Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Tracy L.
Lohr
a,
Zhi
Li
a,
Rajeev S.
Assary
b,
Larry A.
Curtiss
bc and
Tobin J.
Marks
*a
aDepartment of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208, USA. E-mail: t-marks@northwestern.edu
bMaterials Science Division, Argonne National Laboratory, Argonne, IL 60439, USA
cCenter for Nanoscale Materials, Argonne National Laboratory, Argonne, IL 60439, USA
First published on 22nd August 2017
Abstract
Correction for ‘Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism’ by Tracy L. Lohr et al., Energy Environ. Sci., 2016, 9, 550–564.
The authors wish to correct Fig. 4 and associated discussion on p. 555 of the manuscript. The authors discovered a small Excel error in the calculation of Nt. We have therefore replaced Fig. 4 with a new graph that plots % conversion after 1 h. This correction does not affect any other data or change any of the scientific conclusions in the article.
The paragraph on p. 555 should read as follows:
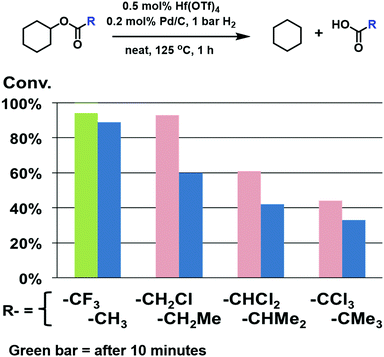
“Substituent effects on the acyl and alkoxy group were also investigated (Fig. 4 and 5). Sequentially replacing the methyl hydrogen atoms of cyclohexyl acetate with methyl groups (R = Me < Et < iPr < tBu; Fig. 4, blue columns) monotonically depresses the conversion after 1 h, under identical reaction conditions, from 89% (Me) to 33% (tBu). Note however that replacing an acyl H with a single electron-withdrawing chloro group increases the conversion to 93% (CH2Cl), while including a second chloro group (CHCl2) depresses the conversion to 61%. Fully chlorinating the methyl group (CCl3) further depresses the conversion to 44%. These results indicate that the most reactive acyl moieties are those with relatively unencumbered electron-withdrawing substituents. Furthermore, appending even more strongly electron-withdrawing substituents such as trifluoro (CF3) substantially increases the conversion relative to R = CH3. In sum, these results indicate that electron-withdrawing groups which stabilize the RCOO− negative charge most enhance the ester hydrogenolysis turnover frequency.”
Fig. 4 should appear as follows:
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2017 |