 1401629 CrossRef.
- J. Huckaba, A. Yella, P. Brogdon, J. S. Murphy, M. K. Nazeeruddin, M. Grätzel and J. H. Delcamp, Chem. Commun., 2016, 52, 8424–8427 RSC.
- J. Feng, Y. Jiao, W. Ma, M. K. Nazeeruddin, M. Grätzel and S. Meng, J. Phys. Chem. C, 2013, 117, 3772–3778 CAS.
- J. H. Delcamp, A. Yella, T. W. Holcombe, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2013, 52, 376–380 CrossRef CAS PubMed.
- M. Urbani, M. Grätzel, M. K. Nazeeruddin and T. Torres, Chem. Rev., 2014, 114, 12330–12396 CrossRef CAS PubMed.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- C. Yi, F. Giordano, N.-L. Cevey-Ha, H. N. Tsao, S. M. Zakeeruddin and M. Grätzel, ChemSusChem, 2014, 7, 1107–1113 CrossRef CAS PubMed.
- M. S. Kang, S. H. Kang, S. G. Kim, I. T. Choi, J. H. Ryu, M. J. Ju, D. Cho, J. Y. Lee and H. K. Kim, Chem. Commun., 2012, 48, 9349–9351 RSC.
- M. S. Kang, I. T. Choi, Y. W. Kim, B. S. You, S. H. Kang, J. Y. Hong, M. J. Ju and H. K. Kim, J. Mater. Chem. A, 2013, 1, 9848–9852 CAS.
- S. H. Kang, M. J. Jeong, Y. K. Eom, I. T. Choi, S. M. Kwon, Y. Yoo, J. Kim, J. Kwon, J. H. Park and H. K. Kim, Adv. Energy Mater., 2016, 1602117 CrossRef.
- S. Mathew, N. A. Astani, B. F. E. Curchod, J. H. Delcamp, M. Marszalek, J. Frey, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, J. Mater. Chem. A, 2016, 4, 2332–2339 CAS.
- T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448–463 RSC.
- P. Docampo, S. Guldin, T. Leijtens, N. K. Noel, U. Steiner and H. J. Snaith, Adv. Mater., 2014, 26, 4013–4030 CrossRef CAS PubMed.
- J. Lu, Y.-C. Chang, H.-Y. Cheng, H.-P. Wu, Y. Cheng, M. Wang and E. W.-G. Diau, ChemSusChem, 2015, 8, 2529–2536 CrossRef CAS PubMed.
- X. Jiang, K. M. Karlsson, E. Gabrielsson, E. M. J. Johansson, M. Quintana, M. Karlsson, L. Sun, G. Boschloo and A. Hagfeldt, Adv. Funct. Mater., 2011, 21, 2944–2952 CrossRef CAS.
- A. Dualeh, R. Humphry-Baker, J. H. Delcamp, M. K. Nazeeruddin and M. Grätzel, Adv. Energy Mater., 2012, 3, 496–504 CrossRef.
- X. Zhang, Y. Xu, F. Giordano, M. Schreier, N. Pellet, Y. Hu, C. Yi, N. Robertson, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, J. Am. Chem. Soc., 2016, 138, 10742–10745 CrossRef CAS PubMed.
- Z. Shen, X. Zhang, F. Giordano, Y. Hu, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, Mater. Chem. Front., 2017, 1, 181–189 RSC.
- Z. Shen, B. Xu, P. Liu, Y. Hu, H. Ding, L. Kloo, J. Hua, L. Sun and H. Tian, J. Mater. Chem. A, 2017, 5, 1242–1247 CAS.
- X. Li, B. Xu, P. Liu, Y. Hu, L. Kloo, J. Hua, L. Sun and H. Tian, J. Mater. Chem. A, 2017 10.1039/C6TA10673K.
- J. Burschka, A. Dualeh, F. Kessler, E. Baranoff, N.-L. Cevey-Ha, C. Yi, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2011, 133, 18042–18045 CrossRef CAS PubMed.
- S. Ahmad, E. Guillén, L. Kavan, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439–3466 CAS.
- T. W. Hamann, R. A. Jensen, A. B. F. Martinson, H. V. Ryswyk and J. T. Hupp, Energy Environ. Sci., 2008, 1, 66–78 CAS.
- D. P. Hagberg, T. Marinado, K. M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Brinck, A. Hagfeldt and L. Sun, J. Org. Chem., 2007, 72, 9550–9556 CrossRef CAS PubMed.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC.
- Y. Wu, X. Zhang, W. Li, Z.-S. Wang, H. Tian and W. Zhu, Adv. Energy Mater., 2012, 2, 149–156 CrossRef CAS.
- Y. Cui, Y. Wu, X. Lu, X. Zhang, G. Zhou, F. B. Miapeh, W. Zhu and Z.-S. Wang, Chem. Mater., 2011, 23, 4394–4401 CrossRef CAS.
- J. Mao, F. Guo, W. Ying, W. Wu, J. Li and J. Hua, Chem. – Asian J., 2012, 7, 982–991 CrossRef CAS PubMed.
- Y.-S. Yen, C.-T. Lee, C.-Y. Hsu, H.-H. Chou, Y.-C. Chen and J. T. Lin, Chem. – Asian J., 2013, 8, 809–816 CrossRef CAS PubMed.
- S. Qu, W. Wu, J. Hua, C. Kong, Y. Long and H. Tian, J. Phys. Chem. C, 2010, 114, 1343–1349 CAS.
- S. Qu, C. Qin, A. Islam, J. Hua, H. Chen, H. Tian and L. Han, Chem. – Asian J., 2012, 7, 2895–2903 CrossRef CAS PubMed.
- S. Qu, C. Qin, A. Islam, Y. Wu, W. Zhu, L. Hua, H. Tian and L. Han, Chem. Commun., 2012, 48, 6972–6974 RSC.
- S. Qu, B. Wang, F. Guo, J. Li, W. Wu, C. Kong, Y. Long and J. Hua, Dyes Pigm., 2012, 92, 1384–1393 CrossRef CAS.
- W. Ying, F. Guo, J. Li, Q. Zhang, W. Wu, H. Tian and J. Hua, ACS Appl. Mater. Interfaces, 2012, 4, 4215–4224 CAS.
- S. Li, K. Jiang, J. Huang, L. Yang and Y. Song, Chem. Commun., 2014, 50, 4309–4311 RSC.
- D. Wang, W. Ying, X. Zhang, Y. Hu, W. Wu and J. Hua, Dyes Pigm., 2015, 112, 327–334 CrossRef CAS.
- F. Li, K. Fan, B. Xu, E. Gabrielsson, Q. Daniel, L. Li and L. Sun, J. Am. Chem. Soc., 2015, 137, 9153–9159 CrossRef CAS PubMed.
- B. D. Sherman, J. J. Bergkamp, C. L. Brown, A. L. Moore, D. Gust and T. A. Moore, Energy Environ. Sci., 2016, 9, 1812–1817 CAS.
- B. D. Sherman, M. V. Sheridan, K.-R. Wee, S. L. Marquard, D. Wang, L. Alibabaei, D. L. Ashford and T. J. Meyer, J. Am. Chem. Soc., 2016, 138, 16745–16753 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |