A comprehensive physico-chemical study on the molecular structure effects of sulfonated polyamide thin-film composites†
Jiahui
Hu
a,
Yang
Liu
a,
Xingzhong
Cao
b,
Peng
Zhang
b,
Junfeng
Zheng
a,
Meng
Li
a,
Xuan
Zhang
*a and
Lianjun
Wang
*a
aJiangsu Key Laboratory of Chemical Pollution Control and Resources Reuse, School of Environmental and Biological Engineering, Nanjing University of Science & Technology, Nanjing 210094, China. E-mail: xuanzhang@mail.njust.edu.cn; wanglj@mail.njust.edu.cn
bMultidiscipline Research Center, Institute of High Energy Physics, CAS, Beijing, China
First published on 10th November 2016
Abstract
Two similar monomers, 4,4′-diaminodiphenyl ether-2,2′-disulfonic acid (ODADS) and benzidine-2,2′-disulfonic acid (BDSA), were selected and used for the fabrication of the corresponding sulfonated thin-film composite (TFC) membranes with trimesoyl chloride (TMC) on the polysulfone substrate via conventional interfacial polymerization. Despite only a slight difference in the chemical structures of these two monomers, the as-prepared membranes exhibited distinctly different separation behaviors during the water desalination process. In brief, the molecular architectures significantly affected the solubility and the diffusion of the small molecules into the organic phase during the IP process. As a result, the corresponding barrier layers of TFC membranes were produced with different densities and thicknesses. Compared to TFC-BDSA, the TFC-ODADS membrane showed relatively lower water permeability but better rejection ability for ions, as confirmed by positron annihilation spectroscopy (PAS). By a dissipative particle dynamics (DPD) simulation approach, it was found that the molecular orientations of the active layer of the two membranes were in opposite directions. Specifically, the sulfonic acid groups in TFC-ODADS membrane surface were mostly facing outward, whereas they were enclosed by the hydrophobic polyamide backbone in the case of the TFC-BDSA membrane. As a consequence, the surface hydrophilicity was greatly enhanced for the former, as indicated by its higher water flux recovery ratio (FRR) of 96.8% for organic pollutants, which was much higher than that of the latter (81.8%).
Design, System, ApplicationThe surface physico-chemical properties of a nanofiltration membrane are crucial since they directly affect the separation performance and the life-time. For this purpose, two similar monomers, 4,4′-diaminodiphenyl ether-2,2′-disulfonic acid (ODADS) and benzidine-2,2′-disulfonic acid (BDSA), were designed and used for the fabrication of the corresponding sulfonated thin-film composite (TFC) nanofiltration membranes. Despite only a slight difference in the chemical structures of these two monomers, the as-prepared membranes exhibited entirely different separation behaviors during the water desalination process. In brief, the molecular architectures significantly affected the solubility and the diffusion of the small molecules into the organic phase during the IP process. By a dissipative particle dynamics (DPD) simulation approach, it was found that the molecular orientations of the active layer of the two membranes were in opposite directions. Specifically, the sulfonic acid groups in the TFC-ODADS membrane surface were mostly facing outward, whereas they were enclosed by the hydrophobic polyamide backbone in the case of the TFC-BDSA membrane. Overall, the physico-chemical properties of the new TFC membrane surface could be well adjusted and controlled based on the molecular design, which paves the way for designing improved NF membrane materials in the future. |
Introduction
Over the past decades, membrane technologies for water purification and desalination have attracted much attention due to the increasing global shortage of clean drinking water.1–6 Due to their lower operation pressure than the conventional reserve osmosis (RO) process as well as their superior ion rejection ability than the common ultrafiltration (UF) procedure, nanofiltration (NF) membranes have been widely studied and used for various purposes, such as municipal water purification or industrial wastewater treatment.7–9The state-of-the-art NF membranes are mostly polyamide thin-film composites (TFC) which are prepared from interfacial polymerization (IP) on a suitable substrate.10–13 Since the IP process relies on the different reactants in the two different solution phases to polymerize at the interface, it is anticipated that the choice of the solutes could largely affect the separation performance of the obtained TFC membranes. Thus, several studies have been devoted to developing improved TFC membranes based on a variety of solutes, including both organic10,11 and organic–inorganic composites.14,15 However, due to their entirely different usage of molecules, polymer matrices or inorganic components, the properties of the corresponding TFC membrane also varied significantly, making it difficult to compare their performance.
The most straightforward way to explore the internal structure–performance relationships of a polymeric material is to perform comparison studies of materials with similar chemical architectures, such as homologues,16,17 isomers,18 or even dendrimers of different generations.19–21 For instance, Jiang's group prepared TFC membranes by using diethylenetriamine (DETA), triethylenetetramine (TETA) or tetraethylenepentamine (TEPA), which served as aqueous monomers.16 Their results showed that different polyamide layers prepared with different monomers did not significantly affect the ion rejection abilities, but had a crucial influence on the water permeability behaviors. In general, the smaller the monomer molecule, the lower the water flux, as it results in materials with smaller pore size and denser barrier layer. Mariñas and coworkers modified the active layer of a commercial nanofiltration membrane with aramide dendrimers of three generations (G1, G2, and G3).19 They found that the NF membranes modified with higher generations exhibited much better rejections than the unmodified one, not only towards positive ions but also towards the organic pollutant, Rhodamine WT. In general, while the results of these studies were interesting, they did not entirely clarify some fundamental issues which still need to be understood, such as the variation in physico-chemical properties with different chemical structures of the membranes.
Our group previously developed a new TFC nanofiltration membrane by using a commercially available monomer, benzidine-2,2′-disulfonic acid (BDSA), to react with trimesoyl chloride (TMC).22 Although the TFC-BDSA membrane was obtained with excellent water permeability and ion rejection ability, its surface nature did not perform according to our intended purpose. The functional “–SO3−” groups were enclosed with the rigid polymer chain, which prevented the membrane surface with improved hydrophilicity, and thus resulted in relatively poor antifouling properties.
Clearly, there is a need to solve the dilemma based on the molecular designs which may help to well control the surface properties. Herein, a new monomer, 4,4′-diaminodiphenyl ether-2,2′-disulfonic acid (ODADS), was synthesized and used to prepare the corresponding TFC membrane. For comparison purposes, BDSA was also used under the same conditions. With only a slight difference in their molecular structures (an ether linkage), these two TFC membranes were systematically studied and compared with respect to their morphologies, free volumes, surface chemistry, antifouling properties and separation performance. Moreover, the molecular orientations of the monomer during the IP process were also studied in detail by means of dissipative particle dynamics (DPD) simulation.
Experimental
Materials
4,4′-Diamino-diphenyl ether (ODA), fuming sulfuric acid (20%), triethylamine (TEA), Congo red (MW: 696.68 Da), methylene blue (MW: 319.86 Da) and humic acid (HA) were purchased from Sinopharm Chemical Reagent Co. Ltd. (China). Trimesoyl chloride (TMC, >99%) was purchased from J&K Chemical Reagent Co. Ltd. (Beijing, China). Polysulfone (PSf) ultrafiltration membrane with a molecular weight cut-off of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da was supplied from RisingSun Membrane Technology (Beijing) Co., Ltd. (Beijing, China) and used as a substrate. Sodium sulfate, magnesium sulfate, magnesium chloride hexahydrate, sodium chloride and n-hexane were purchased from Nanjing Chemical Reagent Co. Ltd. (Nanjing, China). All other chemicals were obtained from commercial sources and used without further purification. Deionized water was used throughout this study.
000 Da was supplied from RisingSun Membrane Technology (Beijing) Co., Ltd. (Beijing, China) and used as a substrate. Sodium sulfate, magnesium sulfate, magnesium chloride hexahydrate, sodium chloride and n-hexane were purchased from Nanjing Chemical Reagent Co. Ltd. (Nanjing, China). All other chemicals were obtained from commercial sources and used without further purification. Deionized water was used throughout this study.
Synthesis of 4,4′-diaminodiphenyl ether-2,2′-disulfonic acid (ODADS)
ODADS was synthesized by direct sulfonation of the parent diamine monomer, 4,4′-diamino-diphenyl ether (ODA), using fuming sulfuric acid. The approach was similar to the one reported in the literature.23Procedure of membrane preparation
Conventional interfacial polymerization technology was utilized to prepare the active skin layer of TFC membrane. A PSf support membrane was immersed in DI water for several hours, then removed from the water and fixed on a plastic plate. Initially, the aqueous-phase solution containing a certain amount of ODADS was prepared with pH 10 adjusted by TEA, and the organic-phase solution was prepared with a certain amount of TMC in n-hexane at room temperature, respectively. Then 100 mL of ODADS solution was poured into the frame and allowed to contact the PSf membrane surface for 5 min before removing the excess aqueous solution. Residual droplets of solution were removed by rolling a rubber roller across the PSf membrane surface. Afterwards, the frame and gasket were reassembled, and 100 mL of TMC solution was poured into it. After 60 s, the TMC solution was drained off and the membrane surface was rinsed with n-hexane (100 mL) to wash away residual reagents. The IP process was implemented at room temperature about 25 °C. Finally the membrane was dried in air at ambient conditions for 30 s, immersed in a weakly acidic aqueous solution (pH around 5.0–6.0) for several hours, and saved in DI water before carrying out the follow-up studies.Membrane characterization
Surface morphology and cross-sections were measured by Field Emission Scanning Electron Microscopy (FE-SEM, FEI Quanta 250F, America). The samples for FESEM were coated with a thin layer of gold to make them conductive. Surface roughness was examined quantitatively using an Atomic Force Microscope (AFM, Mutilmode8, German) equipped with a standard SiN cantilever. Dry membrane samples of dimensions 1 cm × 1 cm were installed on a glass plate using double-sided tape. For better accuracy and precision, measurements were executed at different locations and for variable scan areas. Surface roughness was reflected in terms of the average plane roughness (Ra), root-mean-square (RMS) and the relative surface area. Contact angle measurements were examined using a Contact Angle Measurement Device (KRÜSS DSA30, German) with FAMAS Interface Measurement & Analysis System version 3.1.3. The sessile drop method was utilized to measure the contact angle of a 20 μL water droplet placed carefully on the flat membrane surface. A total of 10 measurements at different locations were carried out for each sample.A streaming potential method was used to detect the charging properties of the membrane surface using an electrokinetic analyzer (SurPASS III Anton Paar, Austria) with KCl (1 mmol L−1) solution as electrolyte solution. The pH dependence of the surface zeta potential was probed via adjusting the pH by NaOH and HCl solutions. The surface zeta potential ζ was identified by the Helmholtz–Smoluchowski equation:
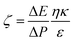 | (1) |
Positron annihilation spectroscopy (PAS)
Experiments on positron annihilation spectroscopy coupled to a variable mono-energy slow positron beam (VMSPB) were carried out in the Institute of High Energy Physics positron research platform.24 The purpose was to probe the variation in the free volume in the multilayer structure of the polyamide TFC membrane as a function of positron incident energy in the range of 0.18–20.18 keV under a vacuum pressure of −10−8 Pa at room temperature. The injection depth of the measuring sample is estimated by the following empirical equation: | (2) |
Doppler broadening energy spectroscopy (DBES) detected γ photons which were generated by positron annihilation through the high-purity germanium detectors. The S parameter is always used to characterize the annihilation nature. The total collected peak energy of the γ spectrum was in the range of 499.5–522.5 keV. The S parameter is the ratio of count in the energy range 510.2–511.8 keV over the count in the total peak (499.5–522.5 keV).
Membrane separation performance tests
The experiments using TFC membranes to treat pure water or salt solution were performed at 6 bar using pure water or 1000 mg L−1 salt or organic solution by self-made cross-flow equipment at room temperature. The effective membrane area is around 12.56 cm2. The TFC membranes were initially subjected to pure water with a pressure of 10 bar for 1 h prior to performing the TFC performance testing experiments. The pure water or salt solution flux, J (L m−2 h−1), of the membrane was determined by direct measurement of the permeate volume, which was calculated by the following equation: | (3) |
The salt solution concentration of permeation was measured by a Conductivity Meter DDS-307 (Shanghai, China). The dye solution concentration of permeation was measured by a UV/VIS Spectrometer (Lambda 25, PerkinElmer). The membrane rejection (R) was calculated by using the following equation:
 | (4) |
To characterize the antifouling properties of membranes, a mixed solution with humic acid (1000 mg L−1) and NaCl (1000 mg L−1) was used. The antifouling performance of the membranes was evaluated by the flux recovery ratio (FRR) and total flux decline ratio (DRt). The specific procedures were described as follows. Firstly, the membranes were compacted at 1.0 MPa for 1 h and the pure water flux Jw1 (L m−2 h−1) was first measured at a transmembrane pressure of 0.6 MPa for 2 h. Then, the foulant aqueous solutions were pressed into the cell filtration system and the solution passed through the membranes at a transmembrane pressure of 0.6 MPa for 6.5 h in order to measure the HA solution flux JP (L m−2 h−1). In third step, the polluted membranes were washed for 2 h with deionized water, and the pure water flux of cleaned membranes Jw2 (L m−2 h−1) for 2.5 h was measured.
The FRR and DRt were used to evaluate the antifouling properties of the membrane, as defined by the following equations:
 | (5) |
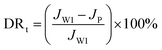 | (6) |
DPD simulations
The details on DPD simulations are similar to our previous report.22 Briefly, the Flory–Huggins parameters (see Tables S1 and S2†) for each bead corresponding to water, n-hexane, and moieties of TMC and ODADS are calculated from the solubility parameter (δ), according to the following equation: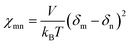 | (7) |
This model was constructed by means of an oil–water two phase interface with a cubic simulation box of 100 Å in length. Two slabs were initially set and filled with water/ODADS and n-hexane/TMC beads, respectively, with an average density of 3.0 for reduced units. The calculations were performed using the Mesocite module in Materials Studio software.
Results & discussion
ODADS was synthesized by direct sulfonation of the diamine precursor, ODA, and fuming sulfuric acid was used as the sulfonating agent. As the amino groups should be fully protonated to form strong electron-withdrawing groups, the sulfonic acid groups are mainly substituted in the meta-position of the phenyl ring with respect to the –NH3+ groups. The chemical structure of ODADS was characterized by its 1H NMR spectrum, as shown in Fig. S1.† The three distinct doublets at 7.00, 6.73 and 6.38 ppm are assigned to the ortho-, meta- and para-aromatic protons next to the sulfonic acid groups, respectively. The broad peak at 4.78 ppm is assigned to the active protons of the amino groups.The reaction process of interfacial polymerization between ODADS and TMC is presented in Scheme 1. The nomenclature used for the series of TFC membranes is: TFC-ODADS-x, where x refers to the concentration of ODADS in the aqueous solution. For comparison purposes, the TFC-BDSA membrane was also prepared and optimized by the IP process according to our previous study.22
 | ||
| Scheme 1 Chemical structures of ODADS and BDSA, and their corresponding interfacial polymerization processes with TMC. | ||
Membrane separation performance
The separation performance of the TFC-ODADS membranes was evaluated, with respect to the water flux and salt rejection ability, using a typical Na2SO4 solution of 1000 ppm. By varying the ODADS and TMC concentrations in the aqueous and organic solutions, respectively, the separation characteristics of the resulting membranes could be systematically optimized. Fig. 1(a) shows the effects of ODADS content on the water flux and rejection properties of the resulting membranes at a fixed TMC concentration of 0.1% (w/v). When the concentration of ODADS was initially kept as 0.3% (w/v), the water flux of the membrane reached up to 33.0 L m−2 h−1 bar−1; however, the Na2SO4 rejection was insufficient at only 66%, indicating a loose polyamide layer formation. With further increase in the ODADS concentration to more than 0.8% (w/v), the water flux started to decrease and reached a constant value of around 8.6 to 8.7 L m−2 h−1 bar−1, while the Na2SO4 rejection increased and reached the highest values of 98.1–98.5%. The effect of TMC concentration on the separation performance of TFC-ODADS is shown in Fig. 1(b), which is quite similar to that of the amine content. The flux gradually decreased and the rejection ability continued to rise with the increase in TMC concentration. The most optimum performance of the TFC-ODADS membrane was finally obtained when the ODADS and TMC contents were 0.8% and 0.1% (w/v), respectively. The optimal membrane displayed flux and rejection as high as 8.7 ± 0.6 L m−2 h−1 bar−1 and 98.1 ± 1.5%, respectively. On the other hand, the water flux and Na2SO4 rejection of the TFC-BDSA membrane (BDSA of 1.0%, TMC of 0.1% (w/v)) are found to be 16.6 L m−2 h−1 bar−1 and 95.4%, respectively, under the same test conditions.22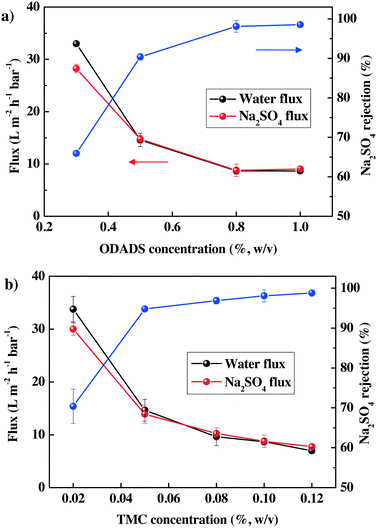 | ||
| Fig. 1 Effects on the separation performance of TFC membranes of (a) ODADS concentration (CTMC in n-hexane was fixed at 0.1% (w/v)); (b) TMC concentration (CODADS in water was fixed at 0.8% (w/v)). | ||
Although there is only a small difference between the chemical structures of the two amine monomers, i.e., an ether linkage, the separation performance of the corresponding membranes was distinctly different from each other. This unusual observation can be explained from a stoichiometric aspect as follows. ODADS and BDSA are expected to have considerably high polarities since they both contain two sulfonic acid groups in a single molecule. Therefore, it would be quite difficult for them to penetrate into the non-polar organic phase from the highly polar aqueous phase during the IP process. In such a case, a high ratio of the “–NH2” to “–COCl” functionality is crucial to form the defect-free polyamide (PA) layer, since the diffusion of these amine solutes might be insufficient compared to some other common monomers, e.g., ethylenediamine (EDA) and PIP.17 Typically, the calculated stoichiometric ratio for ODADS and BDSA to TMC is 5.9 and 7.7, respectively, which are both far larger than the equivalent values, as listed in Table S3.† Additionally, due to the flexible ether linkage, the PA based on ODADS is anticipated to have slightly better solubility in the n-hexane solvent since the polymer chain can undergo free rotation, whereas the fully rigid BDSA causes poorer solubility and earlier precipitation when the two phases are in contact with each other.22,26 Thus, a denser barrier layer with smaller pores is expected to be formed for the TFC-ODADS, which would be responsible for its lower water flux but higher rejections towards ions.
The general separation performance of TFC-ODADS-0.8 (TFC-ODADS, for short in the following discussion) was then evaluated using various inorganic salts and organic dyes, and the results are presented in Fig. 2. The salt rejection ability followed the order of Na2SO4 > NaCl ≥ MgSO4 > MgCl2, which is as expected for a typical negatively charged TFC membrane.27,28 Notably, TFC-ODADS displayed an outstanding rejection of 83.6 ± 4.3% towards NaCl, which is much higher than the 30–50% rejection reported by some previous studies.11,29 In addition, it also showed excellent rejection abilities for two common organic dyes; rejections as high as 99.7% and 97.7% were observed for Congo red (Mw: 696.68 Da) and methylene blue (Mw: 319.86 Da), respectively. The high rejection for organic dyes is attributed to the molecular sieving and charge effects.
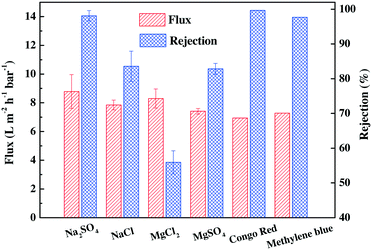 | ||
| Fig. 2 Separation performance of the TFC-ODADS membrane for different salts and dyes. Test conditions: inorganic salt and dye concentration = 1000 mg L−1, 25 °C, 0.6 MPa. | ||
Surface characteristics
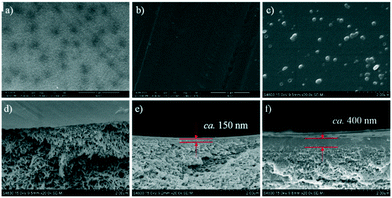 | ||
| Fig. 3 FESEM images of: top surface of (a) PSf membrane; (b) TFC-BDSA membrane; (c) and TFC-ODADS membrane, cross-section of (d) PSf membrane; (e) TFC-BDSA membrane; and (f) TFC-ODADS membrane. | ||
| Membrane | Ra (nm) | RMS (nm) | Water contact angle (degree) |
|---|---|---|---|
| PSf | 1.87 | 2.53 | 72.8 ± 3.2 |
| TFC-BDSA | 2.72 | 3.20 | 86.5 ± 1.9 |
| TFC-ODADS | 6.05 | 13.0 | 56.1 ± 3.8 |
In addition, the thicknesses of the barrier layer for TFC-ODADS and TFC-BDSA membranes were found to be 400 nm and 150 nm, respectively, which is mainly related to the solubility of sulfonated polyamide in the organic phase. As discussed above, the presence of the ether linkages can enhance the flexibility of the polymer chains, thus leading to improvement in solubility. Therefore, the ODADS-based polymer tends to stay homogenously in the organic solution until the final precipitation. However, by then, the thickness of the layer grows to a considerably high degree which inevitably causes the decrease in water permeability.
Contact angle and zeta potential
Fig. 4 shows the water contact angles (WCA) for the PSf substrate, TFC-ODADS and TFC-BDSA membranes between the water and membrane interfaces. The variations in WCA values with respect to different concentrations of diamine monomers were investigated. Our previous studies revealed that the incorporation of sulfonic acid groups did not necessarily result in increase in the surface hydrophilicity;22 TFC-BDSA exhibited relatively hydrophobic nature due to the rigid polymer backbones which enclosed the polar groups. However, this behavior was completely reversed in the case of TFC-ODADS. Its progressively lower WCA values with the increase in ODADS feed concentration indicate the gradually improved surface wettability. Considering the similar chemical compositions of both polyamide systems, this unique phenomenon is probably due to the molecular orientations. In other words, the sulfonic acid groups are likely distributed and exposed on the outer surface of the membrane. | ||
| Fig. 4 Water contact angles for the PSf substrate, TFC-ODADS and TFC-BDSA with different diamine concentrations. | ||
Based on these findings, the zeta potentials of the two membranes were measured to further characterize their surface properties, as shown in Fig. 5. The TFC-ODADS membrane showed lower negative charges than TFC-BDSA over the entire pH range, and the difference became more apparent when the pH was higher than 4.0. Since the “–SO3H” groups present on the surface have been found to greatly enhance the hydrophilicity, the greater number of accumulated sulfonic acid groups in TFC-ODADS are expected to improve the surface negative charges due to the dissociation of hydrogens. Notably, the zeta potential for TFC-ODADS under neutral or weak acidic conditions is about −30.5 to −33.0 mV (pH of 6.0–7.0), which is much lower than that of TFC-BDSA (−15.9 to −16.8 mV, pH of 6.0–7.0). This property is highly responsible for the better rejection abilities of TFC-ODADS towards salts. In particular, as low amounts as 1.9% and 16.4% of Na2SO4 and NaCl penetrated through the membrane, respectively, whereas much higher ion permeability was observed for TFC-BDSA.
Free volume of TFC membranes
The water permeability of TFC membrane is strongly correlated to the free volume properties of the membrane material, especially in polyamide layer. Thus, the PSf, TFC-ODADS and TFC-BDSA membranes were studied by positron annihilation spectroscopy, together with the variable mono-energy slow positron spectroscopy to investigate the changes in the free volume diameters between the two TFC membranes. Fig. 6 shows the S parameters of Doppler broadening energy spectroscopy (DBES) measurements as a function of incident positron energy. It was found that when the incident positron energy was lower than 1 keV, the S values of all three membrane samples increased rapidly, which is a common scattering and diffusion phenomenon after directing an incident positron beam at a material surface. As the positron incident energy increased continuously, the S values of both TFC membranes entered a stage of slow increase, demonstrating that the incident positron beam reached down to the polyamide layers. Finally, the beam reached the porous PSf substrate with the incident positron energy greater than 10 keV, as evident by the constant and close S values. Looking at the curves in the polyamide region at the penetration depth R of 150 nm (ca. 2.79 keV, assuming both materials are of the same density), the TFC-ODADS exhibits significantly lower S values than TFC-BDSA, indicating its lower free volumes. These results clearly confirm our previous analyses regarding the structural influence on the water permeability variation and also support the other experimental observations well. Even though only slight differences exist in the monomer molecules, the polymer architectures may be considerably different due to the different solubilities, penetration behaviors, as well as the chain properties, as mentioned earlier. Therefore, a faster precipitation is likely to occur for TFC-BDSA due to its rigid molecular structure, indicating insufficient polycondensation and resulting in more unreacted sites and larger pores in the polymer matrix. On the other hand, the solubility of TFC-ODADS is much enhanced, resulting in the slower reaction termination, higher molecular weight and a much denser layer formation. | ||
| Fig. 6 S parameter of DBES measurement versus the positron incident energy for the PSf substrate, TFC-ODADS, and TFC-BDSA membranes. | ||
DPD discussion
To explore the relationship between the membrane surface chemistry and separation behavior, the molecular orientation within the membranes was investigated using the DPD method. Consistent with our previous studies, the low repulsion of the phenyl amine and benzoyl chloride groups is the premise of the simulation, indicating the excellent miscibility. To eliminate the system error caused by different concentrations of the diamine monomers, the ODADS concentration in the aqueous phase was set exactly equal to that of BDSA beads. The pair repulsion parameters between each bead were initially calculated using Flory–Huggins parameters from solubility parameters, as listed in Table S2.† The model was then constructed and the initial configuration of TFC-ODADS membrane system was obtained (Fig. 7(a)), in which the beads in both phases were all well dispersed and showed no orderliness.The entire mesoscopic process went through 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps to generate a configuration with lowest energy. Similar to the TFC-BDSA system, the beads in the TFC-ODADS system also moved to the organic–water interface, particularly the beads that were originally located in the water layer, whereas the TMC beads in the organic layer seemed less affected by the repulsions. While the same aggregation phenomena were present in both the simulation models, it can be clearly observed that the molecular orientations were entirely different. The red beads, representative of sulfonic acid groups, all faced up towards the oil phase for the TFC-ODADS, while they all faced downwards in the TFC-BDSA system, as shown in Fig. 7(c) and (f). It is generally accepted that “–SO3H” groups have high polarity and hydrophilicity, and the differences in the solubility parameters were also consistent with these properties. The calculated Δ|δS − δW| value (2.4 (J cm−3)1/2) is much lower than Δ|δS − δH| (30.1 (J cm−3)1/2), suggesting the much better compatibility of “–SO3H” groups with water than with n-hexane. In other words, these polar groups should remain in the water phase, as already observed with TFC-BDSA.22 However, due to the presence of the ether linkage, the polymer backbone of TFC-ODADS becomes more rotatable and flexible, thus allowing the chains to easily turn and become intertwined during the polymerization process. This feature is responsible for the molecular orientation of the formed polymer in the organic solution, which subsequently decides its surface morphology.
000 steps to generate a configuration with lowest energy. Similar to the TFC-BDSA system, the beads in the TFC-ODADS system also moved to the organic–water interface, particularly the beads that were originally located in the water layer, whereas the TMC beads in the organic layer seemed less affected by the repulsions. While the same aggregation phenomena were present in both the simulation models, it can be clearly observed that the molecular orientations were entirely different. The red beads, representative of sulfonic acid groups, all faced up towards the oil phase for the TFC-ODADS, while they all faced downwards in the TFC-BDSA system, as shown in Fig. 7(c) and (f). It is generally accepted that “–SO3H” groups have high polarity and hydrophilicity, and the differences in the solubility parameters were also consistent with these properties. The calculated Δ|δS − δW| value (2.4 (J cm−3)1/2) is much lower than Δ|δS − δH| (30.1 (J cm−3)1/2), suggesting the much better compatibility of “–SO3H” groups with water than with n-hexane. In other words, these polar groups should remain in the water phase, as already observed with TFC-BDSA.22 However, due to the presence of the ether linkage, the polymer backbone of TFC-ODADS becomes more rotatable and flexible, thus allowing the chains to easily turn and become intertwined during the polymerization process. This feature is responsible for the molecular orientation of the formed polymer in the organic solution, which subsequently decides its surface morphology.
In order to determine the exact location of each bead, the concentration gradient profile was analyzed for both systems, as shown in Fig. 8. Unlike the beads of TMC molecules which exhibited slightly higher concentration at the interface, all three beads belonging to ODADS gathered in the middle region. Assuming that the peaks for S, A and E are all in a normal distribution, their most extreme points are found to be around 55, 51 and 48 Å, respectively, suggesting a relatively wide range. By comparing the distances between the peaks, this distribution of the beads could be used to obtain a rough estimate of the thickness of the barrier layer. The effective peaks of TFC-ODADS ranged from 40–70 Å, which is a much wider range than that of the TFC-BDSA peaks (42–62 Å). Although the simulated values are quite different to the actual values obtained from the FESEM results, it could be due to the small dimensional scale of the simulation box. Nevertheless, the DPD method can still provide a reasonable qualitative prediction for the molecular aggregation behavior.
Antifouling properties
Based on the above findings, the antifouling properties of the membranes were investigated since the membrane surface hydrophilicity significantly affects the adhesion ability of the pollutants.30,31 The model fouling agent “HA + NaCl” was chosen to evaluate the antifouling properties of both membranes under the same conditions, as shown in Fig. 9. The normalized flux for both membranes showed significant reduction over the entire fouling process. However, the water flux recovered well for TFC-ODADS after a simple physical rinsing with DI water. In particular, the flux recovery ratio (FRR) was as high as 96.8% for the TFC-ODADS compared to only 81.8% for TFC-BDSA (as listed in Table 2). The superior antifouling properties of TFC-ODADS are mainly attributed to the molecular orientation of its sulfonic acid groups which greatly improved the surface hydrophilicity. With more accumulation of water molecules at the membrane surface, the membrane would be less fouled due to the increase in hydraulic pressure.30,32 | ||
| Fig. 9 Time-dependent normalized fluxes for the TFC-ODADS and TFC-BDSA membranes during filtration of aqueous solution containing 1000 mg L−1 HA + 1000 mg L−1 NaCl, at 25.0 °C. | ||
| Membranes | DRt (%) | FRR (%) |
|---|---|---|
| TFC-ODADS | 38.0 | 96.8 |
| TFC-BDSA | 43.3 | 81.8 |
Considering all of the different properties of the two membranes, it is hard to say which one is better as a general performance membrane. Our opinion is that the end uses of these two membranes would decide their suitability. If the feed is of a complex water quality, e.g., municipal or industrial wastewater treatment, TFC-ODADS might be more suitable due to its better fouling resistance and higher rejection abilities, while the TFC-BDSA would be more appropriate with a high-quality-water inlet, such as the secondary settling tank effluentor drinking water purification.
Conclusions
A novel sulfonated diamine monomer ODADS was synthesized and utilized to fabricate a series of TFC nanofiltration membranes with TMC on the PSf substrate. Due to the introduction of an ether linkage, the ODADS molecule showed better solubility in the organic solvent and thus produced the corresponding polyamide layer with a higher thickness than that prepared from BDSA monomer. Compared to TFC-BDSA, the increase in flexibility of TFC-ODADS membrane led to a decrease in the water permeability, as also confirmed by the free volume detection. The most significant change caused by the polymer structures was the molecular orientation; the sulfonic acid groups in TFC-ODADS membrane surface were mostly located on the outer surface, whereas they were covered by the polyamide backbones in the TFC-BDSA. As a consequence, the surface hydrophilicity and negative charges were greatly enhanced for the former, resulting in much better antifouling properties for TFC-ODADS membrane (96.8% of FRR). In addition, the TFC-ODADS membrane displayed an outstanding rejection of 83.6 ± 4.3% towards NaCl, which suggests its potential use in the further desalination of industrial secondary clarifier effluents. Overall, the physico-chemical properties of the new TFC membrane surface could be well adjusted and controlled based on the molecular design, which paves the way for designing improved NF membrane materials in the future.Acknowledgements
This work was financially supported by NSFC (21406117), the Natural Science Foundation of Jiangsu Province (BK20140782), PAPD and the Fundamental Research Funds for the Central Universities (30915011306).Notes and references
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- G. Geise, H. S. Lee, D. J. Miller, B. D. Freeman, J. E. McGrath and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1685–1718 CrossRef CAS.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- N. Misdan, W. J. Lau and A. F. Ismail, Desalination, 2012, 287, 228–237 CrossRef CAS.
- E. M. V. Hoek and V. V. Tarabara, Encyclopedia of Membrane Science and Technology, John Wiley & Sons, Inc., 2013 Search PubMed.
- L. C. Juang, D. H. Tseng and H. Y. Lin, Desalination, 2007, 202, 302–309 CrossRef CAS.
- A. W. Mohammad, Y. H. Teow, W. L. Ang, Y. T. Chung, D. L. Oatley-Radcliffe and N. Hilal, Desalination, 2015, 369, 226–254 CrossRef.
- B. V. Bruggen, M. Mänttär and M. Nyström, Sep. Purif. Technol., 2008, 63, 251–263 CrossRef.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- J. Jin, D. Liu, D. Zhang, Y. Yin, X. Zhao and Y. Zhang, Desalination, 2015, 355, 141–146 CrossRef CAS.
- X. Li, Y. Cao, H. Yu, G. Kang, X. Jie, Z. Liu and Q. Yuan, J. Membr. Sci., 2014, 466, 82–91 CrossRef CAS.
- C. Wu, S. Liu, Z. Wang, J. Zhang, X. Wang, X. Lu, Y. Jia, W. Hung and K. Lee, J. Membr. Sci., 2016, 517, 64–72 CrossRef CAS.
- D. H. N. Perera, Q. Song, H. Qiblawey and E. Sivaniah, J. Membr. Sci., 2015, 487, 74–82 CrossRef CAS.
- G. S. Lai, W. J. Lau, P. S. Goh, A. F. Ismail, N. Yusof and Y. H. Tan, Desalination, 2016, 387, 14–24 CrossRef CAS.
- X. D. Weng, Y. L. Ji, R. Ma, F. Y. Zhao, Q. F. An and C. J. Gao, J. Membr. Sci., 2016, 510, 122–130 CrossRef CAS.
- Y. F. Li, Y. L. Su, Y. N. Dong, X. T. Zhao, Z. Y. Jiang, R. N. Zhang and J. J. Zhao, Desalination, 2014, 333, 59–65 CrossRef CAS.
- Y. C. Chiang, Y. Z. Hsub, R. C. Ruaan, C. J. Chuang and K. L. Tung, J. Membr. Sci., 2009, 326, 19–26 CrossRef CAS.
- X. Zhang, T. Dong, Y. Pu, T. Higashihara, M. Ueda and L. Wang, J. Phys. Chem. C, 2015, 119, 19596–19606 CAS.
- A. M. S. Jubera, Y. Gao, J. S. Moore, D. G. Cahill and B. J. Mariñas, Environ. Sci. Technol., 2012, 46, 9592–9599 CrossRef PubMed.
- N. Savage and M. S. Diallo, J. Nanopart. Res., 2005, 7, 331–342 CrossRef CAS.
- A. Sarkar, P. I. Carver, T. Zhang, A. Merrington and K. J. Bruzza, J. Membr. Sci., 2010, 349, 421–428 CrossRef CAS.
- J. H. Hu, Z. W. Lv, Y. Z. Xu, X. Zhang and L. J. Wang, J. Membr. Sci., 2016, 505, 119–129 CrossRef CAS.
- J. Fang, X. Guo, S. Harada, T. Watari, K. Tanaka, H. Kita and K. Okamoto, Macromolecules, 2002, 35, 9022–9028 CrossRef CAS.
- Y. Lv, H. C. Yang, H. Q. Liang, L. S. Wan and Z. K. Xu, J. Membr. Sci., 2015, 476, 50–58 CrossRef CAS.
- C. M. Hansen, Hansen Solubility Parameters A User's Handbook, Taylor and Francis, Boca Raton, 2nd edn, 2007 Search PubMed.
- P. W. Morgan and S. L. Kwolek, J. Polym. Sci., Part A: Polym. Chem., 1996, 34, 531–559 CrossRef CAS.
- H. Zhu, A. Szymczyk and B. Balannec, J. Membr. Sci., 2011, 379, 215–223 CrossRef CAS.
- M. B. Wu, Y. Lv, H. C. Yang, L. F. Liu, X. Zhang and Z. K. Xu, J. Membr. Sci., 2016, 515, 238–244 CrossRef CAS.
- T. Ma, Y. Su, Y. Li, R. Zhang, Y. Liu, M. He, Y. Li, N. Dong, H. Wu and Z. Jiang, J. Membr. Sci., 2016, 503, 101–109 CrossRef CAS.
- F. Yan, H. Chen, Y. Lü, Z. Lü, S. Yu, M. Liu and C. Gao, J. Membr. Sci., 2016, 513, 108–116 CrossRef CAS.
- D. Emadzadeh, W. J. Lau, M. Rahbari-Sisakht, A. Daneshfar, M. Ghanbari, A. Mayahi, T. Matsuura and A. F. Ismail, Desalination, 2015, 368, 106–113 CrossRef CAS.
- Y. Li, Y. Su, X. Zhao, R. Zhang, J. Zhao, X. Fan and Z. Jiang, J. Membr. Sci., 2014, 455, 15–23 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6me00078a |
| This journal is © The Royal Society of Chemistry 2017 |



