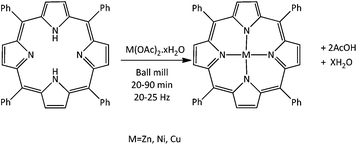
Solventless mechanochemical metallation of porphyrins†
Kathryn
Ralphs
,
Chen
Zhang
and
Stuart L.
James
School of Chemistry and Chemical Engineering, Queens University Belfast, David Keir building, Stranmillis Road, Belfast, BT9 5AG, N. Ireland, UK. E-mail: s.james@qub.ac.uk; k.ralphs@qub.ac.uk
First published on 22nd November 2016
Abstract
A fast solvent-free method for the metallation of porphyrins is presented. ZnTPP, NiTPP, CuTPP and FeTPP have been prepared mechanochemically by ball milling hydrated metal acetate salts with meso-tetraphenylporphyrin (H2TPP) in a shaker mill for as little as 20 min with no added solvent, avoiding the need for undesirable solvents and long reaction times normally used in such reactions.
Mechanochemical synthesis is the induction of chemical reactions through the input of mechanical energy, often with little or no added solvent.1–3 Current environmental concerns over the use of solvents make it very timely to investigate the full scope of this technique, especially in cases where solvents are particularly problematic. As well as being potentially more sustainable than solvent-based synthesis, other advantages of mechanochemical synthesis can include lower reaction temperatures, increased reaction rates, reduced cost and operational simplicity.1–3 The potential applications for mechanochemical synthesis are wide and include organic synthesis,2 inorganic materials,3 pharmaceuticals,4 catalysis,5–7 co-crystals,8 discrete metal complexes,9 macrocycles10 and extended metal organic frameworks (MOFs).11,12 Recent work has shown that screw extrusion techniques can be used to scale-up mechanochemical synthesis.12–15
To date, however, despite their general importance, the synthesis of macrocyclic metal complexes has not been explored mechanochemically to our knowledge. Porphyrins and metallo-porphyrins are amongst the most important macrocyclic complexes and are widely encountered in nature e.g. they form the active sites of proteins such as haemoglobin, myoglobin and cytochromes as well as pigments such as chlorophyll. Applications of metallo-porphyrins include catalysis,16,17 fuel cells,18 medicine19,20 and biomimetic chemistry.21,22
Methods for the metallation of porphyrins typically involve undersirable and highly toxic solvents such as DMF (dimethylformamide), benzene, methanol and chloroform23 and heating to reflux for up to and 5 days.24,25 The need to resort to these solvents and methods may reflect the generally low solubilities of porphyrins and slow kinetics of metallation. In addition to its high toxicity, DMF can decompose on heating to dimethylamine and can cause complicated side reactions. Herein, we describe a simple, direct and potentially more sustainable solvent-free mechanochemical route for the preparation of metallo-porphyrins by ball milling of solid reactants. Analytically pure products are obtained with no work-up other than heating to remove volatile by-products (water and acetic acid).
meso-Tetraphenylporphyrin (H2TPP) and a range of metal acetate monohydrate salts were milled together in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios without solvent for 20–90 min at 25–30 Hz (Fig. 1 and Table 1) using a small commercial shaker mill (Retsch MM400). Following milling, the products were heated in an oven for 2 h at 100 °C to remove any remaining acetic acid and water formed as by-products (Fig. 1) During the milling clear colour changes from dark purple to magenta were observed. To establish the extent of reaction induced by the milling alone, analysis of the products was carried out prior to heating.
1 molar ratios without solvent for 20–90 min at 25–30 Hz (Fig. 1 and Table 1) using a small commercial shaker mill (Retsch MM400). Following milling, the products were heated in an oven for 2 h at 100 °C to remove any remaining acetic acid and water formed as by-products (Fig. 1) During the milling clear colour changes from dark purple to magenta were observed. To establish the extent of reaction induced by the milling alone, analysis of the products was carried out prior to heating.
| Complex | Precursor | Small scale (25.2 mg) | Large scale (151.2 mg) | ||
|---|---|---|---|---|---|
| Time (min) | Speed (Hz) | Time (min) | Speed (Hz) | ||
| ZnTPP | Zn(OAc)2·2H2O | 20 | 20 | 20 | 20 |
| NiTPP | Ni(OAc)2·4H2O | 40 | 25 | 90 | 30 |
| CuTPP | Cu(OAc)2.H2O | 20 | 25 | 20 | 25 |
| FeTPP | Fe(OAc)2 | 180 | 30 | — | — |
The products were characterized by IR, 1H-NMR and UV-visible spectroscopies (NMR spectra could not be obtained for CuTPP and FeTPP due to their paramagnetism). ZnTPP, CuTPP and NiTPP were each successfully prepared on two different scales (∼25 mg and ∼150 mg). In all cases the analytical data were consistent with quantitative reactions to give analytically pure products at both scales. FeTPP was only prepared at the smaller scale and required considerably longer milling. Example spectra are provided here for ZnTPP. Spectra for other products are provided in the ESI.†
IR spectra (see ESI†) of the products following milling confirmed that de-protonation of the N atoms in the ring had occurred through the absence of the N–H stretch in H2TPP at ∼3300 cm−1. Co-ordination of the metal was also supported by the shift of the porphyrin ring vibration from ∼980 cm−1 to ∼1002–1007 cm−1. The fingerprint regions for the metallo-porphyrins prepared matched the data reported in the literature.26
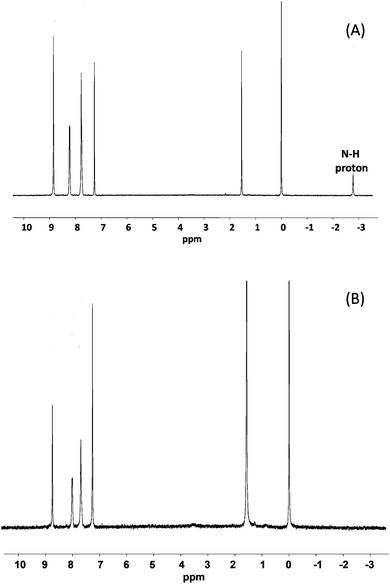
1H-NMR spectra taken after milling showed only peaks corresponding to the target metallo-porphyrins and matched literature data.25Fig. 2 shows spectra for the solid state ZnTPP synthesis and shows that clean quantitative conversion occurred (note the absence of the N–H signal at −2.73 ppm in the product).
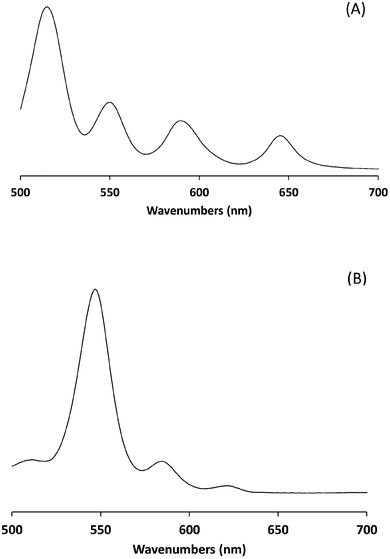
UV-visible analysis was carried out in dichloromethane solution. Metallation of H2TPP is known to simplify its UV-visible absorption spectrum, mainly due to the increased symmetry of the product.26–29 The Q band regions of spectra for H2TPP and ZnTPP are given in Fig. 3. The Soret band remains unaffected (as expected, not shown) and the number of Q bands has been reduced consistent with literature data.26,27,29
Analysis of FeTPP complexes is known to be complicated due to the possibility of varied oxidation states. 1H-NMR spectra could not be obtained for the complex due to the presence of paramagnetic Fe(III). However, the absorption spectrum consisted of a Soret band at 417 nm and Q bands at 509 and 580 nm which is similar to data reported for FeTPPCl,30 suggesting that metallation had also occurred in this case. IR analysis also revealed the absence of the N–H stretch at ∼3317 cm−1 indicating de-protonation and metallation of the porphyrin.
Preparation of ZnTPP and CuTPP was attempted with various alternative metal precursors including ZnO, [Zn5(CO3)2(OH)6] and [Cu(CH3CN)4]PF6. However, these metallations proved unsuccessful even upon the addition of small amounts of solvent such as DMF, MeOH and H2O. Therefore it seems that elimination of acetic acid may be beneficial for these reactions to occur, potentially by acting as an internal solvent. Metallations were also attempted in the ball mill with Au(III), Mg(II), Ag(I), Pt(IV), Li(I), Mn(II) and Co(II) as detailed in the ESI (Table S7†). However, despite using acetate salts in some cases, varying the milling speed and duration as well as adding small amounts of solvents such as DMF or methanol, no reactions were observed for those metal ions. This is surprising given that some of these metal ions are highly labile and highlights the fact that much still remains to be understood regarding mechanochemical synthesis.
For the successful metallations, these simple and efficient methods contrast with conventional methods to metallate porphyrins which include refluxing a divalent metal salt (typically an acetate, halide or hydroxide) with H2TPP in DMF. Alder24 has reported preparing numerous metalloporphryins via this method including ZnTPP, NiTPP and CuTPP. More recently Maqueira et al.31 described the synthesis of ZnTPP and CuTPP by the same process but using a reaction time of 20 min. She et al.30 have described the synthesis of FeTPPCl by refluxing FeCl2 for 6–8 h in DMF. Grant et al.25 prepared ZnTPP and NiTPP by refluxing in chloroform and methanol for 2 h (for ZnTPP) or 5 days (for NiTPP). Purification was also then required involving either washing with water, hydrochloric acid, diethyl ether or methanol, high vacuum drying, recrystallization or column chromatography. As mentioned previously, the solvents used in these reactions are highly toxic and in some cases carcinogenic, and DMF can undergo side reactions at longer reaction durations.23
To summarise, a quick, convenient and clean solvent-free mechanochemical method to metallate porphyrins is described as an alternative to traditional routes that employ highly toxic solvents and in some cases long reaction times. As such this method presents considerable advantages in terms of sustainability and convenience. We note that the only by-products are water and acetic acid which are easily removed by heating, and the method is halide-free and nitrate-free. We also note that Hamilton et al.32 recently described the mechanochemical synthesis of meso-tetrasubstituted porphyrins themselves. Combining those processes with mechanochemical metallation described here should therefore provide very solvent-efficient methods of preparing this important class of compounds directly from simple precursors with little or no solvent.
References
- K. Ralphs, C. Hardacre and S. L. James, Chem. Soc. Rev., 2013, 42, 7701 Search PubMed.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317 Search PubMed.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, G. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. D. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 Search PubMed.
- M. R. Caira, L. R. Nassimbeni and A. F. Wildervanck, J. Chem. Soc., Perkin Trans. 2, 1995, 2213 RSC.
- U. Kamolphop, S. F. R. Taylor, J. P. Breen, R. Burch, J. J. Delgado, S. Chansai, C. Hardacre, S. Hengrasmee and S. L. James, ACS Catal., 2011, 1, 1257 Search PubMed.
- K. Ralphs, C. D'Agostino, R. Burch, S. Chansai, L. F. Gladden, C. Hardacre, S. L. James, J. Mitchell and S. F. R. Taylor, Catal. Sci. Technol., 2014, 4, 531 Search PubMed.
- K. Ralphs, S. Chansai, C. Hardacre, R. Burch, S. F. R. Taylor and S. L. James, Catal. Today, 2015, 246, 198 Search PubMed.
- K. Chadwick, R. Davey and W. Cross, CrystEngComm, 2007, 9, 732 Search PubMed.
- P. J. Nichols, C. L. Raston and J. W. Steed, Chem. Commun., 2001, 1062 Search PubMed.
- M. Pascu, A. Ruggi, R. Scopelliti and K. Severin, Chem. Commun., 2013, 49, 45 Search PubMed.
- A. Pichon, A. L. Garay and S. L. James, CrystEngComm, 2006, 8, 211 Search PubMed.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645 Search PubMed.
- R. S. Dhumal, A. L. Kelly, P. York, P. D. Coates and A. Paradkar, Pharm. Res., 2010, 27, 2725 Search PubMed.
- C. Medina, D. Daurio, K. Nagapudi, K. Nagapudi and F. Alvarez-Nunez, J. Pharm. Sci., 2010, 99, 1693 CrossRef CAS PubMed.
- D. Daurio, K. Nagapudi, L. Li, P. Quan and F. A. Nunez, Faraday Discuss., 2014, 170, 235 RSC.
- W. Sheng, Q. Jiang, W. Luo and C. Guo, J. Org. Chem., 2013, 78(11), 5691 Search PubMed.
- K. Eriksson, E. Göthelid, C. Puglia, J. Bäckvall and S. Oscarsson, J. Catal., 2013, 303, 16 Search PubMed.
- Q. Yang, D. Khvostichenko, J. Atkinson and R. Boulatov, Chem. Commun., 2008, 963 Search PubMed.
- L. Morira, F. Santos, J. Lyon, M. Maftoum-Costa, C. Pacheco-Soares and N. da Silva, Aust. J. Chem., 2008, 61, 741 Search PubMed.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19 Search PubMed.
- O. Zakharieva, A. Trautwein and C. Veeger, Biophys. Chem., 2000, 88, 11 Search PubMed.
- S. M. G. Pines, M. M. Q. Simoes, I. C. M. S. Santos, S. L. H. Rebelo, M. M. Pereira, M. C. P. M. S. Neves and J. A. S. Cavaleino, Appl. Catal., A, 2012, 51, 439 Search PubMed.
- T. Wijesekera and D. Dolphin, Metalloporphyrins in Catalytic Oxidations, Marcel Dekker Inc., 1994 Search PubMed.
- A. D. Alder, F. R. Longo, F. Kampas and J. Kim, J. Inorg. Nucl. Chem., 1970, 32, 2443 Search PubMed.
- M. Strohmeir, A. M. Orendt, J. C. Facelli, M. S. Solum, R. J. Pugmire, R. W. Parry and D. M. Grant, J. Am. Chem. Soc., 1997, 119(30), 7114 Search PubMed.
- A. V. Salker and S. D. Gokakakar, Int. J. Phys. Sci., 2009, 4(6), 377 Search PubMed.
- A. Wahab, M. Bhattacharya, S. Ghosh, A. G. Samuelson and P. K. Das, J. Phys. Chem. B, 2008, 112, 2842 Search PubMed.
- M. J. Gouterman, Mol. Spectrosc., 1961, 6, 138 Search PubMed.
- D. F. Marsh and L. M. Mink, J. Chem. Educ., 1996, 73(12), 1188 Search PubMed.
- Z. Sun, Y. She, Y. Zhou, X. Song and K. Li, Molecules, 2011, 16, 2960 Search PubMed.
- L. Maqueira, A. Iribarrern, A. C. Valdés, C. P. de Melo and C. D. dos Santos, J. Porphyrins Phthalocyanines, 2012, 16, 267 Search PubMed.
- H. Shy, P. Mackin, A. S. Orvieto, D. Gharbharan, G. R. Peterson, N. Bampos and T. D. Hamilton, Faraday Discuss., 2014, 170, 59 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc02420c |
| This journal is © The Royal Society of Chemistry 2017 |