The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats
Rui
Xie
a,
Hong
Zhang
b,
Xing-zhou
Wang
b,
Xiao-zhong
Yang
a,
Shang-nong
Wu
a,
Hong-gang
Wang
a,
Peng
Shen
a and
Tian-heng
Ma
*a
aDepartment of Gastroenterology, Huai'an First People's Hospital, Nanjing Medical University, Huai'an, Jiangsu 223300, P. R. China. E-mail: kxpt_03@163.com; matianheng25@126.com
bDepartment of Endocrinology, Huai'an First People's Hospital, Nanjing Medical University, Huai'an, Jiangsu 223300, P. R. China
First published on 6th December 2016
Abstract
The present study was designed to investigate the protective effect of betulinic acid (BA) on streptozotocin (STZ)-induced diabetic rats. The rats were intraperitoneally injected with STZ (35 mg kg−1). 7 days later, the animals were intragastrically administered with metformin (MET, 150 mg kg−1), BA (20 mg kg−1) or BA (40 mg kg−1) once daily for consecutive 30 days. The blood glucose, the contents of insulin, interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in serum were examined. The levels of IL-6, IL-1β, TNF-α, superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) in kidney tissues were measured. Moreover, the histopathological alteration and the protein expressions of the signaling pathway were detected by hematoxylin and eosin (H&E) staining and western blotting, respectively. BA significantly decreased the levels of serum insulin, IL-6, IL-1β, TNF-α and blood glucose. In addition, BA increased the activities of SOD, CAT and reduced the contents of MDA, IL-6, IL-1β, and TNF-α in kidney tissues. BA also ameliorated the histopathological condition. Furthermore, BA attenuated the phosphorylations of p-adenosine 5′-monophosphate-activated protein kinase (AMPK), nuclear factor kappaB (NF-κB), and an inhibitor of NF-κB (IκBα) and the expressions of NF-E2-related factor 2 (Nrf2) and heme oxygenase (HO)-1. These findings demonstrated that BA exhibited a protective effect on diabetic nephropathy in STZ-induced rats possibly through the AMPK/NF-κB/Nrf2 pathway.
1. Introduction
Diabetes mellitus (DM) is a chronic endocrine disease caused by the inherited or acquired deficiency of insulin production in pancreas.1 The number of humans with diabetes mellitus will increase to 300 million around the world by the year 2025.2 The most frequent pathological features of diabetic complications are diabetic neuropathy, retinopathy, nephropathy and cardiovascular problems. Particularly, diabetic nephropathy (DN) is one of the most common microvascular complications of diabetes mellitus. It is also the primary cause of end-stage kidney disease which is characterized by a series of renal structure abnormalities, such as mesangial expansion, glomerulosclerosis, basement membrane thickening, oxidative stress and inflammatory imbalance.3 Diabetic nephropathy remains a major cause of mortality and morbidity in patients with renal dysfunction. Herein, there is an urgent need to find a safer medicine for the treatment of diabetic nephropathy.Oxidative stress is defined as a status of an imbalance between the cellular anti-oxidative capacity and reactive oxygen species (ROS) formation resulting from the dysregulation of an antioxidant system.4 Thereby, the amelioration of the imbalanced condition by enhancing the cellular antioxidant capacity or scavenging ROS may make some difference for a variety of pathology and disease models. Nuclear factor erythroid-2 related factor-2 (Nrf2), which generally exists as a cap “n” collar basic leucine zipper protective transcription factor, plays a pivotal role in regulating cytoprotective and antioxidant genes triggered by oxidative stress.5 Excessive ROS might lead to the disruption of the Nrf2/Kelch-like ECH associated protein-1 (Keap1) complex and contribute to the Nrf2's activation. The downstream targets of Nrf2 contain HO-1 and the key antioxidant enzymes including CAT and SOD.6 NF-κB, a regulator of inflammatory disorders, is required for the transcription of oxidative stress and sufficient cytokines.7
Traditional Chinese medicine has been used around the world for centuries and is still acknowledged as a major source of medicine.8,9 Betulinic acid (3β-hydroxy-lup-20(29)-en-28-oic acid, BA) is a pentacyclic triterpenoid of plant origin which is distributed throughout the plant kingdom. It is extracted from the outer bark of white birch trees (Betula alba) and exhibits a variety of biological activities including anti-microbial, anti-viral, anti-malarial and anti-inflammatory properties.10,11 Evidence has emerged indicating that BA is beneficial to ischemia/reperfusion-induced renal injury.12 Genet et al. also demonstrated that BA showed a potential anti-diabetic effect.13 However, there has been no available literature focused on the protective effect of BA on diabetic nephropathy. The purpose of the present study was to evaluate the pharmacological effect of BA on STZ-induced diabetic nephropathy and explore its underlying mechanism.
2. Materials and methods
2.1 Reagents
Betulinic acid was purchased from the National Institutes for Food and Drug Control (Beijing, China). Metformin (MET) was purchased from Xiansheng Drug Store (Nanjing, China). Streptozotocin (STZ) was provided by Sigma-Aldrich Co, (St Louis, USA). Blood glucose, insulin, CAT, SOD and MDA commercial kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). IL-1β, IL-6 and TNF-α ELISA kits were purchased from Nanjing KeyGEN Biotech. Co. Ltd (Nanjing, China). All the antibodies were supplied by Cell Signaling Technology (Danvers, USA).2.2 Animals
Adult male Sprague–Dawley rats (180–200 g), purchased from the Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China), were allowed to adapt in their new location for 5 days. The animals were maintained at room temperature (24 ± 1 °C) in a 12 h light–dark natural cycle and were fed with a standard diet and water ad libitum. All the experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.2.3 Ethical statement
Animals were maintained in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and approved by the institutional ethical committee (IEC) of Nanjing Medical University with permission no. IEC/2010-08/05.2.4 Diabetes induction
The rats were intraperitoneally injected with STZ (35 mg kg−1) which were prepared in citrate buffer (pH 4.4, 0.1 M) to induce the diabetic model. Meanwhile, control rats (n = 10) received the same volume of citrate buffer. The blood samples were collected from the orbital venous plexus and the blood glucose levels were estimated by using a commercial kit after 7 days of STZ stimulation. The rats with the blood glucose level of ≥11.1 mmol L−1 were selected for further studies. The diabetic rats were randomly divided into four groups (n = 10): STZ group, STZ + MET (150 mg kg−1) group, STZ + BA (20 mg kg−1) group and STZ + BA (40 mg kg−1) group. As soon as diabetes was confirmed, the rats were intragastrically administered with MET (150 mg kg−1), BA (20 mg kg−1) or BA (40 mg kg−1) once daily for consecutive 30 days. The STZ rats and control rats simultaneously received the same volume of distilled water instead. At the end of the experimental period, the rats were anesthetized with 20% urethane and then sacrificed. Blood samples obtained from hearts were allowed to clot for 20 min at laboratory temperature and then centrifuged at 3000 rpm for 10 min. Three kidney tissues were excised and fixed in normal 10% neutral-buffered formalin for histopathological evaluation, while the other kidney tissues were kept at −70 °C until use.2.5 Measurements of blood glucose and insulin
Blood glucose and insulin in the serum were determined using a glucose kit and ELISA kit according to the manufacturer's protocol.2.6 Measurements of inflammatory cytokines in serum and kidney tissues
The blood was centrifuged at 3000 rpm for 10 min and then the serum samples were stored at −70 °C for pending tests. Additionally, the kidney samples were homogenized with cold normal saline. After being centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, the supernatant of the homogenate was collected in tubes and stored at −70 °C. The protein contents were determined with a BCA protein assay kit (Beyotime, Nanjing, China). The concentrations of IL-6, IL-1β and TNF-α in serum and kidney tissues were analyzed by using ELISA kits according to the manufacturer's instructions.
000 rpm for 10 min at 4 °C, the supernatant of the homogenate was collected in tubes and stored at −70 °C. The protein contents were determined with a BCA protein assay kit (Beyotime, Nanjing, China). The concentrations of IL-6, IL-1β and TNF-α in serum and kidney tissues were analyzed by using ELISA kits according to the manufacturer's instructions.
2.7 Measurements of SOD, CAT and MDA in serum and kidney tissues
The levels of CAT, SOD and MDA in the kidney tissues were determined using test kits purchased from the Nanjing Jiancheng Bioengineering Institute (China, Nanjing). All procedures were carried out according to the manual.2.8 Histopathological observation
The kidney tissues were excised at the end of the experimental procedure. The kidney tissues were fixed in normal 10% neutral-buffered formalin for 48 h. Thereafter, the samples were dehydrated in a graded alcohol, embedded in paraffin wax and stained with hematoxylin and eosin (H&E) according to the standard protocol. After that, the pathological alterations in the kidney tissues were observed with a light microscope in a blinded manner. A kidney inflammatory cell count based on a five point scoring system was performed to estimate the severity of leukocyte infiltration. The scoring system was: 0: no cells, 1: a few cells, 2: a ring of cells with 1 cell layer deep, 3: a ring of cells with 2–4 cell layers deep; and 4: a ring of cells with more than 4 cell layers deep.2.9 Western blotting
The kidney tissues were homogenized, washed with PBS and incubated in lysis buffer (Sigma, St Louis, USA) to obtain extracts of renal proteins. The total protein concentrations were determined with a BCA protein assay (Beyotime, Nanjing, China). Equal amounts of samples were loaded to 10% SDS-PAGE gels and then electrotransferred to nitrocellulose. The blots were incubated with the appropriate concentrations of specific antibodies. After washing, the blots were incubated with horseradish peroxidase-conjugated secondary anti-rabbit antibodies. The membrane was stripped and reblotted with an anti-GAPDH antibody to verify the equal loading of proteins in each lane. Quantification of the protein expression was normalized to GAPDH using a densitometer (Imaging System). Three samples were detected and the representative results were shown.2.10 Statistical analysis
The data were expressed as mean values ± SDs. Differences between the groups were analyzed by one-way ANOVA with Tukey's multiple comparison test, with p < 0.05 considered as significant.3. Results
3.1 Effects of BA on blood glucose and insulin
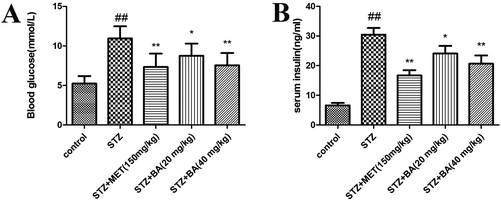
To examine the effects of BA on diabetes, the levels of blood glucose and insulin were detected. As depicted in Fig. 1, the levels of blood glucose and insulin in the diabetic rats were significantly higher than those of control animals (p < 0.01). By contrast, treatments with BA (40 mg kg−1) and MET effectively suppressed the levels of blood glucose and insulin in STZ-stimulated rats (p < 0.01). BA (20 mg kg−1) could also reduce the contents of blood glucose and insulin (p < 0.05). These data suggested that BA exhibited anti-diabetic properties in STZ-induced animals.3.2 Effects of BA on the levels of inflammatory cytokines in serum and kidney tissues
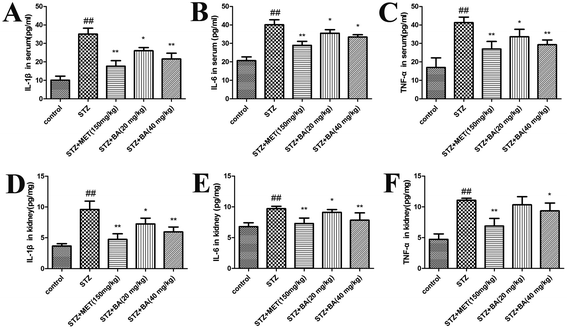
Next, we evaluated the generation of inflammatory cytokines in serum and renal tissues. The levels of TNF-α, IL-1β and IL-6 in the STZ-stimulated group were dramatically elevated compared to those in the control group (p < 0.01). Rats treated with BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1) displayed significant suppression of these cytokines (p < 0.05, p < 0.01, respectively). Moreover, in response to STZ, the contents of TNF-α, IL-1β and IL-6 in kidney tissues were pronouncedly decreased (p < 0.01), whereas the treatments with BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1) remarkably decreased the IL-1β and IL-6 levels (p < 0.05, p < 0.01). The TNF-α content also reduced in the MET-treated group (p < 0.01), which was more efficient than that of the BA (40 mg kg−1) group (p < 0.05). The data indicated that BA might reduce the synthesis and release of inflammatory cytokines in STZ-induced diabetic nephropathy (Fig. 2).3.3 Effects of BA on oxidative stress in serum
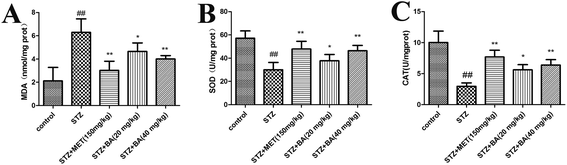
Lipid peroxidation was determined by measuring the levels of MDA, CAT and SOD. As shown in Fig. 3, STZ stimulation significantly declined the activities of SOD and CAT (p < 0.01). While BA (40 mg kg−1) and MET treatments effectively restored the levels of SOD and CAT (p < 0.01), which were more potent than those in the BA (20 mg kg−1) group. In addition, exposure to STZ displayed a strikingly high MDA level (p < 0.01). On the contrary, the administration of BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1) dramatically decreased the MDA content (p < 0.05, p < 0.01, respectively). Our results demonstrated that BA could attenuate the oxidative stress in STZ-stimulated rats.3.4 Effects of BA on histopathological changes in renal tissues
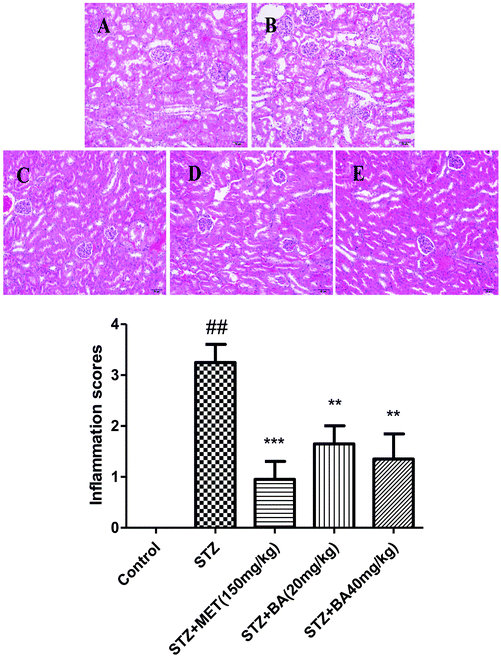
To evaluate the protective role of BA on physiological impairment, hematoxylin and eosin (H&E) staining was performed. Histological examination of kidney tissue from control groups showed a normal cell architecture. By contrast, the kidneys of diabetic rats revealed severe tubular degeneration or necrosis, moderate degrees of glomerular dilatation, vascular wall thickening and interstitial inflammation. Nevertheless, the severity of renal injury was attenuated by BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1). Analytical results indicated that BA obviously ameliorated the histopathological condition in STZ-induced diabetic nephropathy (Fig. 4).3.5 Effects of BA on the protein expressions of Nrf2, HO-1 and phosphorylated AMPK, IκBα, and NF-κB in renal tissues
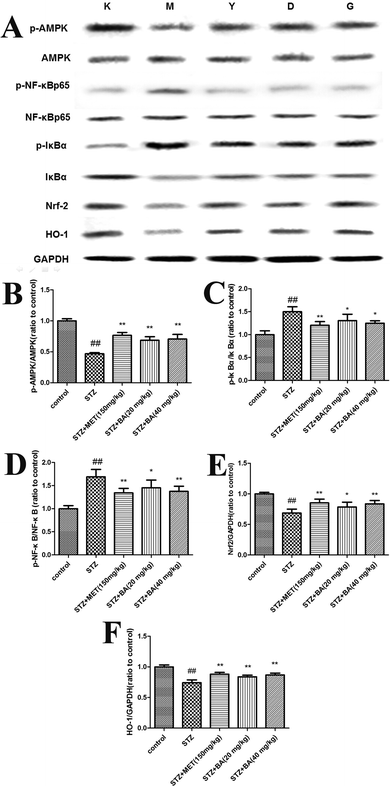
To explore the potential mechanism of BA on STZ-induced diabetic nephropathy, the expressions of Nrf2, HO-1, p-AMPK, p-IκBα and p-NF-κB in kidney tissues were detected. As revealed in Fig. 5, the protein levels of Nrf2, HO-1 and phosphorylated AMPK were markedly decreased in the STZ-challenged group (p < 0.01). However, the treatments with BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1) effectively increased the levels of Nrf2, HO-1 and p-AMPK compared with those in the STZ group (p < 0.05, p < 0.01). In addition, the phosphorylated IκBα and NF-κB were dramatically upregulated (p < 0.01), while the administration of BA (20 mg kg−1, 40 mg kg−1) and MET (150 mg kg−1) notably downregulated the phosphorylations of IκBα and NF-κB (p < 0.05, p < 0.01).4. Discussion
Diabetes mellitus is one of the most common human metabolic diseases which is partially attributed to the derangements in lipid peroxidation. STZ causes pancreatic cell apoptosis and induces an experimental model for type 1 diabetes mellitus. The present study firstly evaluated the protective effect of BA on the renal injury of STZ-induced diabetic rats by its anti-oxidative and anti-inflammatory properties via the AMPK/NF-κB/Nrf2 pathway.The elevated levels of blood glucose and insulin confirmed that the STZ-induced diabetic model was well-established, while the BA treatment decreased the contents of blood glucose and insulin, indicating that BA exerted a protective effect against diabetes. Meanwhile, the histopathological observation of STZ-stimulated kidney sections revealed severe tubular degeneration or necrosis, moderate degrees of glomerular dilatation, vascular wall thickening and interstitial inflammation. Whereas BA obviously attenuated these alterations. Taken together, the results suggested that BA could prevent STZ-challenged diabetic rats.
Inflammatory cytokines including IL-6, IL-1β and TNF-α are involved in the development of diabetic nephropathy. IL-1β is also implicated in the progression of irregularities in intraglomerular hemodynamics related to prostaglandin synthesis.14,15 IL-6 increases the fibronectin level, hastens mesangial cell proliferation, disturbs extracellular matrix dynamics and increases endothelial permeability.16 TNF-α is cytotoxic to glomerular, mesangial and epithelial cells. TNF-α is also able to induce direct renal damage through the generation of reactive free radicals.17 The data suggested that BA significantly reduced the contents of inflammatory cytokines in serum and renal tissues of STZ-induced rats.
Oxidative stress is considered as an imbalance between oxidants and antioxidants. The accumulated reactive oxygen species (ROS) could interact with polyunsaturated fatty acids, lead to the formation of lipid peroxidation in kidney tissues and consequently result in damage or toxicity.18 It is widely acknowledged that oxidative stress is the major factor of diabetic complications including diabetic nephropathy.19 ROS degrades membrane polyunsaturated fatty acids and produces 4-hydroxylnonenal (4-HNE) and malondialdehyde (MDA).20 MDA is a highly unstable aldehyde which could induce oxidative stress by forming a covalent protein adduct which serves as a hallmark of oxidative stress in tissue injury.21 Free radical scavenging enzymes including SOD and CAT are the first defensive line against oxidative damage in mammalian systems. SOD catalyzes the oxidation/reduction/conversion of superoxide radicals (O2−) to molecular oxygen and H2O2.22 Numerous evidence showed that SOD and MDA are closely associated with diabetic nephropathy.23 CAT is regarded as a major renal antioxidant which is involved in the elimination of H2O2 and protects the tissues from highly reactive hydroxyl radicals.24 Previous investigators demonstrated that CAT deficiency accelerated renal injury in diabetes through peroxisomal dysfunction.25 Our results confirmed that BA effectively increased the activities of SOD and CAT and reduced the content of MDA in kidney tissues of STZ-stimulated rats.
AMPK is an energy sensor with the regulation of metabolic homeostasis. AMPK mediates the initiation of renal dysfunction and participates in the attenuation of oxidative stress in diabetic nephropathy.26,27 NF-κB is a crucial target for the treatment of inflammatory and oxidative responses.28 The activation of NF-κB is required for the degradation and phosphorylation of IκB.29,30 According to our knowledge, the induction of NF-κB leads to the imbalance of oxidative mediators and upregulation of pro-inflammatory cytokines including IL-6, IL-1β and TNF-α.31 Former literature studies showed that NF-κB is closely associated with the pathogenesis of oxidative stress in diabetic nephropathy.32,33 Nrf2 is a major factor accounting for prompting the expression of various antioxidant genes in response to a variety of harmful stimulants through the defense against inflammation or oxidative stress.34 HO-1 is the major anti-oxidant and cytoprotective enzyme mediated by Nrf2 activation.35 Nrf2/HO-1 signaling could regulate the oxidative mediators including MDA, SOD and CAT in renal damage of diabetic mice.36 Previous investigators also demonstrated the essential role of Nrf2/HO-1 in diabetic nephropathy.37 Our experimental results indicated that BA effectively attenuated the expressions of Nrf2 and HO-1 and the phosphorylations of AMPK, IκB and NF-κB, which suggested that the renoprotective effect of BA in STZ-induced diabetic rats might be attributed to the AMPK/NF-κB/Nrf2 pathway.
In conclusion, our results indicated that BA exhibited a protective effect by attenuating the oxidative stress and inflammatory condition possibly through the AMPK/NF-κB/Nrf2 signaling pathway. Further studies are warranted to explore the clinical application of BA.
References
- T. Chen, J. Gao and P. Xiang, et al., Protective effect of platycodin D on liver injury in alloxan-induced diabetic mice via regulation of Treg/Th17 balance, Int. Immunopharmacol., 2015, 26(2), 338–348 CrossRef CAS PubMed.
- G. Mahendran, G. Thamotharan and S. Sengottuvelu, et al., Anti-diabetic activity of Swertia corymbosa (Griseb.) Wight ex CB Clarke aerial parts extract in streptozotocin induced diabetic rats, J. Ethnopharmacol., 2014, 151(3), 1175–1183 CrossRef CAS PubMed.
- T. W. C. Tervaert, A. L. Mooyaart and K. Amann, et al., Pathologic classification of diabetic nephropathy, J. Am. Soc. Nephrol., 2010, 21(4), 556–563 CrossRef PubMed.
- P. Xiang, T. Chen and Y. Mou, et al., NZ suppresses TLR4/NF-kappaB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages, Inflammation Res., 2015, 64(10), 799–808 CrossRef CAS PubMed.
- Z. Y. Jiang, H. X. Chu and M. Y. Xi, et al., Insight into the intermolecular recognition mechanism between Keap1 and IKKbeta combining homology modelling, protein-protein docking, molecular dynamics simulations and virtual alanine mutation, PLoS One, 2013, 8(9), e75076 CAS.
- K. Sahin, C. Orhan and Z. Tuzcu, et al., Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail, Food Chem. Toxicol., 2012, 50(11), 4035–4041 CrossRef CAS PubMed.
- W. Jiang, R. Zhou and P. Li, et al., Protective effect of chrysophanol on LPS/d-GalN-induced hepatic injury through the RIP140/NF-κB pathway, RSC Adv., 2016, 6(44), 38192–38200 RSC.
- T. Chen, L. Xiao and L. Zhu, et al., Anti-Asthmatic Effects of Ginsenoside Rb1 in a Mouse Model of Allergic Asthma Through Relegating Th1/Th2, Inflammation, 2015, 38(5), 1814–1822 CrossRef CAS PubMed.
- T. Chen, W. Jiang and H. Zhang, et al., Protective effect of trillin against ethanol-induced acute gastric lesions in an animal model, RSC Adv., 2016, 6(24), 20081–20088 RSC.
- A. Xia, Z. Xue and Y. Li, et al., Cardioprotective effect of betulinic acid on myocardial ischemia reperfusion injury in rats, Evidence-Based Complementary Altern. Med., 2014, 2014 Search PubMed.
- J. Kim, Y. S. Lee and C. S. Kim, et al., Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis, Phytother. Res., 2012, 26(7), 1103–1106 CrossRef CAS PubMed.
- E. Ekşioğlu-Demiralp, E. R. Kardaş and S. Özgül, et al., Betulinic acid protects against ischemia/reperfusion-induced renal damage and inhibits leukocyte apoptosis, Phytother. Res., 2010, 24(3), 325–332 CrossRef PubMed.
- C. Genet, A. Strehle and C. Schmidt, et al., Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes, J. Med. Chem., 2010, 53(1), 178–190 CrossRef CAS PubMed.
- T. Chen, Y. Mou and J. Tan, et al., The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice, Int. Immunopharmacol., 2015, 25(1), 55–64 CrossRef CAS PubMed.
- C. Mora and J. F. Navarro, Inflammation and diabetic nephropathy, Curr. Diabetes Rep., 2006, 6(6), 463–468 CrossRef CAS.
- J. F. Navarro-González and C. Mora-Fernández, The role of inflammatory cytokines in diabetic nephropathy, J. Am. Soc. Nephrol., 2008, 19(3), 433–442 CrossRef PubMed.
- S. R. Kolati, E. R. Kasala and L. N. Bodduluru, et al., BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway, Environ. Toxicol. Pharmacol., 2015, 39(2), 690–699 CrossRef CAS PubMed.
- F. Alarcon-Aguilar, A. Fortis-Barrera and S. Angeles-Mejia, et al., Anti-inflammatory and antioxidant effects of a hypoglycemic fructan fraction from Psacalium peltatum (HBK) Cass. in streptozotocin-induced diabetes mice, J. Ethnopharmacol., 2010, 132(2), 400–407 CrossRef CAS PubMed.
- A. P. Rolo and C. M. Palmeira, Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress, Toxicol. Appl. Pharmacol., 2006, 212(2), 167–178 CrossRef CAS PubMed.
- L. Zhu, T. Wei and X. Chang, et al., Effects of Salidroside on Myocardial Injury In Vivo In Vitro via Regulation of Nox/NF-kappaB/AP1 Pathway, Inflammation, 2015, 38(4), 1589–1598 CrossRef CAS PubMed.
- X. Chang, F. Luo and W. Jiang, et al., Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-kappaB pathway in vivo and in vitro, Int. Immunopharmacol., 2015, 28(1), 604–615 CrossRef CAS PubMed.
- W. Jing, M. Chunhua and W. Shumin, Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro, Toxicol. Appl. Pharmacol., 2015, 285(2), 128–135 CrossRef CAS PubMed.
- N. Giribabu, P. V. Rao and K. P. Kumar, et al., Aqueous extract of Phyllanthus niruri leaves displays in vitro antioxidant activity and prevents the elevation of oxidative stress in the kidney of streptozotocin-induced diabetic male rats, Evidence-Based Complementary Altern. Med., 2014, 2014 Search PubMed.
- T. Chen, R. Wang and W. Jiang, et al., Protective Effect of Astragaloside IV Against Paraquat-Induced Lung Injury in Mice by Suppressing Rho Signaling, Inflammation, 2016, 39(1), 483–492 CrossRef CAS PubMed.
- I. Hwang, J. Lee and J. Y. Huh, et al., Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction, Diabetes, 2012, 61(3), 728–738 CrossRef CAS PubMed.
- M. Kitada, S. Kume and N. Imaizumi, et al., Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway, Diabetes, 2011, 60(2), 634–643 CrossRef CAS PubMed.
- A.-E. Declèves, A. V. Mathew and R. Cunard, et al., AMPK mediates the initiation of kidney disease induced by a high-fat diet, J. Am. Soc. Nephrol., 2011, 22(10), 1846–1855 CrossRef PubMed.
- T. Chen, Q. Guo and H. Wang, et al., Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-small ka, CyrillicB pathways in vivo and in vitro, Free Radical Res., 2015, 49(12), 1459–1468 CrossRef CAS PubMed.
- Q. Jiang, M. Yi and Q. Guo, et al., Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway, Int. Immunopharmacol., 2015, 29(2), 370–376 CrossRef CAS PubMed.
- T. Chen, Z. Ma and L. Zhu, et al., Suppressing Receptor-Interacting Protein 140: a New Sight for Salidroside to Treat Cerebral Ischemia, Mol. Neurobiol., 2016, 53(9), 6240–6250 CrossRef CAS PubMed.
- L. Zhu, T. Chen and X. Chang, et al., Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-kappaB pathway, Neuropharmacology, 2016, 103, 134–142 CrossRef CAS PubMed.
- A. K. Mohamed, A. Bierhaus and S. Schiekofer, et al., The role of oxidative stress and NF-κB activation in late diabetic complications, BioFactors, 1999, 10(2–3), 157–167 CrossRef CAS PubMed.
- S. Mezzano, C. Aros and A. Droguett, et al., NF-κB activation and overexpression of regulated genes in human diabetic nephropathy, Nephrol., Dial., Transplant., 2004, 19(10), 2505–2512 CrossRef CAS PubMed.
- W. Jiang, F. Luo and Q. Lu, et al., The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state, Chem.-Biol. Interact., 2016, 243, 127–134 CrossRef CAS PubMed.
- P. Palsamy and S. Subramanian, Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling, Biochim. Biophys. Acta, Mol. Basis Dis., 2011, 1812(7), 719–731 CrossRef CAS PubMed.
- M. J. Kim and Y. Lim, Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage, Mediators Inflammation, 2013, 2013 Search PubMed.
- T. Jiang, Z. Huang and Y. Lin, et al., The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy, Diabetes, 2010, 59(4), 850–860 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |