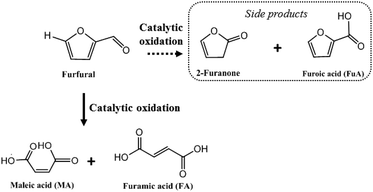
Synthesis of maleic and fumaric acids from furfural in the presence of betaine hydrochloride and hydrogen peroxide†
N.
Araji
a,
D. D.
Madjinza
a,
G.
Chatel
 *a,
A.
Moores
*a,
A.
Moores
 b,
F.
Jérôme
a and
K.
De Oliveira Vigier
b,
F.
Jérôme
a and
K.
De Oliveira Vigier
 *a
*a
aIC2MP UMR CNRS 7285, Université de Poitiers, ENSIP, B1, 1 rue Marcel Doré TSA 41105, 86073 POITIERS Cedex 9, France. E-mail: karine.vigier@univ-poitiers.fr; gregory.chatel@univ-poitiers.fr
bCentre for Green Chemistry and Catalysis, Department of Chemistry, McGill University, 801 Sherbrooke St. W., Montreal, Canada
First published on 1st November 2016
Abstract
Here we report the successful valorisation of furfural into maleic acid (MA) and fumaric acid (FA) with a total yield above 90% using an aqueous solution of betaine hydrochloride (BHC) in the presence of hydrogen peroxide. BHC can be recycled for at least 4 cycles and it can be used to directly convert xylose to MA and FA.
A lot of studies are nowadays devoted to the catalytic conversion of biomass to bio-based chemical platforms, which can be further converted to a wide range of valuable products.1 Among these renewable chemical platforms, furfural is one of the most important building blocks that can be obtained from hemicellulose.2 From this building block, catalytic oxidation affords a number of chemical intermediates and end products.3 Most of the current studies focus on the selective oxidation of furfural to diacids or anhydride acids (succinic acid, fumaric acid (FA), maleic acid (MA) and maleic anhydride). Owing to the fact that MA and FA are used as important intermediates in the chemical industry, their production is constantly increasing; the annual production of MA and FA being 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton per year and 90
000 ton per year and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton per year, respectively. These two C4 dicarboxylic acids are involved in the synthesis of copolymers, surface coatings, lubricants, food and beverage additives, plasticizers, resins (paper, alkyd and unsaturated polyester resins), and agricultural chemicals.4
000 ton per year, respectively. These two C4 dicarboxylic acids are involved in the synthesis of copolymers, surface coatings, lubricants, food and beverage additives, plasticizers, resins (paper, alkyd and unsaturated polyester resins), and agricultural chemicals.4
Industrially, MA is produced via aerobic oxidation of benzene, butane or butadiene.5 FA is essentially manufactured by chemical conversion of maleic anhydride or biological conversion by fungi.6 The production of MA was previously investigated under aerobic conditions or in the presence of hydrogen peroxide in a liquid phase. The exact mechanism of the reaction (role and nature of catalytic sites) is still under discussion.7 MA can be synthesized from furfural via different pathways: (1) abstraction of hydrogen to produce a furfural radical intermediate affording 4-hydroxyfurfural; (2) decarbonylation of furfural to furan, leading to 2-furanone as an intermediate; or by (3) a Baeyer–Villiger oxidation of furfural to furan-2-ol and then to MA through the furan-2(5H)-one intermediate. In all the proposed mechanisms, bifunctional catalysts are required to obtain MA and FA from furfural.
The catalytic aerobic oxidation of furfural was studied by Yin et al. in the presence of a phosphomolybdic acid catalyst (under 20 atm of O2, at 383 K and during 14 h).8 In this study, 69% of selectivity to MA was reached while the conversion of furfural was 50% under the optimized conditions (i.e. 34% yield). In a biphasic system, the oxidation reaction occurred in the aqueous phase while the organic phase constituted a reservoir to gradually release the substrate through the phase equilibrium. The same group reported the combination of phosphomolybdic acid with copper nitrate to selectively oxidize furfural into maleic acid with 51% selectivity and 95% conversion of furfural.9 However, the recycling of this catalytic system was not reported. Hydrogen peroxide has also been used as an oxidant for the oxidation of furfural in the presence of Cr(VI) and Mo(VI)-based catalysts,10 or acid catalysts such as Amberlyst-15, Nafion NR50, Nafion SAC13, hydrochloric acid, acetic acid, sulfuric acid or p-toluenesulfonic acid.11
In a previous study carried out in our group, reaction media containing water and betaine hydrochloride (BHC), a co-product of the sugar beet industry, were used to produce (i) furfural from hemicellulose and (ii) 5-hydroxymethylfurfural (HMF) from fructose and inulin.12 High yields of furfural and HMF were obtained and BHC was recyclable. Based on these results it was interesting to investigate the oxidation of furfural to MA and FA in the presence of an aqueous solution of BHC and hydrogen peroxide.
Here we report that in a BHC/H2O2 system, MA and FA can be obtained with a total yield of 92% (Scheme 1). BHC could be recycled 6 times, and used to directly convert xylose to MA and FA with a total yield of 21% from xylose (i.e. 35% of MA and 8% of FA from furfural). These results are highly appealing because (i) BHC contrary to homogeneous mineral acid was recyclable, (ii) BHC is readily available from biomass, and the process no longer relies on the use of metals; (iii) access to MA and FA directly from sugar in the form of xylose allows one to skip the isolation of furfural. These promising results are very encouraging for the valorization of furfural into valuable acids.13
In a first set of experiments, 0.5 g of furfural was added to 5 g of water containing 40 wt% of BHC (2 g of BHC, pH = 1), in the presence of 10 eq. of H2O2 (35 wt% aqueous solution). The reaction was carried out for 2 h at 90 °C. Yields and conversions were determined by HPLC using the commercial standard. Interestingly, the conversion of furfural was complete, and 44% of MA and 10% of FA were produced after only 30 min of reaction (Table 1, entry 7) along with 10% of FA and 5% of 2-furanone. These results demonstrated that an aqueous solution of BHC and H2O2 is capable of producing C4-dicarboxylic acids from furfural. A kinetic study was then performed (Fig. 1).
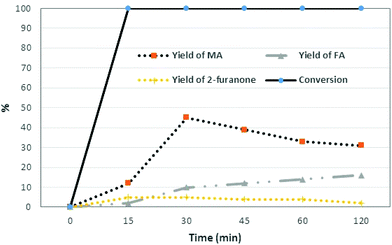 | ||
| Fig. 1 Oxidation of furfural (0.5 g) to MA and FA in the presence of 2.5 eq. of BHC in 5 g of water and 10 eq. of H2O2 at 90 °C (2-one: 2-furanone). | ||
| Entry | H2O2 (eq.) | BHC (wt%) | T (°C) | Time (min) | Conv. (%) | Yield (%) | ||
|---|---|---|---|---|---|---|---|---|
| MA | FA | 2-Furanone | ||||||
| 1 | 10 | 10 | 90 | 30 | 82 | 12 | — | 8 |
| 2 | 60 | 100 | 30 | 5 | 7 | |||
| 3 | 120 | 100 | 29 | 6 | 5 | |||
| 4 | 20 | 30 | 99 | 26 | 3 | 13 | ||
| 5 | 60 | 100 | 27 | 8 | 5 | |||
| 6 | 120 | 100 | 36 | 5 | 4 | |||
| 7 | 40 | 30 | 100 | 44 | 10 | 5 | ||
| 8 | 50 | 30 | 100 | 44 | 4 | 3 | ||
| 9 | 10 | 40 | 50 | 240 | 100 | 49 | 2 | 1 |
| 10 | 10 | 100 | 30 | 100 | 61 | 31 | 3 | |
| 11 | 5 | 100 | 60 | 100 | 36 | 30 | 4 | |
| 12 | 2 | 100 | 60 | 100 | 9 | 20 | 3 | |
| 13 | 2 | 100 | 120 | 100 | 10 | 48 | 2 | |
Under these conditions, the furfural conversion was complete and MA was produced with 12% yield after 15 min of reaction. One can note that after 30 min of reaction, the isomerization of the cis isomer (MA) into the trans isomer (FA) occurred (Fig. 1), since it is known that under acidic conditions and with heating, the reversible addition of proton leads to free rotation about the central C–C bond and formation of more stable and less soluble fumaric acid.14
The effect of the BHC content (Table 1, entries 1–8) was then studied at 90 °C in the presence of 10 eq. of aqueous H2O2. In the presence of an aqueous solution of 10 wt% of BHC, 12% of MA was observed after 30 min of reaction (Table 1, entry 1). Upon a prolonged reaction time (up to 120 min) the yield of MA reached 29% along with a FA yield of 6% (Table 1, entries 2 and 3). When the amount of BHC was increased up to 20 wt%, similar yields were observed (Table 1, entries 4–6). However, a further increase of the BHC content to 40 wt% afforded 56% of MA and 14% of FA (Table 1, entry 7). If the BHC amount was higher than 40 wt%, a decrease in the MA and FA yields was observed due to dominant side decomposition of furfural (Table 1, entry 8).15 It can be concluded that an aqueous BHC content of 40 wt% is optimal to produce FA and MA from furfural with the highest yields. Moreover, “2-furanone was detected in all the experiments suggesting that the reaction mechanism could involve the in situ production of furan as described previously.16 In order to confirm this hypothesis, 2-furanone was used as a substrate instead of furfural and after 30 min under optimized conditions, only 6% of MA and 1% of FA were obtained. A prolonged reaction time up to 17 h led to an increase of the conversion to 50% but only 3% of MA and 7% of FA were observed. These results suggest that 2-furanone is not the main intermediate in the production of MA and FA from furfural using BHC and H2O2 aqueous solution”.
The effect of the reaction temperature was also studied (Table 1, entries 9 and 10). It was shown that a decrease of the reaction temperature from 90 to 50 °C led to a similar yield of MA (49% vs. 44%) while the yield of FA remained rather low (2% at 50 °C). The reaction time was higher (240 min) at 50 °C than at 90 °C (30 min) to reach a similar yield of MA. However, the decrease of the reaction temperature inhibited the formation of FA since the FA yield decreased from 14 to 2% when going from 90 to 50 °C; the total yield (MA + FA) being smaller at 50 °C than at 90 °C. An increase of the temperature increases the selectivity to C4 acids. This claim was further supported by the increase of the reaction temperature up to 100 °C which afforded 61% of MA along with 31% of FA. It can be pointed out that at 100 °C a total yield of FA and MA over 90% was observed. If the amount of H2O2 is decreased from 10 to 5 eq., the yield of MA decreased from 61 to 36% while the yield of FA was similar, around 30%. A further decrease of the amount of H2O2 from 5 to 2 eq. led to a decrease of the MA yield to 10% while the FA yield increased up to 48%, the main product formed being FA. These results show that H2O2 plays an important role in the isomerization reaction as well as in the selectivity of the reaction. The decrease of the H2O2 content led to a decrease in the selectivity of the reaction to MA and FA with an increase of the FA yield. In order to selectively produce fumaric acid, a low amount of hydrogen peroxide and high reaction temperature are required.
To the best of our knowledge, this is the first time that such yields of MA and FA are reported in the literature.
BHC is a strong organic acid, providing pH values around 1 under the studied conditions. In order to understand the role of BHC in the reaction, we compared its activity with two comparable inorganic acids, HCl and H2SO4. All the reactions were carried out under reflux at 90 °C in the presence of 10 eq. of aqueous H2O2 for 30 min.
In a reaction test in the absence of BHC, the pH was adjusted to 1 by addition of HCl or H2SO4 (Table 2, entries 2 and 3) before the reaction was performed. This reaction provided lower yields of MA and FA compared to the ones observed in the presence of BHC. These results suggest that the main role of BHC is to act as a strong acid.
| Entry | Acid | Yield (%) | |
|---|---|---|---|
| MA | FA | ||
| 1 | BHC (pH ∼ 1) | 44 | 10 |
| 2 | HCl (pH ∼ 1) | 35 | 2 |
| 3 | H2SO4 (pH ∼ 1) | 30 | 7 |
The recycling of BHC was explored, to show the benefit of using BHC instead of HCl (Fig. 2). The recycling of BHC was evaluated under optimized conditions (100 °C under reflux, 10 eq. of H2O2, 2 g of BHC in 5 mL of water (40 wt% of BHC in water)).
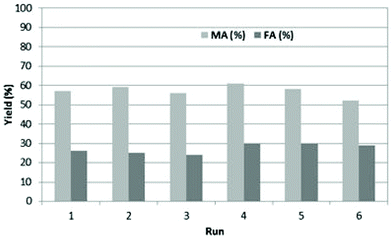 | ||
| Fig. 2 Recyclability of BHC in the oxidation of furfural (0.5 g) to MA and FA in the presence of 2.5 eq. of BHC in 5 g of water and 10 eq. of H2O2 at 100 °C. | ||
After 30 min of reaction, BHC was recovered by precipitation upon addition of acetone with high purity in the reaction media. BHC was separated by filtration and reused as collected. We were pleased to see that up to 6 cycles can be performed without significant loss in MA and FA yields. After the 6th recycling, BHC was recovered and analyzed by 1H NMR and the pH of the aqueous solution of BHC was measured. Similar NMR spectra were obtained between fresh and used BHC (Fig. S1†) and the pH was only 1. Moreover, titration of chlorine in BHC was also performed and no change in the chlorine content was observed between commercial BHC and recovered BHC. These results show that BHC was not degraded during the reaction.
As we have established before that BHC was efficient towards the conversion of xylose to furfural, we explored the direct transformation of xylose into MA and FA.12 To this end the dehydration of xylose to furfural was carried out in the presence of 0.1 g of xylose and 1 g of BHC and 5 mL of water at 180 °C according to our previous results. A furfural yield of 48% was obtained after 60 min of reaction. The reaction media was cooled down to room temperature and 5.8 g of H2O2 was added as well as 1 g of BHC. The reaction temperature was increased up to 90 °C and 17% of MA along with 4% of FA (yield in accordance with the xylose content) were observed after 30 min of reaction (Scheme 2).
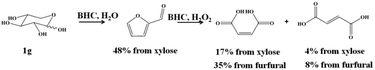 | ||
| Scheme 2 Synthesis of MA and FA from xylose in the presence of BHC and hydrogen peroxide in a one-pot two-step process. | ||
It can be pointed out that if the yield of MA was calculated from furfural produced from xylose, 35% of MA was obtained, showing a similar yield to those observed from commercial furfural, the BHC content in water being 40 wt%.
Conclusions
Here, we have demonstrated that BHC can be used to selectively convert furfural to maleic and fumaric acids, with high yields (above 90%). BHC yields similar results to HCl, but its main advantage relies on its recyclability. Particularly, BHC was recyclable up to 6th cycle without significant loss in MA and FA yields. The analysis of BHC after the recycling study has shown that BHC was not decomposed during the reaction proving the significance of this media for this reaction. Moreover, BHC is capable of producing MA and FA directly from xylose in a one-pot two-step process, thus streamlining the production of important industrial intermediates directly from sugars.Acknowledgements
The authors would like to thank the French Ministry of National Education, Higher Education and Research as well as the France-Canada Research Fund for the funding of the PhD grant of NA. The International Consortium on Eco-conception and Renewable Resources (FR CNRS INCREASE 3707) is also acknowledged for its financial support for this study.References
- (a) L. Hu, G. Zhao, W. Hao, X. Tang, Y. Sun, L. Lin and S. Liu, RSC Adv., 2012, 2, 11184 RSC; (b) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC; (c) S. Dutta, S. De, B. Saha and M. I. Alam, Catal. Sci. Technol., 2012, 2, 2025 RSC; (d) P. Gallezot, Catal. Today, 2007, 121, 76 CrossRef CAS; (e) I. Agirrezabal-Telleria, I. Gandarias and P. L. Arias, Catal. Today, 2014, 234, 42 CrossRef CAS.
- (a) J. P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150 CrossRef CAS PubMed; (b) A. S. Mamman, J. M. Lee, Y. C. Kim, I. T. Hwang, N. J. Park, Y. K. Hwang, J. S. Chang and J. S. Hwang, Biofuels, Bioprod. Biorefin., 2008, 2, 438 CrossRef CAS; (c) K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663 CrossRef CAS.
- (a) R. Cao, C. Liu and L. Liu, Org. Prep. Proced. Int., 1996, 28, 215 CrossRef CAS; (b) M. Krystof, M. Perez-Sanchez and P. Dominguez de Maria, ChemSusChem, 2013, 6, 826 CrossRef CAS PubMed; (c) J. Lan, Z. Chen, J. Lin and G. Yin, Green Chem., 2014, 16, 4351 RSC; (d) N. Alonso-Fagúndez, I. Agirrezabal-Telleria, P. L. Arias, J. L. G. Fierro, R. Mariscal and M. L. Granados, RSC Adv., 2014, 4, 54960 RSC; (e) N. Alonso-Fagundez, M. L. Granados, R. Mariscal and M. Ojeda, ChemSusChem, 2012, 5, 1984 CrossRef CAS PubMed; (f) H. Choudhary, S. Nishimura and K. Ebitani, Appl. Catal., A, 2013, 458, 55 CrossRef CAS; (g) F. Cherubini, Energy Convers. Manage., 2010, 51, 1412 CrossRef CAS; (h) R. A. Sheldon, Green Chem., 2014, 16, 950 RSC.
- C. A. Roa Engel, A. J. J. Straathof, T. W. Zijlmans, W. M. van Gulik and L. A. M. van der Wielen, Appl. Microbiol. Biotechnol., 2008, 78, 379–388 CrossRef CAS PubMed.
- K. Lohbeck, H. Haferkorn, W. Fuhrmann and N. Fedtke, Maleic and Fumaric Acids, in Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- Z. Zhou, G. Du, Z. Hua, J. Zhou and J. Chen, Bioresour. Technol., 2011, 102, 9345–9349 CrossRef CAS PubMed.
- R. Wojcieszak, F. Santarelli, S. paul, F. Dumeignil, F. Cavani and R. V. Gonçalves, Sustainable Chem. Processess, 2015, 3, 9 CrossRef.
- H. Guo and G. Yin, J. Phys. Chem. C, 2011, 115, 17516 CAS.
- S. Shi, H. Guo and G. Yin, Catal. Commun., 2011, 12, 731 CrossRef CAS.
- (a) L. A. Badovskaya, V. M. Latashko, V. V. Poskonin, E. P. Grunskaya, Z. I. Tyukhteneva, S. G. Rudakova, S. A. Pestunova and A. V. Sarkiisyan, Chem. Heterocycl. Compd., 2002, 38, 1040–1048 CrossRef CAS; (b) E. P. Grunskala, L. A. Badovskaya, V. V. Poskonin and Y. F. Yakuba, Chem. Heterocycl. Compd., 1998, 34, 775–780 CrossRef.
- H. Choudhary, S. Nishimura and K. Ebitani, Chem. Lett., 2012, 41, 409–411 CrossRef CAS.
- (a) F. Liu, F. Boissou, A. Vignault, L. Lemée, S. Marinkovic, B. Estrine, K. De Oliveira Vigier and F. Jérôme, RSC Adv., 2014, 4, 8836–28841 Search PubMed; (b) K. De Oliveira Vigier, A. Benguerba, J. Barrault and F. Jérôme, Green Chem., 2012, 14, 285 RSC.
- M. D. Sutton and J. B. D. Peterson, J. Sugar Beet Res., 2001, 38, 19–34 CrossRef.
- K. Nozaki and R. Ogg Jr., J. Am. Chem. Soc., 1941, 63, 2583 CrossRef CAS.
- D. Montané, J. Salvadó, C. Torras and X. Farriol, Biomass Bioenergy, 2002, 22, 295 CrossRef.
- J. Lan, Z. i. Chen, J. Lin and G. Yin, Green Chem., 2014, 16, 4351 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General procedure for the syntheses of MA and FA and BHC characterization after recycling. See DOI: 10.1039/c6gc02620f |
| This journal is © The Royal Society of Chemistry 2017 |