Synthesis and biological activity evaluation of novel peroxo-bridged derivatives as potential anti-hepatitis B virus agents†
Menglu
Jia‡
a,
Rui
Zhao‡
a,
Bing
Xu
a,
Wenqiang
Yan
a,
Fuhao
Chu
a,
Hongshun
Gu
b,
Tianxin
Xie
a,
Hongjun
Xiang
a,
Jian
Ren
a,
Dagang
Chen
c,
Penglong
Wang
*a and
Haimin
Lei
*a
aSchool of Chinese Pharmacy, Beijing University of Chinese Medicine, Beijing 100102, China. E-mail: hm_lei@126.com; wpl581@126.com
bDepartment of Pharmacology, Xuanwu Hospital of Capital Medical University, Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Beijing 100053, China
cBeijing lam ze biological technology co, LTD, 102500, China
First published on 19th October 2016
Abstract
Previous studies have demonstrated that natural steroid compounds containing a peroxide bridge exhibited potential anti-hepatitis B virus activity. To continue our research, a simple and regioselective methodology, using Eosin Y as a clean photosensitized oxidation catalyst, was developed for the synthesis of a peroxide bridge in steroids. The method that using Eosin Y as the catalyst was exposed to visible light and furbished in high yields, did not involve tedious work-up or purification, and avoided using environmentally hazardous solvents. It can be regarded as a green protocol. Moreover, a series of cholesterol and β-sitosterol derivatives containing a peroxide bridge were synthesized using this method and screened for their anti-HBV activity. Among the compounds synthesized in this research, 5α,8α-cyclicobioxygen-6-vinyl-3-oxo-cholesterone (1f, 3.13 μg ml−1) had the most potent activity with inhibition rates of 77.45% ± 6.01% and 58.73% ± 8.64% on the secretion of HBsAg and HBeAg antigens, respectively, after 8 days. Further acute toxicity test showed that the LD50 value of compound 1f was 362.46 mg kg−1 after an intraperitoneal injection in mice. Moreover, structure–activity relationships of cholesterol and β-sitosterol derivatives were briefly discussed.
Introduction
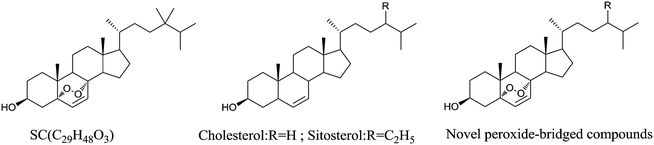
Steroids, a widespread class of natural organic compounds occurring in animals, plants and fungi, play a highly significant role in drug discovery.1–3 These compounds have shown remarkable biological activities, including antiviral, anti-tumor, antihypertensive and anticholinergic.4–8 In previous studies, a new steroid compound (alcohol from ascidian, SC) with a peroxide bridge was discovered, which exhibited potent anti-hepatitis B virus activity.9–11 However, due to the low content of SC in Styela clava,12 it stimulated our interest in synthesizing its structural analogs. Cholesterol and β-sitosterol have the same parent nucleus as SC (Fig. 1). Therefore, they are expected to be anti-HBV drugs by building peroxide bridges.In our present study, a new method was established to synthesize a peroxide bridge using eosin Y as a photosensitive catalytic oxidizer, which has been patented.13 The method was operated at room temperature under visible light, which was simple and environmentally friendly compared to the conventional synthetic methods.14–16 The experimental results from the synthesis indicated that eosin Y could combine with oxygen in air and then oxidize dienes into peroxide bridges so that the dye might be regarded as a specific catalytic to diene ring in the 5,8 position of cholesterol and β-sitosterol. Therefore, we will continue to focus on its application in other steroids and optimize chemistry technology for synthesizing a peroxide bridge.
In our continuous effort, a series of novel cholesterol and β-sitosterol derivatives with peroxide bridges were synthesized using the new method. All the new derivatives were fully characterized by 1H-NMR, 13C-NMR, 2D-NMR spectroscopy (HMQC, HMBC and 1H-1H COSY) and HRMS. Their inhibitory effects on the expression of HBsAg and HBeAg in the HepG2.2.15 cells were evaluated by MTS and ELISA assays. Moreover, the acute toxic test, as a part of the safety evaluation of peroxo-bridged derivatives, was carried out to investigate the potential toxicity by an intraperitoneal injection in ICR mice. In addition, the structure–activity relationships of these novel compounds were also briefly discussed.
Results and discussion
Chemistry
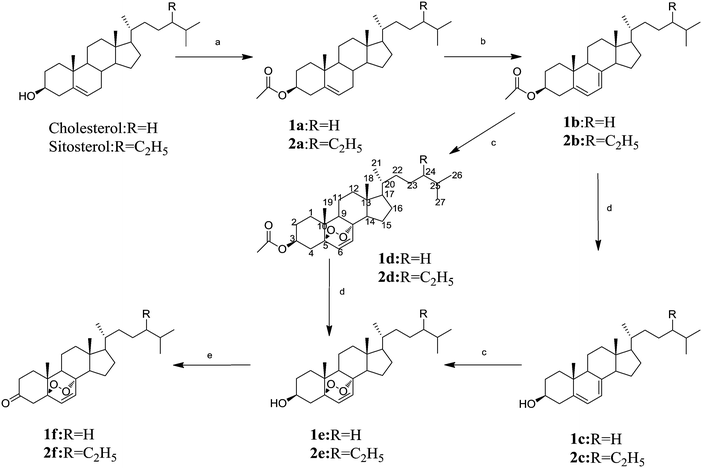
All designed derivatives were synthesized via the routes outlined in Scheme 1. Compounds 1a and 2a were prepared via acetylation reaction of cholesterol and β-sitosterol, which then underwent free radical elimination reaction with N-bromosuccinimide (NBS) to afford compounds 1b and 2b. Subsequently, products 1c and 2c were obtained after deacetylation reaction of 1b and 2b. Then 1b and 2b were further reacted with eosin Y under visible light to generate the target compounds 1d and 2d. Compounds 1e and 2e were obtained via two different routes (1): a–b–c–d and (2): a–b–d–c with different yields. Compounds 1f and 2f were eventually synthesized from 1e and 2e with chromic acid solution in acetone. All new derivatives were fully characterized with 1H-NMR, 13C-NMR, 2D-NMR spectra (HMQC, HMBC and 1H-1H COSY) and HRMS.The molecular formula of 1f was assigned as C27H42O3 on the basis of the positive high-resolution mass spectrometer (HR-MS) m/z 437.30087 [M + Na]+. Product 1f was further identified by HMQC, HMBC and 1H-1H COSY 2D-NMR spectra. Combined with the significant HMQC, HMBC and 1H-1H COSY correlations, CH-6 [δH 6.53 (d, 1H, J = 8.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–)] was connected with δC 83.4 (C-5), and CH-7 [δH 6.27 (d, 1H, J = 8.5 Hz,
CH–)] was connected with δC 83.4 (C-5), and CH-7 [δH 6.27 (d, 1H, J = 8.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–)] was connected with δC 80.0 (C-8), which were similar with 1b. Furthermore, there were δC 83.4 (C-5) and δC 80.0 (C-8) in the 13C-NMR spectrum of compound 1f and δC 141.6 (C-5) and δC 138.6 (C-8) in the 13C-NMR spectrum of 1b. The abovementioned analysis suggests that the peroxide bridge of compound 1f was synthesized. This inference could also be applied to compounds 1d and 1e.
CH–)] was connected with δC 80.0 (C-8), which were similar with 1b. Furthermore, there were δC 83.4 (C-5) and δC 80.0 (C-8) in the 13C-NMR spectrum of compound 1f and δC 141.6 (C-5) and δC 138.6 (C-8) in the 13C-NMR spectrum of 1b. The abovementioned analysis suggests that the peroxide bridge of compound 1f was synthesized. This inference could also be applied to compounds 1d and 1e.
Within the framework of green chemistry, solvents occupy a strategic place. Green chemistry aims to replace hazardous and/or harmful solvents with more environmentally friendly alternatives. In our experiment, eosin Y, a type of dye,17 was used as a photosensitive catalytic oxidizer.18,19 Eosin Y as a photosensitive catalytic oxidizer simplifies the reaction and avoids the use of H2SO4, H2O2 and expensive materials, such as MTO and Re2O7,20,21 which is regarded as a relatively green process. In addition, the possible mechanism involved in the reaction is that eosin Y activated oxygen in air, and then the structure of conjugated dienes was peroxidized to obtain corresponding products. Furthermore, the synthetic structures of the products indicate that the Eosin Y might catalyze diene specifically.
Biological activity
| Compound | 4 d | 8 d | ||
|---|---|---|---|---|
| HBsAg (%) | HBeAg (%) | HBsAg (%) | HBeAg (%) | |
| 1d | 12.96 ± 3.45 | 3.51 ± 2.65 | 13.77 ± 4.23 | 7.56 ± 4.78 |
| 1e | 1.20 ± 4.67 | 1.35 ± 4.87 | 1.56 ± 3.12 | 2.55 ± 5.01 |
| 1f | 72.28 ± 6.00 | 54.10 ± 5.06 | 77.45 ± 6.01 | 58.73 ± 8.64 |
| 2d | 26.67 ± 2.98 | 25.64 ± 3.23 | 28.79 ± 4.34 | 25.78 ± 3.54 |
| 2e | 19.01 ± 2.65 | 2.88 ± 5.45 | 20.04 ± 3.12 | 10.35 ± 3.56 |
| 2f | 1.91 ± 4.65 | 3.28 ± 4.98 | 5.70 ± 4.13 | 2.36 ± 5.37 |
| 3TC | 5.68 ± 3.78 | 4.94 ± 4.99 | −0.44 ± 3.85 | −9.22 ± 6.32 |
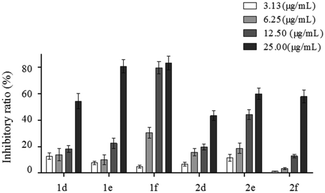
For example, activity of 1f was more remarkable than that of 1d and 1e, which suggested that 3-carbonyl may promote the anti-HBV activity of cholesterol derivatives with a peroxide bridge. However, compound 2d exhibited stronger activity than that of 2e and 2f, which demonstrated that 3-acetyl may play a key role in β-sitosterol derivatives. It thus appeared that diverse sterol matrices had different working groups. This phenomenon could be related to their biogenetic derivation.
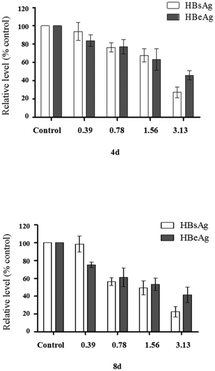
Further study on antigens inhibition of compound 1f was performed by setting four concentration gradients. As shown in Fig. 3, dose- and time-effect relationship was found. After 8 days, 1f showed significant inhibitory effects on HBsAg secretion, with the highest inhibition at 77.45% ± 6.01%. Similarly, the secretion of HBeAg was significantly reduced with the highest inhibition at 58.73% ± 8.64%.
Moreover, we calculated the therapeutic index of 1f to indicate its anti-hepatitis B activity (Table 2).
| Time | Antigen | IC50 | TC50 | TC50/IC50 |
|---|---|---|---|---|
| 4 d | HBeAg | 1.78 ± 0.24 | 5.79 ± 0.52 | |
| HBsAg | 1.86 ± 0.09 | 5.51 ± 0.18 | ||
| 8 d | HBeAg | 2.59 ± 0.15 | 10.25 | 3.40 ± 0.24 |
| HBsAg | 2.95 ± 0.35 | 3.47 ± 0.37 |
| Dose (mg kg−1) | Mice number start/end | Death rate (%) | LD50 (mg kg−1) | 95% CIs |
|---|---|---|---|---|
| 1000 | 6/6 | 100 | 362.46 | 233.83–561.85 |
| 750 | 6/6 | 100 | ||
| 422 | 6/4 | 67 | ||
| 350 | 6/3 | 50 | ||
| 178 | 6/0 | 0 | ||
| 56 | 6/0 | 0 |
The LD50 value (362.46 mg kg−1) can be worked out through the regression equation: y (Probit) = −17.114 + 8.6408 log(D), which showed the safe dose range of 1f.
(Statement: all experiments were performed in compliance with the relevant laws and institutional guidelines, and the institutional committee(s) that have approved the experiments).
Conclusions
A new method to synthesize a peroxo bridge was established using eosin Y as a photosensitive catalytic oxidizer. Compared with the conventional synthetic methods, the new method was in lower consumption and more convenient. Therefore, our synthesis of peroxide bridges was optimized by the special catalyzer. A series of novel steroid derivatives containing peroxide bridge were synthesized using this method, and their bioactivity tests showed that most derivatives had anti-HBV activities. In particular, compound 1f exhibited high inhibitory capacity against HBsAg and HBeAg secretion in HepG2.2.15 cells, whereas nearly no cytotoxicity on HepG2.2.15 cells at 3.13 μg mL−1 was observed, showing good therapeutic index.The structures were significantly different between steroid derivatives and 3TC. Although lamivudine was no longer recommended as first-line therapy for chronic hepatitis B, using lamivudine judiciously on the clinical and scientific research was paramount.22 Unlike the metabolism antagonism of 3TC, steroids might indicate yet another mechanism that is related with the capacity of steroid hormones to intercalate into cell membrane bilayer and alter membrane fluidity.23 This property of the steroids would affect the insertion of viral glycoproteins further inhibiting the assembly of virus particles,4 which requires further study. Therefore, these compounds were expected in collaboration with 3TC for the treatment of hepatitis B diseases. In summary, the steroid derivatives with peroxide bridges demonstrated potential anti-HBV activity, and it provided us new ideas to discover anti-HBV leading compounds.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81173519) and the Innovation Team Project Foundation of Beijing University of Chinese Medicine (Lead Compound Discovering and Developing Innovation Team Project Foundation).Notes and references
- D. W. Gilroy, T. Lawrence, M. Perretti and A. G. Rossi, Nat. Rev. Drug Discovery, 2004, 3, 401–416 CrossRef CAS PubMed.
- H. Li, X. Zhao, J. Wang, Y. Dong, S. Meng, R. Li, X. Niu and X. Deng, Sci. Rep., 2015, 5, 17668, DOI:10.1038/srep17668.
- J. P. Munafo Jr and T. J. Gianfaqan, Nat. Prod. Rep., 2015, 32, 454–477 RSC.
- V. Castilla, J. Ramirez and E. C. Coto, Curr. Med. Chem., 2010, 17, 1858–1873 CrossRef CAS PubMed.
- J. A. Salvador, J. F. Carvalho, M. A. Neves, S. M. Silvestre, A. J. Leitão, M. M. C. Silva and M. L. S. Melo, Nat. Prod. Rep., 2013, 30, 324–374 RSC.
- H. Li, Y. Jiang and P. Li, Nat. Prod. Rep., 2006, 23, 735–752 RSC.
- A. Ali, M. Asif, H. Khanam, A. Mashrai, M. A. Sherwani, M. Owais and Shamsuzzaman, RSC Adv., 2015, 5, 75964–75984 RSC.
- Z. Xu, K. A. Harvey, T. Pavlina, G. Dulot, M. Hise, G. P. Zaloga and R. A. Siddiqui, Nutrients, 2012, 4, 904–921 CrossRef CAS PubMed.
- C. Cai, H. Lei, Q. Li and T. Ren, Chin. J. Pharm. Anal., 2006, 26, 1211–1213 CAS.
- X. Ma, S. Chi and B. Li, Zhongguo Haiyang Yaowu, 2006, 25, 53 Search PubMed.
- J. Hu and X. Wang, Zhongguo Yiyuan Yaoxue Zazhi, 2004, 4, 4 Search PubMed.
- C. Cai, H. Lei and T. Ren, Zhongguo Haiyang Yaowu, 2003, 22, 22–23 CAS.
- H. Lei and D. Chen, CN Pat., 101619089, 2010 Search PubMed.
- I. A. Yaremenko, A. O. Terent'ev, V. A. Vil, R. A. Novikov, V. V. Chernyshev, V. A. Tafeenko, D. O. Levitsky, F. Fleury and G. I. Nikishin, Chem. – Eur. J., 2014, 20, 10160–10169 CrossRef CAS PubMed.
- S. Malik, S. A. Khan, P. Ahuja, S. K. Arya, S. Sahu and K. Sahu, Med. Chem. Res., 2013, 22, 5633–5653 CrossRef CAS.
- S. Hatano and J. Abe, Phys. Chem. Chem. Phys., 2012, 14, 5855–5860 RSC.
- E. Slyusareva, M. Gerasimova, A. Plotnikov and A. Sizykh, J. Colloid Interface Sci., 2014, 417, 80–87 CrossRef CAS PubMed.
- W. Zhang, Y. Li, S. Peng and X. Cai, Beilstein J. Nanotechnol., 2014, 5, 801–811 CrossRef PubMed.
- D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688–6699 RSC.
- N. Kumar, S. I. Khan, H. Atheaya, R. Mamgain and D. S. Rawat, Eur. J. Med. Chem., 2011, 46, 2816–2827 CrossRef CAS PubMed.
- P. Ghorai and P. H. Dussault, Org. Lett., 2008, 11, 213–216 CrossRef PubMed.
- V. Soriano and B. McMahon, Antiviral Res., 2013, 100, 435–438 CrossRef CAS PubMed.
- K. P. Whiting, C. J. Restall and P. F. Brain, Life Sci., 2000, 67, 743–757 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00344c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |