The influence of flaxseed gum on the microrheological properties and physicochemical stability of whey protein stabilized β-carotene emulsions
Duoxia
Xu
 a,
Yameng
Qi
a,
Xu
Wang
a,
Xin
Li
a,
Shaojia
Wang
a,
Yanping
Cao
*a,
Chengtao
Wang
a,
Baoguo
Sun
a,
Eric
Decker
b and
Atikorn
Panya
c
a,
Yameng
Qi
a,
Xu
Wang
a,
Xin
Li
a,
Shaojia
Wang
a,
Yanping
Cao
*a,
Chengtao
Wang
a,
Baoguo
Sun
a,
Eric
Decker
b and
Atikorn
Panya
c
aBeijing Advanced Innovation Center for Food Nutrition and Human Health (BTBU), School of Food & Chemical Engineering, Beijing Engineering and Technology Research Center of Food Additives, Beijing Higher Institution Engineering Research Center of Food Additives and Ingredients, Beijing Key Laboratory of Flavor Chemistry, Beijing Laboratory for Food Quality and Safety, Beijing Technology & Business University, Beijing, China. E-mail: xdxbtbu@126.com; Fax: +86-10-68985645; Tel: +86-10-68985645
bDepartment of Food Science, University of Massachusetts, Amherst, MA, USA
cFood Biotechnology Research Unit, National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Phaholyothin Rd., Khlong Nuang, Khlong Luang, Pathumthani 12120, Thailand
First published on 19th December 2016
Abstract
The impact of flaxseed gum (FG) on the microrheological properties and physicochemical stability of whey protein isolate (WPI) stabilized β-carotene emulsions at pH 3.0 was studied. A layer-by-layer electrostatic deposition method was used to prepare multilayered β-carotene emulsions with interfacial membranes consisting of WPI and FG. The microrheological behavior of the multilayered β-carotene emulsions was measured through the diffusive wave spectroscopy technique. WPI alone and WPI–FG (concentration of FG = 0.1, 0.2, 0.3 wt%) stabilized β-carotene emulsions were purely viscous giving a mean square displacement that scaled linearly with decorrelation time (τ). The presence of 0.01, 0.02, and 0.05 wt% FG in the WPI-stabilized emulsions caused them to exhibit viscoelastic properties. Meanwhile, the increase in τ reflected the increase in the length scale of connectivity in the emulsions until a “cluster” was formed and the droplets were not free to move due to droplet–network interaction. The apparent increase in the macroscopic viscosity and elasticity index and decrease in the solid lipid balance and fluidity index of emulsions with lower concentrations (0.01, 0.02, 0.05 wt%) of FG indicated that the bridging flocculation of FG had a much more appreciable influence on the microrheological properties than depletion flocculation (higher concentrations, 0.1, 0.2, 0.3 wt%). Droplet size, zeta-potential, and transmission profiles using the centrifugal sedimentation technique and β-carotene degradation during storage were also characterized. With the addition of FG, the zeta-potential of WPI coated β-carotene droplets decreased from positive to negative, and an increase in the apparent droplet size was also noted. LUMISizer analysis exhibited an improvement in physical stability with the addition of 0.1 wt% FG. FG also helped to chemically stabilize the WPI emulsions against β-carotene degradation mainly by slowing down the mobility of the droplets.
1. Introduction
β-Carotene is now widely used as a pigment in food processing and may prevent a number of serious health disorders such as cancer, heart disease and colorectal adenomas.1 However, food enrichment with β-carotene encounters great difficulties because β-carotene is insoluble in water, and only slightly soluble in oil at room temperature.2 Meanwhile, the instability of β-carotene negatively influences product quality and consumer acceptance resulting in the potential rejection of β-carotene fortified foods.3β-Carotene can be dispersed into a lipid phase and incorporated into a suitable delivery system. Oil-in-water (O/W) emulsions as a delivery system can produce physically stable β-carotene dispersions, which are more chemically stable during storage and have increased bioavailability.4,5 The emulsion is formed by small oil droplets dispersed in an aqueous continuous phase, and each oil droplet is surrounded by emulsifier molecules. It has been suggested that proteins and polysaccharides have significant effects on the physicochemical properties of emulsions.6 Proteins act as emulsifiers because of their ability to adsorb at the oil–water interface and to increase emulsion stability.7,8 Most polysaccharides behave like emulsion stabilizers by forming an extended network in the continuous phase which thus becomes highly viscous.9
One of the most promising methods of emulsifying bioactive compounds with proteins and polysaccharides is the sequential adsorption of polyelectrolytes, called the layer-by-layer technique.10,11 The layer-by-layer technique is a powerful tool that can be used to control the interaction, composition, charge, thickness and permeability of protein–polysaccharide films. The multilayer emulsion is formed on a charged surface by adding polysaccharides to oppositely charged protein coated droplets.12–14 It modulates the functional properties of the emulsions such as droplet distribution, microstructure, rheological behavior and chemical stability. It has been reported that emulsions stabilized by a multilayer surface of β-lactoglobulin, casein, whey protein isolate, lactoferrin, alginate, chitosan, pectin, soybean soluble polysaccharide, etc. have better physical and chemical stabilities due to the formation of a barrier interfacial layer.15–17 Multilayer emulsion technology has also provided a way to design emulsions with specific controlled digestibility.
Whey protein isolate (WPI) has been widely used in food products due to its high nutritional value and excellent emulsifying properties. It is reported that WPI can stabilize emulsions against flocculation and coalescence through a combination of electrostatic and steric interactions.18 The limited emulsifying stability of WPI as an emulsifier lies in its thin interfacial membrane. Flaxseed gum (FG) is composed mainly of a neutral and an anionic fraction, including L-galactose, D-xylose, L-rhamnose, and D-galacturonic acid.19 FG is an anionic biopolymer that has many potential applications in the food, cosmetic, and pharmaceutical industries because of its unique nutritional and physicochemical properties.20,21 In particular, FG can be used in beverages and juices as a thickener due to its high viscosity. FG as an emulsifying agent was used in combination with WPI at neutral pH.22 Although there are extensive publications about emulsions stabilized with protein and polysaccharide, the properties of multilayered β-carotene emulsions with interfacial membranes consisting of WPI and FG have never been reported. We hypothesize that FG could bind to WPI coated β-carotene droplets and improve their physicochemical stability.
Viscoelastic properties are usually measured with shear rheometers that probe macroscopic samples in various deformation geometries. Shear rheometry depends on the extent of strain and the magnitude of the shear modulus to be measured. However, these mechanical rheometers may destroy or affect the internal structure of the samples. Microrheological techniques are a nondestructive method of measuring viscoelastic properties. The main principle of microrheology is tracing of particle motion through the diffusing wave spectroscopy technique, which depends on the viscoelastic structure of the sample.23
Therefore, the purpose of this study was to investigate the influence of FG on the microrheological properties and physicochemical stability of WPI stabilized β-carotene emulsion. The present paper was an attempt to use microrheological properties to trace the motion of β-carotene emulsion droplets. Physicochemical stability was assessed by average droplet size, zeta-potential, and transmission profiles using the centrifugal sedimentation technique and chemical stability in order to better understand the interactions occurring during structuring of emulsion and β-carotene degradation during storage. Ultimately our goal is to use protein-FG to protect bioactive components and to extend the application of bioactive components in the food industry.
2. Materials and methods
2.1 Materials
Whey protein isolate (WPI) was purchased from Jinan SAN Chemical Co., Ltd (Jinan, China). The product contained 97.6% protein (dry basis), as determined by the supplier's standard proximate analysis procedures. β-Carotene powder (purity >97%) was purchased from Nanjing Ze Lang Pharmaceutical Technology Co., Ltd (Nanjing, China). Medium-chain triglyceride (MCT) oil was obtained from Lonza Inc. (Allendale, NJ, USA). Flaxseed gum (FG) was purchased from Beijing Pinellia Technology Development Co., Ltd (Beijing, China) and sodium azide was purchased from Sigma-Aldrich (St Louis, MO). All other chemicals were of analytical grade.2.2 Preparation of WPI–FG stabilized β-carotene emulsion
WPI and FG were first dispersed in 10 mM phosphate buffer at pH 7.0, separately. The solutions were kept overnight to ensure complete dispersion and dissolution. Sodium azide (0.01 wt%) was added to prevent microbial growth.WPI stabilized β-carotene emulsion (1 wt% protein) was prepared with 10 wt% medium chain triacylglycerol (MCT) oil containing β-carotene (β-carotene was first dissolved in MCT oil at 140 °C for several seconds, 0.02 wt% in the final emulsion) as the dispersed phase and 90 wt% aqueous phase solution at room temperature. The β-carotene emulsions were prepared using an Ultra-Turrax (B25 model, Shanghai Beierte experimental equipment Co., Ltd) at a speed of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min to form coarse emulsions, which were subsequently homogenized using a M110-PS Microfluidizer processor (Microfluidics International Corp., Newton, MA) at an operational pressure of 50 MPa three times. After preparation, the pH was adjusted to 3.0 using 1.0 M HCl.
000 rpm for 3 min to form coarse emulsions, which were subsequently homogenized using a M110-PS Microfluidizer processor (Microfluidics International Corp., Newton, MA) at an operational pressure of 50 MPa three times. After preparation, the pH was adjusted to 3.0 using 1.0 M HCl.
The WPI–FG bilayer emulsions were prepared by slow addition of a WPI-stabilized β-carotene emulsion (1 wt% WPI, 0.02 wt% β-carotene, 10 wt% MCT) to a solution of FG ranging in concentration from 0 to 0.6 wt% at pH 3.0, respectively. The final mixture was rehomogenized using the conditions as described above for the WPI stabilized β-carotene emulsion. Final emulsion samples (0.01 wt% β-carotene, 5 wt% MCT, 0.5 wt% WPI and 0–0.3 wt% FG) were transferred into screw-capped brown bottles flushed with nitrogen.
2.3 Measurement of zeta-potential
The WPI-stabilized β-carotene emulsions with different concentrations of FG were diluted to 0.04 wt% using a buffer solution at pH 3.0 prior to analysis. Diluted emulsions were then injected into the measurement chamber of a particle electrophoresis instrument. The zeta-potential of the droplets was then determined by a particle electrophoresis instrument using a Zetasizer Nano-ZS90 (Malvern Instruments, Worcestershire, UK), and the zeta-potential was determined by measuring the direction and velocity of droplet movement in the applied electric field.2.4 Determination of droplet size
The average droplet size of WPI-stabilized β-carotene emulsions with different concentrations of FG was determined by dynamic light scattering using a Zetasizer Nano-ZS90 (Malvern Instruments, Worcestershire, UK) at a fixed detector angle of 90°. Emulsions were diluted using a buffer solution at pH 3.0 to minimize multiple scattering effects prior to each measurement. The averaged intensity droplet size was obtained by a CONTIN mode analysis. The results were described as cumulative mean diameter (size, μm) for droplet size.2.5 Microrheological behavior
The microrheometer Rheolaser Lab (Formulaction, France) used for the measurements of the microrheology of β-carotene emulsions is based on diffusing wave spectroscopy (DWS). It corresponds to dynamic light scattering in concentrated media and measures the particles’ Brownian motion, which depends on the viscoelastic structure of the sample. In the experiment, 20 mL of an emulsion were carefully transferred with a plastic pipette along the wall of a flat-bottomed cylindrical glass tube (diameter 25 mm). The tube was immediately placed in the sample chamber of the Rheolaser at 25 °C. A microrheology test of 1 h 40 min was carried out on each sample of WPI stabilized β-carotene emulsion containing different concentrations of FG.The instrument measures the Brownian motion of the emulsion droplets, reported as mean square displacement (MSD) versus time. The mean square displacement (MSD) vs. decorrelation time (τ) curves, macroscopic viscosity index (MVI) and elasticity index (EI), solid lipid balance (SLB) and fluidity index (FI) parameters of the samples were obtained by using the software RheoSoft Master 1.4.0.0.
2.6 Physical stability analyzed by LUMISizer
The physical stability of β-carotene emulsions with various concentrations of FG was measured with a LUMISizer (L.U.M. GmbH, Berlin, Germany). The instrument employs centrifugal sedimentation to accelerate the occurrence of instability phenomena such as sedimentation, flocculation or creaming.24,25 Emulsion samples were subjected to centrifugal force, while near-infrared light illuminated the entire sample cell to measure the intensity of transmitted light as a function of time and position over the entire sample length simultaneously. The instrumental parameters used for the measurement were as follows: volume, 1.8 mL of dispersion; 3000 rpm; time, 7650 s; time interval, 30 s; temperature, 25 °C.26,272.7 Chemical stability
β-Carotene emulsion samples were diluted 10 times with buffer solution (pH 3.0) and then transferred into brown screw-capped bottles flushed with nitrogen and stored in the dark at 55 °C to accelerate β-carotene degradation. The β-carotene content was measured during the storage.The content of β-carotene in the emulsion was determined following the method of Yuan et al.28 The β-carotene was extracted with ethanol and n-hexane and then the absorbance at 450 nm was measured with a Shimadzu UVmini-1240UV-vis spectrophotometer. The content of β-carotene was obtained by referring to a standard curve of β-carotene prepared under the same conditions. The β-carotene content was expressed as the relative β-carotene C in percentage: C(t)/C0, where C(t) is the β-carotene content after storage for a period t and C0 is the β-carotene content at the time of preparation.
2.8 Statistical analysis
All emulsions were prepared in duplicate, and all measurements were performed three times. Data were subjected to analysis of variance (ANOVA) using the software package SPSS 12.0 for Windows (SPSS Inc., Chicago, IL).3. Results and discussion
3.1 Zeta-potential
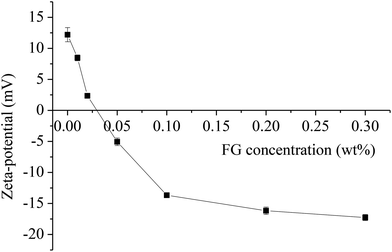
Fig. 1 depicts the influence of FG concentration (0–0.3 wt%) on the zeta-potential of WPI-stabilized β-carotene emulsions at pH 3.0. In the absence of FG, the net charge on the WPI coated β-carotene droplets was around 12.2 ± 1.1 mV. The isoelectric point of WPI is around pH 5.0, hence at pH 3.0 the WPI-coated β-carotene droplet was positively charged. The electrical charge on the droplets became increasingly less positive as the FG concentration in the emulsion was increased (Fig. 1), which suggested that the negatively charged FG molecules adsorbed to the surfaces of the positively charged emulsion droplets forming WPI–FG membranes by the layer-by-layer technique.When not enough FG was present, the emulsions containing 0.01–0.05 wt% FG showed that the zeta-potential value was close to zero. This indicated that FG at a lower content might bind to one or more emulsion droplets at the same time and was not enough to form strong electrostatic repulsion between droplets, which led to the increase of MVI and EI and decrease of SLB and FI of the emulsions (shown in section 3.3). At a high enough concentration of FG (0.1–0.3 wt%), the β-carotene emulsion droplet became negatively charged. Finally, the droplet charge reached a plateau and showed a value of −17.3 ± 0.5 mV when the FG concentration was 0.3 wt%, suggesting that the droplets had become saturated with FG. The ability of charged polyelectrolytes to adsorb onto the surface of oppositely charged colloidal particles has been well evaluated in the literature.9
3.2 Droplet size
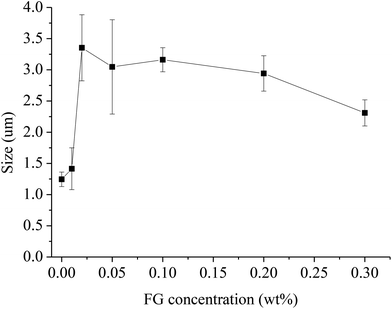
Fig. 2 illustrates the mean droplet size of WPI-stabilized β-carotene emulsions as a function of FG concentration. Without the presence of FG, the mean droplet size of WPI stabilized β-carotene emulsion was relatively small, being 1.2 ± 0.11 μm. In the presence of FG, the emulsions’ mean droplet size was increased. At lower FG concentrations (0.02 wt% and 0.05 wt%), there were significant increases in the mean droplet size. The high measurement errors indicated that the emulsions had a wide size distribution attributed to flocculation of the β-carotene droplets. The increase of droplet size could explain the increase in MVI and EI values mentioned in the following. With the further addition of FG (0.3 wt%), there was a relative decrease but no significant difference in the mean droplet size. Similar phenomena were also found of two different varieties (Emerson and McDuff) of FG being able to interact with proteins, and an increase in apparent particle size was also noted with increasing FG.20The results could be explained by the fact that while FG at low concentration interacted with WPI on the surface of the β-carotene droplets, destabilization occurred when the droplet charges were neutralized. It was also due to insufficient FG to cover the entire droplets in the emulsion. Thus, FG may act as a bridge to interact with WPI molecules between the surfaces of the β-carotene droplets by attractive electrostatic forces. When the concentration of FG increased, the large droplet size was a clear indication of the flocculation and aggregation of the droplets. The increase in size also proved the interaction of FG with the WPI-covered β-carotene droplets and corresponded directly with the differences in the drop of zeta-potential described in Fig. 1. The difference (p < 0.05) in droplet size between the WPI–FG (0.3 wt% FG) β-carotene emulsion (with a much bigger droplet size) and WPI emulsion suggested that a part of the FG was present at the droplet interface. It has been reported that polysaccharide covers the droplet with a thin monolayer. Therefore, the formation of a FG layer at the droplet surface should result in an increase in hydrodynamic radius of less than 0.1 μm.29 The addition of 0.1, 0.2 and 0.3 wt% FG caused a droplet size increase of more than 0.1 μm, suggesting that a part of FG probably did not adsorb at the droplet interface or that depletion flocculation had occurred.
3.3 Microrheological properties
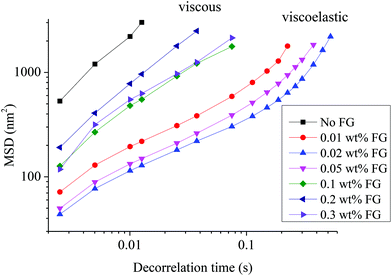
The influence of FG addition on the microrheological properties of WPI stabilized β-carotene emulsions was measured using the Rheolaser lab. If the measurement is not in the linear viscoelastic region, the classical mechanical rheology technique is reported to be a cause of a dramatic shear induced structural breakdown, leading to droplet deflocculation.30 Therefore, a nondestructive technique is needed to analyse the rheological properties of the emulsions. The Rheolaser lab technique does not provide any kind of modification of emulsion properties compared with shear rheology. It is a direct method of monitoring the Brownian motion of droplets and interpreting it in terms of microrheology.31Microrheology typically measures the motion of emulsion droplets and traces the droplet interactions. The representative mean square displacement (MSD) vs. decorrelation time curves for WPI stabilized β-carotene emulsions as a function of FG concentrations is shown in Fig. 3. The MSD of the droplets is a direct measure of the dynamic properties of the droplets and the polymers in which they are embedded. The MSD curve enables the characterization of the viscous and viscoelastic properties of the sample. WPI alone and WPI–FG (concentration of FG, 0.1, 0.2, 0.3 wt%) stabilized β-carotene emulsions were purely viscous over the range of decorrelation time (τ), giving a MSD that scaled linearly with τ: MSD = Aτ. It was found that the MSDs of these emulsions with addition of 0.01, 0.02, and 0.05 wt% FG were not linear over the range of decorrelation time. The presence of 0.01, 0.02, and 0.05 wt% FG in the WPI-stabilized emulsions led to critical power law scaling of the MSD ≈ τn. This indicated that these three emulsions (in the presence of 0.01, 0.02 and 0.05 wt% FG) were viscoelastic, meaning that the MSDs tended to reach a plateau as the β-carotene emulsion droplets became trapped. In the presence of 0.01, 0.02, and 0.05 wt% FG, τ was increased, reflecting the increase in the length scale of connectivity in the emulsions until a “cluster” was formed and the droplets were not free to move due to the droplet–network interaction.
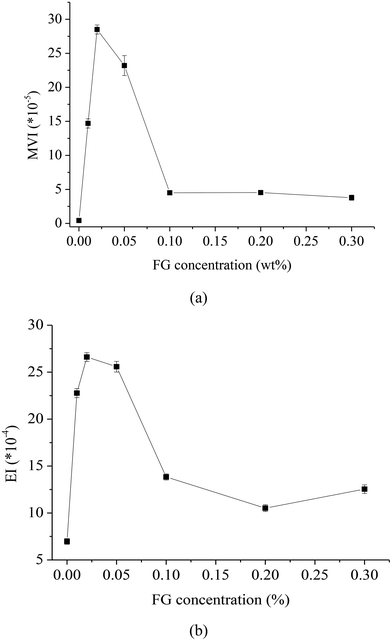
The macroscopic viscosity index (MVI) value quantifies the macroscopic viscosity at zero-shear. It corresponds to the inverse of the speed of the particles for long times (inverse of the linear slope of the MSD for long times). Fig. 4 shows the effect of different FG concentrations on the MVI value of WPI stabilized β-carotene emulsion at 25 °C. The MVI of β-carotene emulsions prepared with WPI–FG was increased compared with emulsions prepared with WPI alone. The increase in MVI value was more pronounced for emulsions formed with lower concentrations (0.01, 0.02, 0.05 wt%) of FG, which could be related to the droplet–network interaction and higher degree of flocculation. When the concentration of FG was 0.02 wt%, the MVI value significantly increased 67.4 times (p < 0.05) compared with WPI emulsion. It was interesting to find that the MVI of β-carotene emulsion containing more than 0.1 wt% FG was decreased compared with the presence of relatively lower concentrations of FG (0.01, 0.02, 0.05 wt%). With further increase in FG concentration, the MVI value of the WPI–FG (0.3 wt%) emulsion significantly increased 3.6 times (p < 0.05) compared with the WPI emulsion. This suggested that the increased MVI did not depend on the effect of FG viscosity but on the interaction between the FG and the droplets. This agreed with Moschakis and co-worker's report about the effect of xanthan gum on the microrheology of emulsion.32
The elasticity index (EI) value represents the elasticity strength of the samples. It corresponds to the inverse of the distance travelled by the particles before interacting with the network. Fig. 4b shows the effect of different FG concentrations on the EI value of WPI stabilized β-carotene emulsion. The EI of β-carotene emulsions prepared by WPI–FG was increased and then decreased with the addition of FG. When the concentration of FG was 0.05 wt%, the EI value dramatically increased 3.7 times compared with the one prepared by WPI alone. When the concentration of FG was less than 0.1 wt%, the EI value was dramatically increased, which was typically attributed to the emulsions flocculation, strongly aggregated droplets and three dimensional network formations at the relatively low FG content. When the concentration of FG was more than 0.1 wt%, the increase in the EI of the β-carotene emulsion with the further increase of FG concentration was not so remarkable.
The apparent increase in the MVI and EI of emulsions with lower concentrations (0.01, 0.02, 0.05 wt%) of FG indicated that the bridging flocculation of FG had a much more appreciable influence on microrheological properties than depletion flocculation (higher concentrations, 0.1, 0.2, 0.3 wt%). It can be inferred that bridging flocculation could dominate and strengthen droplet–droplet resistance interaction and aggregation, which resulted in dense network formation and spatial rearrangement of the β-carotene droplets.
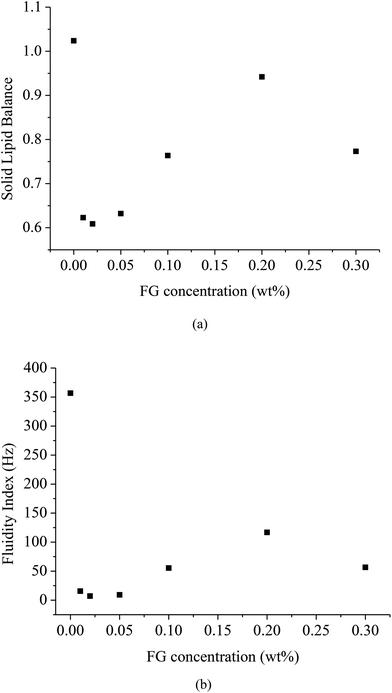
The SLB value (∼tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′) is the ratio between the solid-like and liquid-like behavior of the studied sample. It corresponds to the MSD slope at short τ. The fluidity index (FI) is obtained as the inverse of τ and is measured in Hz. The influence of FG concentration on the SLB and FI of emulsions is shown in Fig. 5a and b. WPI-stabilized β-carotene emulsions with and without addition of FG showed an SLB > 0.5, which means that the liquid behavior dominated. The SLB of WPI-stabilized β-carotene emulsion was close to 1, which proved that the sample was purely viscous/liquid-like. After addition of FG (0.01, 0.02, 0.05 wt%), the SLB of emulsions was decreased. This suggested that the rheological behavior of β-carotene emulsions changed from a strongly liquid-like behavior to a slightly liquid-like behavior. As shown in Fig. 5b, this phenomenon was consistent with the fact that the β-carotene emulsion was undergoing a substantial change from a rather dispersed fluid state (FI = 356.7 for WPI emulsion) to a highly structured lipid state (FI = 15.6, 7.1, 9.2 for WPI–FG emulsion with the FG content of 0.01, 0.02, 0.05 wt%, respectively) resulting from the establishment of the “network structure” due to the electrostatic interaction between the droplets and the bridging effect of FG (0.01, 0.02, 0.05 wt%). It can be seen that the values of SLB and FI were increased further with the increase of the FG content (0.1, 0.2, 0.3 wt%), causing a mainly viscous/liquid-like behavior and higher mobility. This suggested that the elastic characteristic of the β-carotene emulsion was weakened by the further incorporation of FG. These experimental results could be explained in terms of an increase in the mobility of the emulsions as the FG concentration and/or the droplet electrostatic repulsion increased. It proved that enough FG did adsorb to WPI-stabilized droplets, leading to a dramatic increase of droplets mobility compared with the lower content of FG. When FG concentration was 0.3 wt%, SLB and FI exhibited a slight decrease. This indicated that the droplets were suppressed, leading to loss of mobility probably due to the aggregate formation by depletion flocculation.
δ = G′′/G′) is the ratio between the solid-like and liquid-like behavior of the studied sample. It corresponds to the MSD slope at short τ. The fluidity index (FI) is obtained as the inverse of τ and is measured in Hz. The influence of FG concentration on the SLB and FI of emulsions is shown in Fig. 5a and b. WPI-stabilized β-carotene emulsions with and without addition of FG showed an SLB > 0.5, which means that the liquid behavior dominated. The SLB of WPI-stabilized β-carotene emulsion was close to 1, which proved that the sample was purely viscous/liquid-like. After addition of FG (0.01, 0.02, 0.05 wt%), the SLB of emulsions was decreased. This suggested that the rheological behavior of β-carotene emulsions changed from a strongly liquid-like behavior to a slightly liquid-like behavior. As shown in Fig. 5b, this phenomenon was consistent with the fact that the β-carotene emulsion was undergoing a substantial change from a rather dispersed fluid state (FI = 356.7 for WPI emulsion) to a highly structured lipid state (FI = 15.6, 7.1, 9.2 for WPI–FG emulsion with the FG content of 0.01, 0.02, 0.05 wt%, respectively) resulting from the establishment of the “network structure” due to the electrostatic interaction between the droplets and the bridging effect of FG (0.01, 0.02, 0.05 wt%). It can be seen that the values of SLB and FI were increased further with the increase of the FG content (0.1, 0.2, 0.3 wt%), causing a mainly viscous/liquid-like behavior and higher mobility. This suggested that the elastic characteristic of the β-carotene emulsion was weakened by the further incorporation of FG. These experimental results could be explained in terms of an increase in the mobility of the emulsions as the FG concentration and/or the droplet electrostatic repulsion increased. It proved that enough FG did adsorb to WPI-stabilized droplets, leading to a dramatic increase of droplets mobility compared with the lower content of FG. When FG concentration was 0.3 wt%, SLB and FI exhibited a slight decrease. This indicated that the droplets were suppressed, leading to loss of mobility probably due to the aggregate formation by depletion flocculation.
 | ||
| Fig. 5 Fluidity index (FI, a) and solid lipid balance (SLB, b) values for WPI stabilized β-carotene emulsion (0.5 wt% WPI, 5 wt% MCT, 0.01 wt% β-carotene) as a function of FG concentrations. | ||
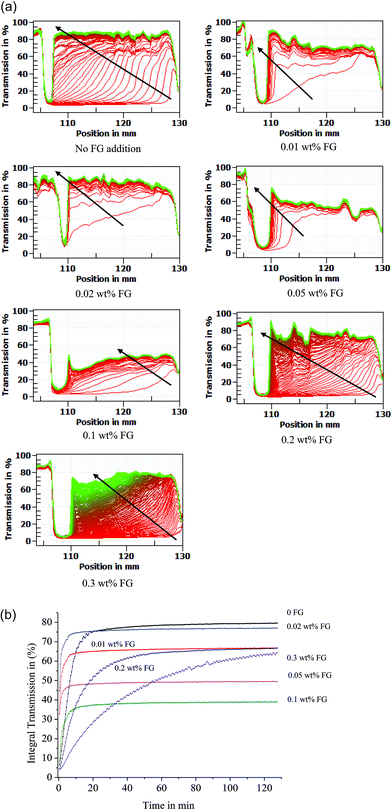
3.4 Physical stability
The physical stability of WPI stabilized β-carotene emulsion as a function of FG concentration was detected by LUMISizer measurements. The stability was expressed as the evolution of the integrated transmission–time profiles determined at 3000 rpm over 7650 s at 25 °C. The position in the transmission profiles at about 105 mm corresponded to the filling height of the emulsions. The position of the cell bottom was at 130 mm. The transmission profiles were representative of the variation of the droplet concentration inside the emulsion. A high transmission means a low concentration of droplets and a low transmission means a high concentration of droplets.24 All these first profiles lay at the bottom; the last profiles lay at the top (Fig. 6a).From Fig. 6a, it can be seen that the first profile taken after 30 s exhibited low transmissions along the sample length for the WPI-stabilized emulsion, except the bottom part. It was found that with increasing the FG concentration from 0.01 wt% to 0.05 wt%, the first profile taken after 30 s exhibited high transmissions along the sample length. During centrifugation a sharp front moved towards the cell top. The sharp front means that nearly all droplets are moving as a collective (zone creaming). This indicated that already after 30 s a creaming layer was formed. The structure of the creaming can therefore be considered as a flocculated network.
Above 0.1 wt% FG, the first profile taken after 30 s exhibited lower transmissions along the sample length. Instead of a sharp front creaming was observed. The profiles were nearly evenly spaced with a considerably smaller distance compared with WPI and WPI–FG (0.01 wt%, 0.02 wt%, 0.05 wt%) emulsions. This indicated that resistance against compaction was steadily increasing. This showed that the last profiles taken after 7650 s exhibited lowest transmissions along the emulsion length for adding 0.1 wt% FG.
Fig. 6b exhibits the time-dependent integral transmission profiles of β-carotene emulsions with different FG concentrations. The higher integral transmission exhibits the much lower stability of the emulsion. As can be seen the stability of WPI emulsions can be markedly increased by addition of high amounts of FG. At the beginning of the 10 min, the stability of β-carotene emulsions with different concentrations of FG was in the order of 0.3 > 0.2 > 0.1 ≥ 0 > 0.05 > 0.01 > 0.02 wt%. Emulsions with FG concentrations of 0.01–0.05 wt% were very unstable, because they exhibited a remarkable increase in transmission due to creaming and flocculation during centrifugation. When the FG concentration was above 0.1 wt%, the emulsion became stable, as there were very few changes in integral transmission.
As shown in Fig. 6b, it was evident that the integral transmission behavior of the emulsions was time-dependent. After the integral transmission reached a plateau, the stability of β-carotene emulsions with different concentrations of FG was in the order of 0.1 > 0.05 > 0.3 > 0.2 ≥ 0.01 > 0.02 > 0 wt%. This demonstrated that WPI coated β-carotene emulsion at acidic pH against creaming was very poor with a transmission value of 79.6%. It can be seen that the physical stability of WPI stabilized β-carotene emulsion was improved with the addition of FG. In the presence of FG, the mobility of β-carotene emulsion droplets was slowed down. This indicated that the physical stability (with transmission value of 39.1%) of WPI stabilized β-carotene emulsion was markedly improved with the proper addition of FG at 0.1 wt%. The improved stability of WPI–FG coated droplets with addition of FG of 0.1 wt% can be attributed to the relatively strong electrostatic repulsion between the droplets due to their high electrical charge. On the other hand, the stability of the WPI–FG coated β-carotene emulsions can also be attributed to the strong steric repulsion between droplets resulting from the relatively thick FG layer adsorbed to their surfaces.33 Similar results were also reported by Sun et al.,34 who studied the effect of xanthan on the stability of WPI coated droplets, and they proved that xanthan at a concentration of 0.5 wt% showed little or no flocculation. In the remainder of this study, we prepared WPI β-carotene emulsion with an FG concentration of 0.1 wt% because the droplets had a relatively high zeta-potential and a lower creaming index, since the droplets appeared to be saturated with FG at this concentration. This indicated that the presence of 0.1 wt% FG improved physical stability by the proper combination of optimum electrostatic repulsion interaction, droplet size, interfacial adsorption and microrheological properties.
From Fig. 6b it can be seen that at lower concentration of FG (less than 0.1 wt%), β-carotene emulsions showed higher integral transmission and formed flocculated emulsion droplets. Although the mobility of β-carotene emulsion droplets was slowed down, the improved MVI and EI values of the β-carotene emulsion were not enough to improve its physical stability. This could be attributed to a resultant attractive interaction between the droplets. In these systems, repulsive electrostatic interaction would be smaller, mainly due to the greater charge neutralization between WPI and FG at the interfacial membrane. At higher concentrations of FG (more than 0.1 wt%), more FG was present in solution and increased the depletion flocculation, leading to an increase in the creaming rate. The decreased stability due to excess polysaccharide in solution has been reported before.35
LUMISizer was well suited to characterise the process of β-carotene emulsion physical stabilisation and the related effect of FG. Even more, it can be used for optimisation of the FG concentration. In the following the effect of FG was investigated for chemical stability (at fixed FG concentration of 0.1 wt%).
3.5 Chemical stability
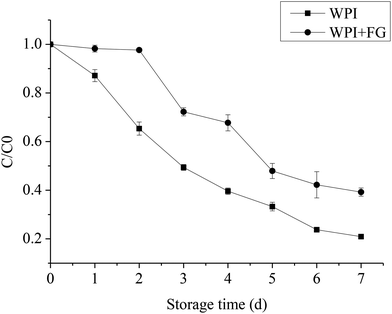
The chemical stability of the β-carotene emulsion was examined since the unsaturated pigment is known to be unstable to oxidation.2 In theory, the interfacial layer surrounding β-carotene droplets can be engineered to improve their chemical stability by controlling their composition, charge, thickness, etc. Therefore, the influence of 0.1 wt% FG (WPI versus WPI–FG) on the chemical stability of the β-carotene emulsion was examined (Fig. 7). Emulsions containing β-carotene droplets coated by WPI or WPI–FG were prepared and stored for 7 days at 55 °C. The droplet size and zeta-potential did not significantly change after storage (data not shown) indicating that the emulsions were physically stable. The β-carotene content was measured during the storage. There were clear differences in the chemical stability of the emulsions in the presence and absence of FG. In the WPI coated emulsion, a steep decrease in the β-carotene content was observed during the storage, with loss of 79.1 ± 0.4% after a storage of 7 days. In the presence of FG, there was a slight degradation of β-carotene during a 2 day storage, followed by a steep decrease after a 3 day storage, with a 60.7 ± 1.6% loss after a storage of 7 days. Thus, the β-carotene droplets surrounded by a WPI–FG membrane significantly increased the chemical stability of the β-carotene emulsions (p < 0.05).FG may improve the chemical stability of β-carotene emulsions through a number of different physicochemical mechanisms. Firstly, the increased EI, MVI and FI could be explained by the slowing down of the mobility of the β-carotene droplets, therefore reducing the interaction with the transition metal and free radical and reducing the β-carotene degradation rates.36 Secondly, the droplet size of emulsions could influence the β-carotene degradation rates; WPI–FG coated β-carotene droplets with a bigger droplet size, having a smaller surface area, would be expected to reduce the possibility of attracting transition metals. Thirdly, based on the charge analysis, an WPI–FG emulsion with negative charges might decrease the chemical stability of β-carotene compared with an WPI emulsion which is positively charged. Because the negative charge of WPI–FG emulsion may have the ability to attract transition metals, such as iron and copper. Therefore, the reverse result proved that the droplet charge did not play an important role in the chemical stability of β-carotene emulsion. Fourthly, the FG may improve the chemical stability of the emulsions through an increment in the thickness of the adsorbed FG layer which would inhibit the transition metal ions from reaching the core of the β-carotene droplets. Finally, the non-adsorbed FG may also prevent β-carotene from degradation due to their ability to chelate transition metals and free radicals at negatively charged sites, thereby preventing them from coming into close contact with the β-carotene. FG is also likely to have free radical scavenging activity like other polysaccharides due to its ability to donate hydrogen and probably act as a radical chain breaking antioxidant. Further work is needed to identify the other potential mechanisms.
4. Conclusions
Combining the multi techniques of DWS, dynamic light scattering, and centrifugal sedimentation, it was possible to fully understand the effect of FG on the microrheological properties and stability of WPI coated β-carotene emulsion. The results demonstrated that in an acidic environment, the microrheological properties and physical stability of WPI coated β-carotene emulsions were strongly affected by the concentration of FG. This suggested that the increased MVI did not depend on the effect of FG viscosity but on the interaction between the FG and the droplets. The apparent increase in the MVI and EI of the emulsions with lower concentrations of FG indicated that the bridging flocculation of FG had a much more appreciable influence on microrheological properties than depletion flocculation. It can be inferred that bridging flocculation could dominate and strengthen the droplet–droplet resistance interaction and aggregation, which resulted in dense network formation and spatial rearrangement of the β-carotene droplets. The SLB proved that the rheological behavior of β-carotene emulsions with addition of FG changed from a strongly liquid-like behavior to a slightly liquid-like behavior. It was evident that 0.1 wt% FG improved the physical stability by the appropriate combination of optimum electrostatic repulsion interaction, droplet size, interfacial adsorption and microrheological properties. Finally, it proved that β-carotene droplets surrounded by a WPI–FG membrane clearly increased the chemical stability of the emulsions mainly by slowing down the mobility of the β-carotene droplets. The results of this study provide practical information for using FG to protect bioactive compounds and improve nutritional profiles at acidic pH.Acknowledgements
This research was funded by the National Natural Science Foundation of China (31501486), Beijing Natural Science – SANYUAN jointly Foundation (15S00016), 13th Five-Year the State Key Development Program (2016YFD0400802), Cultivation of Excellent Talents in Beijing City (2014000020124G032), Development of Innovative Teams and Teachers’ Occupation Advancement Project of Beijing Municipal Universities and Colleges (IDHT20130506). The authors are also grateful to Yuwei He, Guangbin Chen and Mathias Fleury for the use of Rheometer LAB (Formulaction, France) and analysis of the microrheology.References
- S. Kiokias and M. H. Gordon, Food Rev. Int., 2004, 20, 99–121 CrossRef CAS.
- C. S. Boon, D. J. McClements, J. Weiss and E. A. Decker, Crit. Rev. Food Sci. Nutr., 2010, 50(6), 515–532 CrossRef CAS PubMed.
- L. Mao, J. Yang, D. Xu, F. Yuan and Y. Gao, J. Dispersion Sci. Technol., 2010, 31(7), 986–993 CrossRef CAS.
- Y. Liu, Z. Hou, F. Lei, Y. Chang and Y. Gao, Innovative Food Sci. Emerging Technol., 2012, 15, 86–95 CrossRef.
- R. Zhang, Z. Zhang, L. Zou, H. Xiao, G. Zhang, E. Decker and D. J. McClements, Food Funct., 2016, 7(1), 93–103 CAS.
- B. Chen, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2010, 58, 3779–3784 CrossRef CAS PubMed.
- E. Bouyer, G. Mekhloufi, V. Rosilio, J. Grossiord and F. Agnely, Int. J. Pharm., 2012, 436, 359–378 CrossRef CAS PubMed.
- E. Dickinson, Soft Matter, 2008a, 4, 932–942 Search PubMed.
- E. Dickinson, Food Hydrocolloids, 2008b, 23, 1473–1482 Search PubMed.
- M. J. Fabra, M. L. Flores-Lopez, M. A. Cerqueira, D. J. de Rodriguez, J. M. Lagaron and A. A. Vicente, Food Bioprocess Technol., 2016, 9(3), 471–480 CrossRef CAS.
- E. M. Shchukina and D. G. Shchukin, Curr. Opin. Colloid Interface Sci., 2012, 17, 281–289 CrossRef CAS.
- M. Klein, A. Aserin, I. Svitov and N. Garti, Colloids Surf., B, 2010, 77, 75–81 CrossRef CAS PubMed.
- A. S. L. Lim, Z. Burdikova, J. J. Sheehan and Y. H. Roos, Innovative Food Sci. Emerging Technol., 2016, 34, 310–319 CrossRef CAS.
- T. B. Tan, W. C. Chu, N. S. Yussof, F. Abas, H. Mirhosseini, Y. K. Cheah, I. A. Nehdi and C. P. Tan, Food Funct., 2016, 7(4), 2043–2051 CAS.
- S. A. Fioramonti, M. J. Martinez, A. M. R. Pilosof, A. C. Rubiolo and L. G. Santiago, Food Hydrocolloids, 2015, 43, 8–17 CrossRef CAS.
- D. J. McClements, Soft Matter, 2011, 7, 2297–2316 RSC.
- F. Liu, D. Wang, C. Sun and Y. Gao, Food Hydrocolloids, 2016, 52, 661–669 CrossRef CAS.
- E. Bouyer, G. Mekhloufi, I. L. Potier, T. Kerdaniel, J. Grossiord, V. Rosilio and F. Agnely, J. Colloid Interface Sci., 2011, 354, 467–477 CrossRef CAS PubMed.
- H. H. Chen, S. Y. Xu and Z. Wang, J. Food Eng., 2006, 77, 295–303 CrossRef CAS.
- S. Khalloufi, M. Corredig, H. D. Goff and M. Alexander, Food Hydrocolloids, 2009, 23, 611–618 CrossRef CAS.
- D. Xu, J. Zhang, Y. Cao, J. Wang and J. Xiao, LWT–Food Sci. Technol., 2016, 66, 590–597 CrossRef CAS.
- K. R. Kuhn, F. G. D. Silva and F. M. Netto, Food Res. Int., 2014, 59, 89–97 CrossRef.
- E. Sarmiento-Gomez, I. Santamaria-Holek and R. Castillo, J. Phys. Chem. B, 2014, 118, 1146–1158 CrossRef CAS PubMed.
- T. Sobisch and D. Lerche, Colloids Surf., A, 2008, 331, 114–118 CrossRef CAS.
- G. Petzold, C. Goltzsche, M. Mende, S. Schwzrz and W. Jaeger, J. Appl. Polym. Sci., 2009, 114, 696–704 CrossRef CAS.
- D. Xu, X. Wang, J. Jiang, F. Yuan and Y. Gao, Food Hydrocolloids, 2011, 25, 258–266 Search PubMed.
- F. Yuan, D. Xu, X. Qi, J. Zhao and Y. Gao, Food Bioprocess Technol., 2013, 6(4), 1024–1031 CrossRef CAS.
- Y. Yuan, Y. Gao, J. Zhao and L. Mao, Food Res. Int., 2008, 41, 61–68 CrossRef CAS.
- T. J. Wooster and M. A. Augustin, J. Colloid Interface Sci., 2006, 303, 564–572 CrossRef CAS PubMed.
- A. P. Batista, A. Raymundo, I. Sousa and J. Empis, Food Hydrocolloids, 2006, 20, 44–52 CrossRef CAS.
- M. Corredig and M. Alexander, Trends Food Sci. Technol., 2008, 19, 67–75 CrossRef CAS.
- T. Moschakis, B. S. Murray and E. Dickinson, Langmuir, 2006, 22, 4710–4719 CrossRef CAS PubMed.
- R. Charoen, A. Jangchud, K. Jangchud, T. Harnsilawat, O. Naivikul and D. J. McClements, J. Food Sci., 2011, 76(1), E165–E172 CrossRef CAS PubMed.
- C. Sun, S. Gunasekaran and M. P. Richards, Food Hydrocolloids, 2007, 21(4), 555–564 CrossRef CAS.
- E. T. Grotenhuis, M. Paques and G. Aken, J. Colloid Interface Sci., 2000, 227(2), 495–504 CrossRef PubMed.
- T. Waraho, D. J. McClements and E. A. Decker, Trends Food Sci. Technol., 2011, 22(1), 3–13 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |