Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the zT value of thermoelectrics by nanostructuring: tuning the nanoparticle morphology of Sb2Te3 by using ionic liquids
Julian
Schaumann
ab,
Manuel
Loor
ab,
Derya
Ünal
a,
Anja
Mudring
 *ac,
Stefan
Heimann
b,
Ulrich
Hagemann
d,
Stephan
Schulz
*ac,
Stefan
Heimann
b,
Ulrich
Hagemann
d,
Stephan
Schulz
 *b,
Franziska
Maculewicz
e and
Gabi
Schierning
*ef
*b,
Franziska
Maculewicz
e and
Gabi
Schierning
*ef
aInorganic Chemistry III – Materials Synthesis and Characterization, Ruhr-Universität Bochum, DE-44801, Bochum, Germany. E-mail: mudring@iastate.edu
bFaculty of Chemistry and Center for NanoIntegration (CENIDE), University of Duisburg-Essen, DE-45117 Essen, Germany. E-mail: stephan.schulz@uni-due.de
cDepartment of Materials Science and Engineering, Iowa State University and Ames Laboratory, U.S. Department of Energy, Ames, IA 50011, USA
dInterdisciplinary Center for Analytics on the Nanoscale (ICAN), NETZ, University of Duisburg-Essen, Carl-Benz-Str. 199, 47047 Duisburg, Germany
eFaculty of Engineering and Center for NanoIntegration (CENIDE), University of Duisburg-Essen, Bismarckstr. 81, DE-47057 Duisburg, Germany
fInstitute for Metallic Materials, IFW Dresden, P.O. Box 270116, D-01171 Dresden, Germany. E-mail: g.schierning@ifw-dresden.de
First published on 22nd December 2016
Abstract
A systematic study on the microwave-assisted thermolysis of the single source precursor (Et2Sb)2Te (1) in different asymmetric 1-alkyl-3-methylimidazolium- and symmetric 1,3-dialkylimidazolium-based ionic liquids (ILs) reveals the distinctive role of both the anion and the cation in tuning the morphology and microstructure of the resulting Sb2Te3 nanoparticles as evidenced by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), and X-ray photoelectron spectroscopy (XPS). A comparison of the electrical and thermal conductivities as well as the Seebeck coefficient of the Sb2Te3 nanoparticles obtained from different ILs reveals the strong influence of the specific IL, from which C4mimI was identified as the best solvent, on the thermoelectric properties of as-prepared nanosized Sb2Te3. This work provides design guidelines for ILs, which allow the synthesis of nanostructured thermoelectrics with improved performances.
Introduction
Thermoelectric generators (TEG) directly convert heat fluxes into useable electrical energy and therefore provide a wear- and noiseless power source.1 The efficiency of a thermoelectric material is defined by the dimensionless figure of merit zT ((α2σ/κ)T), where α is the Seebeck coefficient, σ the specific electrical conductivity, κ the thermal conductivity as the sum of the electronic κel and the lattice κL contribution and T the absolute temperature in Kelvin. It is assumed that at least a zT ≅ 1.5 is necessary for most technical applications to become efficient and commercially viable.2 Unfortunately, the electrical and thermal transport coefficients are interrelated and cannot easily be optimized independently from each other. Metals naturally show high electrical and thermal conductivities, whereas both of these are small for insulators such as ceramics. The best choices of materials for technical applications in thermoelectric devices are semiconducting materials which contain heavy elements. This inherently minimizes the thermal conductivity due to a low speed of sound of such materials, while still a sufficiently high electronic conductivity is obtained. For technical applications near room temperature, Sb2Te3 and Bi2Te3 as well as their solid ternary solutions (SbxBi1−x)2Te3 are currently the most efficient materials due to their high electrical conductivities and high Seebeck coefficients combined with low thermal conductivities.3Sb2Te3 is a tetradymite-type layered material, which has been investigated for decades since it is a narrow band-gap (Egap 0.26 eV) semiconductor with good thermoelectric characteristics near room temperature.4 More recently, interest in Sb2Te3 increased due to its capability to serve as a topological insulator.5 Nanostructuring has been demonstrated theoretically and experimentally to greatly improve the figure of merit by effectively reducing the lattice contribution to the thermal conductivity6 while the electrical conductivity of the material is mostly unaffected. Different types of scattering centres for the heat carrying phonons such as nanoscale precipitates or grain boundaries and other interfaces have been employed for optimizing thermoelectric materials this way.7 Even a hierarchical design of the nano- and microstructure was developed to effectively scatter the broad spectrum of phonon wavelengths, which led to record-high zT values.8
Our general interest in thermoelectric materials prompted us to investigate the synthesis of binary (Sb2Te3, Bi2Te3) and ternary ([SbxBi1−x]2Te3) materials both in solution9 and via gas phase based processes such as atomic layer deposition (ALD)10 and metal organic chemical vapour deposition (MOCVD)11 using single-source and dual-source precursor approaches. The microwave-assisted decomposition of the single source precursor (Et2Sb)2Te 1 in an ionic liquid (IL) had been shown to produce highly stoichiometric Sb2Te3 nanoparticles,12a while Bi2Se3, Bi2Te3 and (SbxBi1−x)2Te3 nanoparticles were synthesized by using specific reactive ILs.12b,c The Sb2Te3 nanoparticles showed exceptionally high figures of merit of up to 1.5 at 300 °C, without the need of alloying or electronic doping. This new synthetic strategy allowed an effective decoupling of electronic and phononic transport properties.12a In our studies we made the observation that the Sb2Te3 particle morphology changed depending on the chemical identity of the ionic liquids, which prompted us to study their influence on the microwave-assisted decomposition of 1 in more detail and look for correlations with the thermal and electronic transport properties in the obtained material.
We herein report on our systematic study on the decomposition of 1 in different ILs, in which both the anion and the cation were systematically varied, using microwave-assisted techniques. In addition, the results from detailed transport measurement of the resulting Sb2Te3 nanoparticles are reported that allow for a structure–property analysis.
Results and discussion
We have recently developed a synthetic protocol that enabled us to access Sb2Te3 nanomaterials with a record-figure of merit by the decomposition of 1 in the ionic liquid C4mimBr (C4mim = 1-butyl-3-methylimidazolium) under microwave (MW) irradiation.12a As the IL acted in this reaction not only as the solvent but also as the heat transfer medium, we herein study the specific role of the IL as the nanotemplating agent by investigating a set of ILs based on 1,3-dialkylimidazolium cations. Starting from the most prominent IL cation, 1-butyl-3-methylimidazolium (C4mim+), first the counter anion was varied from Cl−, Br−, I− to NTf2− (NTf2− = bis(trifluoromethanesulfonyl)amide). Variation of the IL anion not only leads to a change in fundamental physical properties of the IL such as the melting point or viscosity but also its solvation properties such as polarity. Moreover, the chosen anions range from relatively strongly coordinating (Cl−) to quite weakly coordinating (NTf2−) anions. In the context of nanomaterial synthesis the capabilities of the IL ions to interact with the as-formed nuclei and crystal seeds is especially important as this allows for the morphology13 and even the phase control14 of nanomaterials. The Lewis basicity of these ILs decreased in the order of Cl−, Br−, I− to NTf2−.15 Similarly, variations of the cation influence the overall IL properties. Generally an increase of the melting point with increasing chain length of the alkyl group is observed for imidazolium based ILs. Symmetrically substituted imidazolium ILs typically exhibit higher melting points than asymmetrical ILs.16 Again, in the context of tuning the nanostructure of a material through the templating effect of the IL, the interaction of the IL cation with the nanomaterial needs to be considered. Imidazolium cations can interact not only electrostatically, but, as they bear acidic protons (the 2H proton of the imidazolium ring is especially acidic) and an aromatic π-system, can also undergo secondary bonding interactions such as hydrogen bonding and π-bonding. This has been found especially important in the synthesis of nanosized oxide materials.17 However, the cation size can critically influence these bonding capabilities.18 For this reason, the alkyl-chain of the C4 × C1mim+ imidazolium cation was varied from three to eight carbon atoms.In addition to the set of 1-methyl-n-alkylimidazolium bromides, the corresponding set of symmetrically substituted cations (CnCnmim+) with n = 4, 6 and 8 were explored. Ionic liquids are known to be highly structured solvents,19 which can impact nanoparticle formation critically.20 In particular, for imidazolium cations with longer alkyl chains a highly ordered structure of the IL can be expected,21i.e. imidazolium-based ILs with more than eleven carbon atoms in the side chain tend to form thermotropic liquid crystalline phases. The use of ordered phases as the template in nanoparticle synthesis has already been reported.22
To obtain Sb2Te3 nanoparticles from various ionic liquids, in a typical reaction, 1 was added to the respective IL at 90 °C and stirred for 5 min until a homogeneous dispersion or solution was formed, which was then heated in a laboratory microwave oven first for 30 s at 100 °C, then for 5 s at 150 °C and finally for 5 min at 170 °C. The resulting colloidal solution was centrifuged (2000 rpm), washed with 10 mL of acetonitrile (7×) to completely remove the by-product SbEt3 (Scheme 1) and dried at ambient temperature under reduced pressure. Black precipitates were obtained, which were characterized by powder X-ray diffraction (PXRD), energy dispersive X-ray analysis (EDX), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS).
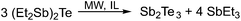 | ||
| Scheme 1 Synthesis of Sb2Te3 nanoparticles by microwave-assisted decomposition of the single source precursor 1. | ||
General product characterization
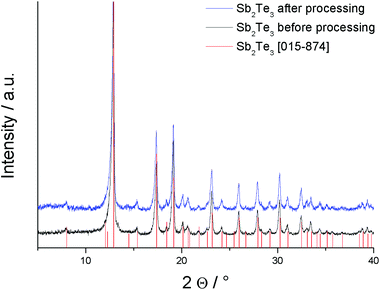
PXRD measurements confirmed the formation of phase-pure Sb2Te3 in all ILs (see Fig. 1 for a representative PXRD pattern). All observed diffraction peaks can be indexed to the database pattern of Sb2Te3 (JCPS file 015874) and the lattice parameters were refined to a = 4.266(9) Å and c = 30.456(6) Å.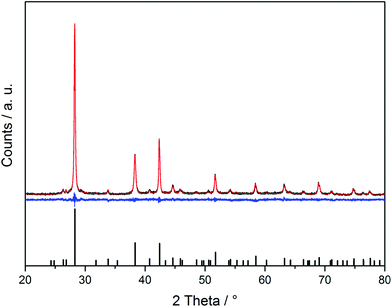 | ||
| Fig. 1 Representative powder X-ray diffraction pattern of Sb2Te3 nanoparticles (with Cu Kα radiation) including Rietveld refinements. | ||
A small texture effect was observed since the intensity of the 1010 reflex was somewhat smaller compared to the reference. Our samples show an intensity ratio of the 015 (28.3°)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1010 (38.5°)
1010 (38.5°)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 (42.5°) reflex of 1
110 (42.5°) reflex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26
0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35, whereas this ratio in the reference was 1
0.35, whereas this ratio in the reference was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35
0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33. A size determination of the nanoparticles typically yielded sizes of >300 nm, but these values should be taken with care due to their plate-like structure (see Fig. 4 and 5).
0.33. A size determination of the nanoparticles typically yielded sizes of >300 nm, but these values should be taken with care due to their plate-like structure (see Fig. 4 and 5).
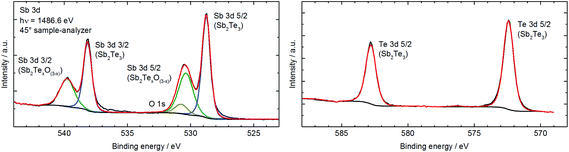
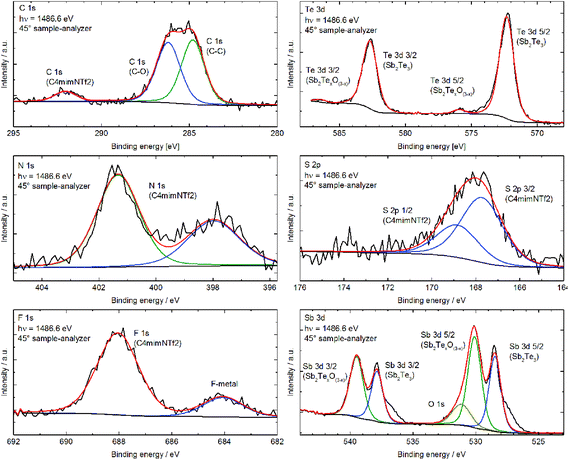
EDX analysis confirmed within standard deviations the stoichiometric composition of the products. In addition, no signals originating from the IL or contaminations, i.e. oxidation or hydrolysis products, were detected. These results were confirmed by infrared (IR) spectroscopy, showing no absorption band of the respective ILs on the particle surface. In contrast, the nanoparticles were shown to be partially oxidized at the surface by XPS, which is a much more surface sensitive analytical method compared to EDX and IR. Fig. 2 exemplarily shows the XPS spectra of Sb2Te3 nanoparticles prepared in C4mimI, while Fig. 3 displays XPS spectra of a sample obtained in C4mimNTf2.
The XPS spectra of the sample prepared in C4mimI (Fig. 2) and C4mimNTf2 (Te and Sb spectra at the top right and bottom right in Fig. 3) clearly showed that both Sb and Te are partially oxidized, as is clearly visible from the metal oxide peaks at 530.1 eV binding energy for the Sb 3d5/2 and at 575.9 eV for the Te 3d5/2 peaks. These findings are in good agreement with the literature values.12b,c,23,24 However, while only around 3% of the Te are present as an oxide in the case of Sb roughly 40% (prepared in C4mimI) to 60% (prepared in C4mimNTf2) of the Sb is oxidized. The ratio of elemental Sb to elemental Te gives exactly the expected ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. This means that there is an excess of Sb at the surface and that this Sb is present as an oxide. Comparable surface oxidation reactions have been very recently observed for binary and ternary bismuth chalcogenide nanoparticles, in which Bi2Te3 and Bi2Te2Se were found to easily oxidize upon exposure to air while Bi2Se3 was significantly more stable toward oxidation.12b,c,31 In addition, Sb2Te3 thin films were found to be easily oxidized after exposure to atmosphere and a post-deposition treatment was therefore suggested by the authors as an effective method to promote the formation of the Sb–Te bond and prevent oxidation of the thin film surface. As a consequence, the nanoparticles have to be stored and handled under inert gas conditions to avoid surface oxidation reactions. In addition, N, S, F, C and O (Fig. 3) are also found on the surface, which can be attributed to the residues of the ionic liquid (C4mimNTf2) and the washing solvent (CH3CN), which can also coordinate to the nanoparticle surface.
3. This means that there is an excess of Sb at the surface and that this Sb is present as an oxide. Comparable surface oxidation reactions have been very recently observed for binary and ternary bismuth chalcogenide nanoparticles, in which Bi2Te3 and Bi2Te2Se were found to easily oxidize upon exposure to air while Bi2Se3 was significantly more stable toward oxidation.12b,c,31 In addition, Sb2Te3 thin films were found to be easily oxidized after exposure to atmosphere and a post-deposition treatment was therefore suggested by the authors as an effective method to promote the formation of the Sb–Te bond and prevent oxidation of the thin film surface. As a consequence, the nanoparticles have to be stored and handled under inert gas conditions to avoid surface oxidation reactions. In addition, N, S, F, C and O (Fig. 3) are also found on the surface, which can be attributed to the residues of the ionic liquid (C4mimNTf2) and the washing solvent (CH3CN), which can also coordinate to the nanoparticle surface.
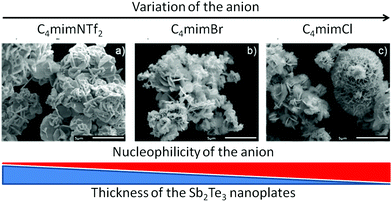
Role of the anion (An) of 1-alkyl-3-methyl-imidazolium based ionic liquids C4mimAn. The role of the anion (Cl−, Br−, I−, NTf2−) of the 1-butyl-3-methyl-imidazolium based ionic liquid in tuning the composition and the morphology of the resulting nanoparticles was investigated by SEM. All four samples show the formation of hexagonally shaped Sb2Te3 nanoplates with diameters ranging between 300–2000 nm and varying in thickness between 65–120 nm (Fig. 4).
 | ||
| Fig. 4 SEM images of Sb2Te3 nanoparticles synthesized in various ionic liquids. The nanoparticles in (A) were synthesized in C4mimCl, (B) in C4mimBr, (C) in C4mimI and (D) in C4mimNTf2. | ||
These platelets form larger agglomerates. Both the dimensions of the nanoplates and the type of agglomeration are strongly influenced by the IL anion. The thickness of the individual hexagonal platelets increased while changing the IL anion from Cl−, Br−, I− to NTf2−. Also, the association of these platelets changed from individual sandrose-type spherical aggregates over more aggregated spheres of platelets to less spherical, less extended aggregates. This observation could be correlated with the coordination ability of the IL anion. Chloride is a strongly Lewis basic, coordinating anion whereas the NTf2 anion has a weak coordination ability. Thus, ionic liquids with rather strongly coordinating anions force the formation of thinner platelets as the vertical particle growth is hindered through the interaction of the IL anion with the particle surface. An IL with a less coordinating anion not only hinders the particle growth less, resulting in the formation of thicker platelets, but also stabilizes the particles less against further agglomeration and, in consequence, larger agglomerates are found in C4mimNTf2.
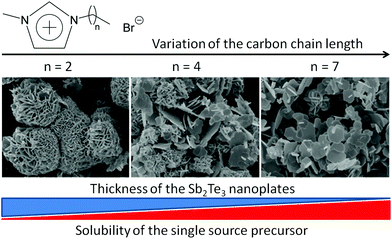
Influence of the alkyl chain length of asymmetrical 1-n-alkyl-3-methylimidazolium bromide ionic liquids CnmimBr (n = 3–8). To investigate the influence of the IL cation on the morphology of the Sb2Te3 nanoparticles, a set of 1-n-alkyl-3-methylimidazolium bromides was synthesized and explored as the reaction medium in the synthesis of Sb2Te3 nanoparticles through a microwave reaction.
The chain length of the 1-n-alkyl-3-methylimidazolium cation was systematically varied from three to eight carbon atoms. Bromide was chosen in these experiments as the anion in order to be comparable with the results of previous studies.12a In C3mimBr exclusively isolated spherical aggregates of small platelets with diameters of 2–5 μm were formed. By increasing the side chain length of the alkyl group of the 1-n-alkyl-3-methylimidazolium cation, the size and number of these aggregates shrink. At the same time individual larger hexagonal plates of Sb2Te3 are formed, which have a smaller tendency to aggregate. When C8mimBr is used in the synthesis, almost exclusively hexagonal plates are observed (Fig. 5).
 | ||
| Fig. 5 SEM images of Sb2Te3 nanoparticles synthesized in 1-n-alkyl-3-methylimidazolium bromide CnmimBr with (a) n = 3, (b) n = 4, (c) n = 5, (d) n = 6, (e) n = 7, and (f) n = 8. | ||
It is obvious that the IL cation has a strong influence on the nanostructure of the obtained material and two factors appear to be important: solubility of the precursor in the IL and structural order of the IL. The solubility of the precursor increases with increasing alkyl-chain length of the cation, which can be correlated to the decreasing polarity of the IL. Whilst in ILs with short alkyl chains such as C3mimBr and C4mimBr only dispersions of (Et2Sb)2Te in the IL were obtained, a full solubility of the precursor was observed for C8mimBr. As a consequence, the tendency of the formation of inhomogeneously distributed micro-drops of 1 in the IL increases with decreasing alkyl chain length of the IL, which obviously facilitates the formation of ball-like agglomerates upon thermolysis. In contrast, thermolysis of a homogeneous solution of 1 in the IL containing long alkyl chains leads to a steady growth of the Sb2Te3 nanoparticles, which consequently form large sheets. In addition, it is known for 1-alkyl-3-methylimidazolium bromides that an increasing alkyl chain length of the cation leads to an increasing structural order, which may lead to the formation of lamellar, smectic liquid crystalline structures which could act as a template.21b,c Therefore, a set of symmetrically substituted 1-n-alkyl-3-n-alkylimidazolim bromides was tested as the reaction medium.
Influence of the alkyl chain length of symmetrical 1,3-n-alkylimidazolium bromide ionic liquids CnCnimBr. The synthesis of Sb2Te3 from 1 in CnCnimBr with n = 4, 6 and 8 yielded in all cases a phase pure material. However, while carrying out the synthesis in C4C4imBr, only a dispersion of the single source precursor was obtained, whilst in C6C6imBr and C8C8imBr homogeneous solutions were formed (Fig. 6). The nanostructures of the material obtained from the different ILs show distinct differences. The trend in the change of the morphology, however, is similar to the observations made for asymmetrical imidazolium bromides.
The nanoparticles synthesized in C4C4imBr (Fig. 7A) consist of strongly agglomerated Sb2Te3 nanoplates. Predominately sandrose-like structures with sizes between 1 and 4 μm are formed by the aggregation of individual Sb2Te3 particles, whose diameters range from 300 to 1200 nm. The diameter of the individual Sb2Te3 platelets was found to increase with increasing alkyl-chain lengths of the IL cation. Individual nanoplates with diameters between 300 and 1500 nm were found in C6C6imBr (Fig. 7B), while those obtained from C8C8imBr (Fig. 7C) range from 300 to 2500 nm. In addition, the SEM images of the resulting nanoparticles clearly prove a decreasing agglomeration tendency of the hexagonal Sb2Te3 nanoplates with increasing chain length and hence increasing steric demand and coordination strength of the IL as were observed for the Sb2Te3 nanoparticles obtained from unsymmetrical ILs (see Fig. 5). While compact ball-like agglomerates were formed with C4C4imBr, the nanoparticles obtained in C6C6imBr show loosely agglomerated card structures, and nanoparticles synthesized in C8C8imBr consist of single Sb2Te3 sheets and to some extent slightly crooked card structures (Fig. 7). With increasing alkyl chain length of the cation, the tendency of the formation of sandrose-like structures decreases. Instead, 3D agglomeration increases until finally in C8C8imBr predominately large extended plates are formed. This confirms that an interplay of the precursor solubility and microstructure and the coordination ability of the IL strongly influence the microstructure formation.
 | ||
| Fig. 7 SEM images of Sb2Te3 nanoparticles synthesized in C4C4imBr (A), C6C6imBr (B) and C8C8imBr (C). | ||
Whenever the single source precursor 1 has poor solubility in the IL, sandrose-like aggregates are formed. This potentially occurs due to the formation of micro-droplets, which can act as individual micro-reaction compartments. In contrast, thermolysis of homogeneously dissolved solutions of 1 in ILs of higher hydrophobicity, which increases with increasing alkyl chain length, leads to a steady growth of the Sb2Te3 nanoparticles. Finally the microstructure of the IL can help to guide the particle growth. C8C8imBr prefers the formation of a lamellar structure and thus favours the sheet-like growth of Sb2Te3 nanoplates.
Variation of the different alkyl-chain lengths of symmetric imidazolium-based ILs CnCnimBr. Since the influence of the alkyl chain lengths was observed for both the unsymmetrically and symmetrically substituted imidazolium derivatives, detailed transport characterization was performed with the nanoparticles obtained from the symmetrically substituted ILs.
Fig. 8 shows the cross-section SEM images of the three pellets as-obtained from samples synthesized in C4C4imBr (C4) (Fig. 8A), C6C6imBr (C6) (Fig. 8B) and C8C8imBr (C8) (Fig. 8C), respectively. Distinct differences between the characteristic microstructure of the three samples after the cold pressing compaction can be seen, which can be directly correlated to the morphology of the Sb2Te3 nanoparticles from the IL.
In C4C4imBr the formation of sandroses (Fig. 7A) prevailed and this microstructure is maintained in the cold pressed samples where individual spheres can be made out (Fig. 9A). In C6C6imBr random three dimensional aggregations of these particles occurred (Fig. 7B) and this also shows in the compacted sample (Fig. 9B). In C8C8imBr the formation of large, extended nanosheets took place (Fig. 7C) and the SEM image of the cross section pellets shows still individual sheets that are stacked parallel (Fig. 9C). The microstructure evoked by the individual particle morphology and aggregation impacts directly the densities of the compacted samples. The density of the samples is 5.3 g cm−3 (82%) for C4C4imBr, 5.7 g cm−3 (86%) for C6C6imBr, and 4.9 g cm−3 (75%) for C8C8imBr, respectively.
Fig. 10 shows the thermoelectric transport properties of the three samples between room temperature and 573 K. Table 1 summarizes the thermoelectric transport data of these three pellets at room temperature. The Seebeck coefficients range from 140 μV K−1 to 180 μV K−1. The decomposition of 1 was shown to produce Sb2Te3 nanoparticles with a highly stoichiometric composition and low anti-site defect concentration, resulting in high values of the Seebeck coefficient as observed in our previous study.12a This is observed here, too.
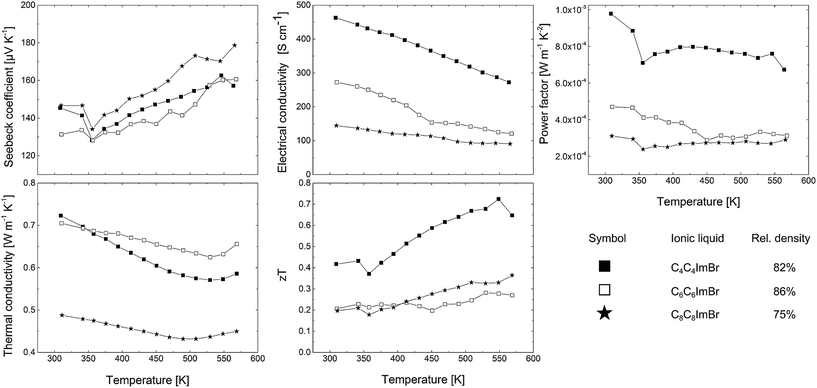 | ||
| Fig. 10 Thermoelectric transport properties of three Sb2Te3 bulk samples synthesized in C4C4imBr, C6C6imBr and C8C8imBr. | ||
| Sample | C4 | C6 | C8 |
|---|---|---|---|
| Ionic liquid | C4C4imBr | C6C6imBr | C8C8imBr |
| Density [g cm−3] | 5.3 | 5.7 | 4.9 |
| Relative density [%] | 82 | 86 | 75 |
| n H [×1019 cm−3] – overall | 5.5 | 4.9 | 3.1 |
| μ H [cm2 V−1 s−1] – overall | 53 | 35 | 29 |
| – In electrically active volume | 64 | 41 | 39 |
| σ [S cm −1] | 463 | 272 | 145 |
| κ [W m−1 K−1] | 0.72 | 0.71 | 0.49 |
| κ L [W m−1 K−1] | 0.44 | 0.59 | 0.39 |
| α [μV K−1] | 145 | 131 | 147 |
The Hall carrier concentrations nH are 5.5 × 1019 cm−3 (C4C4imBr), 4.9 × 1019 cm−3 (C6C6imBr) and 3.1 × 1019 cm−3 (C8C8imBr). The samples show a high variation in the electrical conductivity σ. The highest σ of 463 S cm−1 at room temperature was observed for the sample synthesized in C4C4imBr and is smaller for the samples prepared in C6C6imBr (272 S cm−1) and C8C8imBr (145 S cm−1).
From the electrical conductivity and the Hall carrier concentration, we obtained the Hall mobility of the charge carriers, μH, which was corrected for the electrically active volume of the material (Fig. 11).
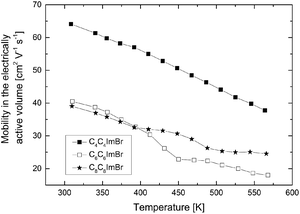 | ||
| Fig. 11 Mobility in the electrically active volume plotted as a function of temperature for samples obtained from the respective ionic liquids. | ||
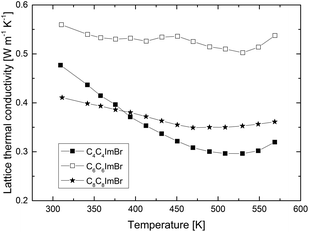
For this, the value was normalized to the relative density of the samples.25 With this correction for the density, the Hall mobilities μH of 64 cm2 V−1 s−1 (C4C4imBr), 41 cm2 V−1 s−1 (C6C6imBr) and 39 cm2 V−1 s−1 (C8C8imBr) were found. There is no evident trend of the Hall mobility and the electrical conductivity with respect to the varying densities of the three samples, instead the mobility decreases with increasing chain length. Due to the nanostructure of the samples, the thermal conductivity could be reduced from 5.6 W m−1 K−1 parallel ∥ and 1.6 W m−1 K−1 ⊥ perpendicular to the c-direction26 for a single crystalline Sb2Te3 in the range of 0.49–0.72 W m−1 K−1, comparable with values previously reported by Mehta et al. for Sb2Te3 nanoparticles.27 At 490 K the thermal conductivity exhibits a minimum in all samples and increases at higher temperatures. To calculate the lattice thermal conductivity, we subtracted the electronic contribution κe from the total thermal conductivity, estimated from the Wiedemann–Franz dependence, κe = σLT. For this, the literature value of the Lorenz number (L = 1.72 × 10−8 W Ω K−2 Single Parabolic Band Model)28 was used considering a temperature independent L.
Fig. 12 clearly shows that the lattice thermal conductivity still increases. This is most likely caused by the bipolar effect known to appear in this temperature range for semiconductors with a small band gap (Sb2Te3: band gap Eg = 0.28 eV29): at a certain temperature electron–hole-pairs are generated and an additional contribution for the thermal conductivity κ from the bipolar thermal conductivity κb is given. While the thermal conductivity data points towards a contribution of the bipolar effect, in principal this effect should also influence the other transport coefficients, i.e. decrease the Seebeck coefficient and increase the electrical conductivity due to minority carriers, which is not seen here.
The most promising property combination of the transport properties is found for samples synthesized in C4C4imBr, which exhibited the highest charge carrier concentration, the highest charge carrier mobility and the lowest lattice thermal conductivity. The figure of merit zT reaches a maximum value of 0.72 at 550 K for the C4C4imBr sample (Fig. 10).
From this we conclude that the formation of individual sandrose nanostructures of Sb2Te3 that can be maintained in the compacted samples, gives the best combination of properties leading to high zT values. Thus, short chain length IL cations are beneficial for this. To check this hypothesis, the thermoelectric transport properties of samples obtained from ILs with short chain imidazolium cations (C4mim) in combination with various anions that gave sandrose-like nanostructures are investigated.
Role of the anion of 1-butyl-3-methyl-imidazolium based ILs in thermoelectric properties. In order to investigate the role of the anion in the thermoelectric properties of the resulting Sb2Te3 nanoparticles in more detail, four Sb2Te3 samples were prepared under analogous reaction conditions in C4mimCl (A), C4mimBr (B), C4mimI (C) and C4mimNTf2 (D), respectively, and then compacted to Sb2Te3 pellets using the same protocol. Cross section pictures (Fig. 13) of the resulting cold pressed pellets clearly demonstrate that the agglomerate structure as observed in the SEM pictures of the nanoparticles is preserved within the microstructure of the pellets (Fig. 13A–D). Sb2Te3 synthesized in C4mimNTf2 shows only a few agglomerates in the microstructure (Fig. 13D), whilst for those observed from C4mimCl shows that those rose-like structures are still preserved.
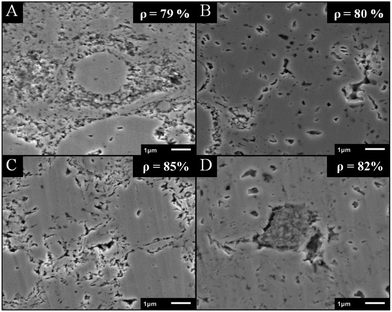 | ||
| Fig. 13 Cross section SEM images of four cold pressed Sb2Te3 bulk samples synthesized in C4mimCl (A), C4mimBr (B), C4mimI (C) and C4mimNTf2 (D). | ||
Table 2 summarizes the thermoelectric transport properties for the four samples synthesized in C4mimCl, C4mimBr, C4mimI and C4mimNTf2 at 300 K. The density of the compressed pellets are 5.1 g cm−3 (79%, C4mimCl), 5.2 g cm−3 (80%, C4mimBr), 5.5 g cm−3 (85%, C4mimI) and 5.3 g cm−3 (82%, C4mimNTf2), respectively.
| Sample | Cl | Br | I | NTf2 |
|---|---|---|---|---|
| Ionic liquid | C4mimCl | C4mimBr | C4mimI | C4mimNTf2 |
| Density [g cm−3] | 5.1 | 5.2 | 5.5 | 5.3 |
| Relative density [%] | 79 | 80 | 85 | 82 |
| n H [×1019 cm−3] – overall | 9.7 | 3.8 | 5.7 | 7.1 |
| μ H [cm2 V−1 s−1] – overall | 19 | 43 | 95 | 35 |
| – In electrically active volume | 24 | 54 | 112 | 42 |
| σ [S cm−1] | 291 | 264 | 869 | 393 |
| κ [W m−1 K−1] | 0.56 | 0.72 | 0.89 | 1.1 |
| α [μV K−1] | 150 | 133 | 135 | 150 |
In Fig. 14 the thermoelectric transport properties are presented. The Seebeck coefficient for all samples ranges from 130 to 170 μV K−1 in the temperature range between room temperature and 573 K, which is comparable to the values of the samples discussed before. The highest electrical conductivity of 870 S cm−1 at room temperature was found for the sample synthesized in C4mimI, whereas that prepared in C4mimNTf2 (397 S cm−1), C4mimCl (293 S cm−1) and C4mimBr (264 S cm−1) showed significantly lower values. The thermal conductivity is 1.1 W m−1 K−1 for the sample obtained from C4mimNTf2, 0.89 W m−1 K−1 for that from C4mimI, 0.72 W m−1 K−1 for that from C4mimBr and 0.56 W m−1 K−1 for that from C4mimCl. The electrical and thermal conductivities show a dependence on the density of the samples. The highest values for σ and κ are measured for the samples with densities of 85% (C4mimI) and 82% (C4mimNTf2) and are smaller for the Sb2Te3 pellets with 80% (C4mimBr) and 79% (C4mimCl). The highest zT value of 0.93 at 260 °C is reached for C4mimI, and for the other samples the zT values are between 0.35 (C4mimBr) and 0.44 (C4mimNTf2, C4mimCl).
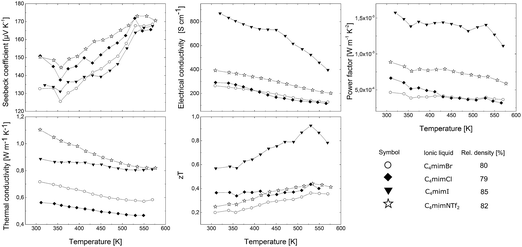 | ||
| Fig. 14 Thermoelectric transport properties of four Sb2Te3 bulk samples synthesized in C4mimBr, C4mimCl, C4mimI, and C4mimNTf2. | ||
By correlating the thermal transport data with the particle morphologies it is evident that the concept of controlling the thermal conductivity through phonon phase boundary scattering by a nanotemplating effect of the IL has been successful: in the case where small, individual nano-sandroses could be obtained by using an ionic liquid of high polarity with a strongly coordinating anion, the compacted material exhibited the lowest thermal conductivity.
The electrical Hall mobility shows a clear trend for the samples synthesized in the ionic liquids C4mimCl, C4mimBr, and C4mimI, with increasing μH from 24 cm2 V−1 s−1 to 112 cm2 V−1 s−1. This correlates with the trend found in the morphology of the respective nanoparticles which show an increasing thickness of the nanoparticle platelets with increasing atomic number of the halide anion (compare Fig. 4). It is assumed that the nanoparticle platelets orient – at least partly – during the compaction process perpendicular to the pressing direction. All transport properties are characterized in the pressing direction of the pellets. Therefore, with increasing thickness of the platelets, there are less scattering events for both, electrons and phonons, and consequently the highest values for the electrical Hall mobility and also the thermal conductivity are reached. However, looking at the ionic radii of the IL anions used for the synthesis, it becomes clear that the ionic radius of an I− ion (220 pm) is very similar to that of a Te2− ion (221 pm).30 Thus, it appears possible that small amounts of I− can replace Te2− in the structure of Sb2Te3, which could also influence the electronic transport properties. More theoretical and experimental evidence will be needed to further substantiate this hypothesis.
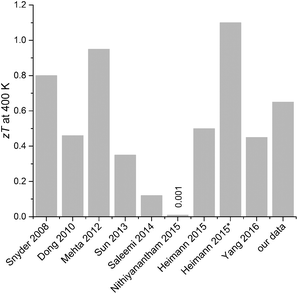
Comparison of zT values. Within the following paragraph we compare our results with the literature state of the art. Table 3 shows the zT of nanostructured Sb2Te3 samples for different synthesis routes.
| Synthesis method | zT (300 K) | zT (400 K) | Ref. |
|---|---|---|---|
| a This paper. b EG = ethylene glycol. c SSP = single source precursor. The marked values (*) were interpolated from the literature data for 300 K for comparison. | |||
| Thermal decomposition of SSP in the IL C4mimI | 0.59 | 0.65 | |
| Commercial alloys, mostly based on Sb2Te3 | 1 | 0.8 | Snyder (2008)31 |
| Solvothermal (MW-assisted); SbCl3 + Na2TeO3 + N2H4 hydrate in EGb | 0.15 | 0.46 | Dong (2010)32 |
| S-doped Sb2Te3 NPs | 0.7 | 0.92 | Mehta (2012)27 |
| Solvothermal, template-free | 0.22* | 0.35 | Sun (2013)33 |
| Co-precipitation followed by wet Chemical reduction | 0.03* | 0.12 | Saleemi (2014)34 |
| Thermal decomposition of SSPc in IL | Heimann (2015)12a | ||
| (a) Thermal (oil bad) | 0.4 | 0.5 | |
| (b) MW-assisted | 0.75 | 1.1 | |
| Solvothermal; SbCl3 + Te powder + alkaline 2,7-DHN in the presence of CTAB | 0.001 | Nithiyanantham (2015)35 | |
| Physical vapor deposition (PVD); Sb2Te3−xSx platelets | Thankamma (2015)36 | ||
| -Without doping x = 0 | 0.22 | ||
| -With the S-content x = 0.3 | 0.54 | ||
| Solvothermal; Sb2Te3 wires; Sb + Te in EG | 0.32* | 0.44 | Yang (2016)37 |
Within this comparison, Snyder and Toberer31 report the zT data by Marlow Industries that reach zT ≅ 0.8 at 400 K for Sb2Te3-based alloys (not further specified). By co-doping Sb2Te3 with sulphur, Mehta et al. demonstrate zT ≅ 0.92 at 400 K.27 Phase pure Sb2Te3, without any alloying or co-coping, was investigated by Heimann et al. within an earlier work of this group.12a Hereby, the microwave-assisted decomposition of the SSP (Et2Sb)2Te in ionic liquids enhanced the zT value from zT ≅ 0.5 to zT ≅ 1.1 at 400 K. Within this work we systematically studied the influence of different ILs and report zT values that match this range of literature values, see graphical compilation in Fig. 15.
Conclusions
The morphology of Sb2Te3 nanoparticles synthesized in 1-alkyl-3-methylimidazolium- and 1,3-dialkylimidazolium-based ILs strongly depends on the chain length of the alkyl group of the IL cation (Fig. 16) and the Lewis basicity of the IL anion (Fig. 17).An increasing chain length resulted in better solubility of the single source precursor (Et2Sb)2Te, which enhanced the formation of less aggregated nanoparticles. In addition, the role of the anion is mainly attributed to its basicity and its capability to bind to the growing nanoparticle surface. Stronger bases were found to more effectively block the surface, resulting in the formation of thin Sb2Te3 nanoplates, while the formation of thicker nanoparticles was observed with decreasing basicity. As a consequence, the thermoelectric properties of the resulting Sb2Te3 nanoplates strongly differed. Identification of the distinctive roles of the IL anion and cation may help to further improve the figure of merit for these types of materials in the near future.
Experimental
Synthetic procedures
Synthetic procedures and thermolysis experiments were performed under inert conditions (Ar atmosphere) in a glovebox or using standard Schlenk techniques. Solvents were dried over Na/K alloy and degassed prior to use. 1 was prepared according to a literature method.38 1-N-Methylimidazole (99%, Sigma Aldrich), 1-halobutanes (99%, Acros) and CH3CN (99.9+%, Extra Dry, Acros) were commercially available. 1-Alkyl-3-methylimidazolium (Cnmim; n = 3–8) and 1,3-dialkyl-imidazolium-based ILs (CnCnim; n = 4, 6 and 8) were prepared by literature methods.12,39Materials and methods
Material characterization
Acknowledgements
S. Schulz and G. Schierning gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft DFG within the priority program SPP 1708. The authors like to thank M. Sc. Georg Bendt (Faculty of Chemistry, University of Duisburg-Essen) for Rietveld refinements of selected samples.Notes and references
- M. H. Elsheikh, D. A. Shnawah, M. F. M. Sabri, S. B. M. Said, M. H. Hassan, M. B. A. Bashir and M. Mohamad, Renewable Sustainable Energy Rev., 2014, 30, 337 CrossRef.
- L. E. Bell, Science, 2008, 321, 1457 CrossRef CAS PubMed.
- (a) D. M. Rowe, CRC Handbook of Thermoelectrics, CRC Press, Boca Raton, FL, 1995 Search PubMed; (b) J. R. Drabble and C. H. L. Goodman, J. Phys. Chem. Solids, 1958, 5, 142 CrossRef CAS.
- (a) R. Venkatasubramanian, E. Siivola, T. Colpitts and B. O'Quinn, Nature, 2001, 413, 597 CrossRef CAS PubMed; (b) T. C. Harman, P. J. Taylor, M. P. Walsh and B. E. LaForge, Science, 2002, 297, 2229 CrossRef CAS PubMed; (c) D. Hsieh, Y. Xia, D. Qian, L. Wray, F. Meier, J. H. Dil, J. Osterwalder, L. Patthey, A. V. Fedorov, H. Lin, A. Bansil, D. Grauer, Y. S. Hor, R. J. Cava and M. Z. Hasan, Phys. Rev. Lett., 2009, 103, 146401 CrossRef CAS PubMed.
- (a) H. Zhang, C.-X. Liu, X.-L. Qi, X. Dai, Z. Fang and S.-C. Zhang, Nat. Phys., 2009, 5, 438 CrossRef CAS; (b) L. He, X. Kou and K. L. Wang, Phys. Status Solidi RRL, 2013, 7, 50 CrossRef CAS; (c) J. J. Cha, K. J. Koski and Y. Cui, Phys. Status Solidi RRL, 2013, 7, 15 CrossRef CAS; (d) G. Wang, X. Zhu, J. Wen, X. Chen, K. He, L. Wang, X. Ma, Y. Liu, X. Dai, Z. Fang, J. Jia and Q. Xue, Nano Res., 2010, 3, 874 CrossRef CAS; (e) C. Pauly, G. Bihlmayer, M. Liebmann, M. Grob, A. Georgi, D. Subramaniam, M. R. Scholz, J. Sánchez-Barriga, A. Varykhalov, S. Blügel, O. Rader and M. Morgenstern, Phys. Rev. B: Condens. Matter, 2012, 86, 235106 CrossRef.
- (a) J. P. Heremans, M. S. Dresselhaus, L. E. Bell and D. T. Morelli, Nat. Nanotechnol., 2013, 8, 471 CrossRef CAS PubMed; (b) J. Yang, H.-L. Yip and A. K.-Y. Jen, Adv. Energy Mater., 2013, 3, 549 CrossRef CAS.
- (a) N. Mingo, D. Hauser, N. P. Kobayashi, M. Plissonnier and A. Shakouri, Nano Lett., 2009, 9, 711 CrossRef CAS PubMed; (b) B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto, D. Vashaee, X. Chen, J. Liu, M. S. Dresselhaus, G. Chen and Z. Ren, Science, 2008, 320, 634 CrossRef CAS PubMed; (c) Z. Wang, J. E. Alaniz, W. Jang, J. E. Garay and C. Dames, Nano Lett., 2011, 11, 2206 CrossRef CAS PubMed.
- K. Biswas, J. He, I. D. Blum, C.-I. Wu, T. P. Hogan, D. N. Seidman, V. P. Dravid and M. G. Kanatzidis, Nature, 2012, 489, 414 CrossRef CAS PubMed.
- (a) S. Schulz, S. Heimann, J. Friedrich, M. Engenhorst, G. Schierning and W. Assenmacher, Chem. Mater., 2012, 24, 2228 CrossRef CAS; (b) G. Bendt, A. Weber, S. Heimann, W. Assenmacher, O. Prymak and S. Schulz, Dalton Trans., 2015, 44, 14272 RSC.
- S. Zastrow, J. Gooth, T. Boehnert, S. Heiderich, W. Toellner, S. Heimann, S. Schulz and K. Nielsch, Semicond. Sci. Technol., 2013, 28, 035010 CrossRef.
- (a) G. Bendt, S. Schulz, S. Zastrow and K. Nielsch, Chem. Vap. Deposition, 2013, 19, 235 CrossRef CAS; (b) G. Bendt, S. Zastrow, K. Nielsch, P. S. Mandal, J. Sánchez-Barriga, O. Rader and S. Schulz, J. Mater. Chem. A, 2014, 2, 8215 RSC; (c) S. Schulz, G. Bendt, J. Sonntag, A. Lorke, U. Hagemann and W. Assenmacher, Semicond. Sci. Technol., 2015, 30, 085021 CrossRef.
- (a) S. Heimann, S. Schulz, J. Schaumann, A. Mudring, J. Stötzel and G. Schierning, J. Mater. Chem. C, 2015, 3, 10375 RSC; (b) M. Loor, G. Bendt, U. Hagemann, C. Wölper, W. Assenmacher and S. Schulz, Dalton Trans., 2016, 45, 15326 RSC; (c) M. Loor, G. Bendt, J. Schaumann, U. Hagemann, M. Heidelmann, C. Wölper and S. Schulz, Z. Anorg. Allg. Chem. DOI:10.1002/zaac.201600325.
- (a) C. Lorbeer, J. Cybinska and A.-V. Mudring, Cryst. Growth Des., 2011, 11, 1040 CrossRef CAS; (b) T. Alammar and A.-V. Mudring, ChemSusChem, 2011, 12, 1796 CrossRef PubMed; (c) T. Alammar, O. Shekhah, J. Wohlgemuth and A.-V. Mudring, J. Mater. Chem., 2012, 22, 18252 RSC.
- (a) T. Alammar, H. Noei, Y. Wang and A.-V. Mudring, Nanoscale, 2013, 5, 8045 RSC; (b) C. Lorbeer and A.-V. Mudring, Chem. Commun., 2014, 50, 13282 RSC.
- (a) L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790S RSC; (b) S. Pitula and A.-V. Mudring, Phys. Chem. Chem. Phys., 2010, 12, 7056 RSC; (c) J. Bartosik and A.-V. Mudring, Phys. Chem. Chem. Phys., 2010, 12, 4005 RSC; (d) A. Babai, G. Kopiec, A. Lackmann, B. Mallick, S. Pitula, S.-F. Tang and A.-V. Mudring, J. Mol. Liq., 2014, 92, 191 CrossRef.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS PubMed.
- (a) T. Alammar, K. Chow and A.-V. Mudring, New J. Chem., 2015, 39, 1339 RSC; (b) T. Alammar, H. Noei, Y. Wang, W. Grünert and A.-V. Mudring, ACS Sustainable Chem. Eng., 2015, 3, 42 CrossRef CAS; (c) T. Alammar, I. Hamm, M. Wark and A.-V. Mudring, Appl. Catal., B, 2015, 178, 20 CrossRef CAS.
- P. Ghosh, S.-F. Tang and A.-V. Mudring, J. Mater. Chem., 2011, 21, 8640 RSC.
- (a) A. Mele, C. D. Tran and S. H. De Paoli Lacerda, Angew. Chem., Int. Ed., 2003, 42, 4364 CrossRef CAS PubMed; (b) F. Castiglione, R. Simonutti, M. Mauri and A. Mele, J. Phys. Chem. Lett., 2013, 4, 1608 CrossRef CAS PubMed; (c) J. C. Araque, J. J. Hettige and C. J. Margulis, J. Phys. Chem. B, 2015, 119, 12727 CrossRef CAS PubMed; (d) O. Russina, W. Schroer and A. Triolo, J. Mol. Liq., 2015, 210, 161 CrossRef CAS; (e) W. D. Amith, J. J. Hettige, E. W. Castner and C. J. Margulis, J. Phys. Chem. Lett., 2016, 7, 3785 CrossRef CAS PubMed.
- (a) J. Canongia Lopes, M. F. Costa Gomes and A. A. H. Pádua, J. Phys. Chem. B, 2006, 110, 16816 CrossRef PubMed; (b) M. Yang, P. Campbell, C. Santini and A.-V. Mudring, Nanoscale, 2014, 6, 3367 RSC; (c) R. Hayes and G. G. Warr, Chem. Rev., 2015, 115, 6357 CrossRef CAS PubMed.
- (a) M. Yang, B. Mallick and A.-V. Mudring, Cryst. Growth Des., 2013, 13, 3068 CrossRef CAS; (b) M. Yang, B. Mallick and A.-V. Mudring, Cryst. Growth Des., 2014, 14, 1561 CrossRef CAS; (c) A. Getsis and A.-V. Mudring, Cryst. Res. Technol., 2008, 43, 1187 CrossRef CAS; (d) M. Yang, K. Stappert and A.-V. Mudring, J. Mater. Chem. C, 2014, 2, 458 RSC; (e) M. Yang, B. Mallick and A.-V. Mudring, Cryst. Growth Des., 2013, 13, 3068 CrossRef CAS.
- A. Taubert, Angew. Chem., Int. Ed., 2004, 43, 5380 CrossRef CAS PubMed.
- Sb values: (a) G. Chen, J. Zhou, J. Zuo and Q. Yang, Appl. Mater. Interfaces, 2016, 8, 2819 CrossRef CAS PubMed; (b) C. D. Wagner, A. V. Naumkin, A. Kraut-Vass, J. W. Allison, C. J. Powell and J. R. Rumble Jr., NIST Standard Reference Database 20, Version 3.4 (web version), (http://srdata.nist.gov/xps/), 2003 Search PubMed; (c) G. Gupta and J. Kim, Dalton Trans., 2013, 42, 8209 RSC; (d) M.-J. Shin, D.-J. Choi, M.-J. Kang, S.-Y. Choi, I.-W. Jang, K.-N. Lee and Y.-J. Park, J. Korean Phys. Soc., 2004, 44, 10 CAS. Te values: (e) C. D. Wagner, A. V. Naumkin, A. Kraut-Vass, J. W. Allison, C. J. Powell and J. R. Rumble Jr., NIST Standard Reference Database 20, Version 3.4 (web version), (http:/srdata.nist.gov/xps/), 2003 Search PubMed; (f) H. Bando, K. Koizumi, Y. Oikawa, K. Daikohara, V. A. Kulbachinskii and H. Ozaki, J. Phys.: Condens. Matter, 2000, 12, 5607 CrossRef CAS; (g) C. R. Thomas, M. K. Vallon, M. G. Frith, H. Sezen, S. K. Kushwaha, R. J. Cava, J. Schwartz and S. L. Bernasek, Chem. Mater., 2016, 28, 35 CrossRef CAS; (h) E. J. Menke, M. A. Brown, Q. Li, J. C. Hemminger and R. M. Penner, Langmuir, 2006, 22, 10564 CrossRef CAS PubMed.
- B. Lv, S. Hu, W. Li, X. Di, L. Feng, J. Zhang, L. Wu, Y. Cai, B. Li and Z. Lei, Int. J. Photoenergy, 2010, 476589 Search PubMed.
- D. Gautam, M. Engenhorst, C. Schilling, G. Schierning, R. Schmechel and M. Winterer, J. Mater. Chem. A, 2015, 3, 189 CAS.
- H. Scherrer and S. Scherrer, in CRC Thermoelectrics Handbook, ed. D. M. Rowe, CRC Press, 2006, vol. 1, p. 407 Search PubMed.
- R. J. Mehta, Y. Zhang, C. Karthik, B. Singh, R. W. Siegel, T. Borca-Tasciuc and G. Ramanath, Nat. Mater., 2012, 11, 223 CrossRef PubMed.
- A. F. May and G. J. Snyder, in Materials, preparation, and characterization in thermoelectric, 1, ed. D. M. Rowe, CRC Press, 2012, p. 11 Search PubMed.
- R. Sehr and L. R. Testardi, J. Phys. Chem. Solids, 1962, 23, 1219 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751 CrossRef.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed.
- G.-H. Dong, Y.-J. Zhu and L.-D. Chen, J. Mater. Chem., 2010, 20, 1976 RSC.
- S. Sun, J. Peng, R. Jin, S. Song, P. Zhu and Y. Xing, J. Alloys Compd., 2013, 558, 6 CrossRef CAS.
- M. Saleemi, A. Ruditskiy, M. S. Toprak, M. Stingaciu, M. Johnsson, I. Kretzschmar, A. Jacquot, M. Jägle and M. Muhammed, J. Electron. Mater., 2014, 43, 1927 CrossRef CAS.
- U. Nithiyanantham, S. R. Ede, M. Fevzi Ozaydin, H. Liang, A. Rathishkumarb and S. Kundu, RSC Adv., 2015, 5, 89621 RSC.
- G. Thankamma and A. G. Kunjomana, J. Cryst. Growth, 2015, 415, 65 CrossRef CAS.
- H. Q. Yang, L. Miao, C. Y. Liu, X. Y. Wang, Y. Peng, A. J. Zhang, X. Y. Zhou, G. Y. Wang, C. Li and R. Huang, Dalton Trans., 2016, 45, 7483 RSC.
- (a) H. J. Breunig and H. Jawad, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1982, 37, 1104 Search PubMed; (b) S. Heimann, S. Schulz, D. Bläser and C. Wölper, Eur. J. Inorg. Chem., 2013, 28, 4909 Search PubMed.
- (a) E. R. Parnham and R. E. Morris, Chem. Mater., 2006, 18, 4882 CrossRef CAS; (b) J. M. Obliosca, S. D. Arco and M. H. Huang, J. Fluoresc., 2007, 17, 613 CrossRef CAS PubMed; (c) N. H. Khan, S. Agrawal, R. I. Kureshy, S. H. R. Abdi, A. Sadhukhan, R. S. Pillai and H. C. Bajaj, Catal. Commun., 2010, 11, 907 CrossRef CAS.
- A. S. Pashinkin, A. S. Malkova and M. S. Mikhailova, Russ. J. Phys. Chem. A, 2008, 82, 878 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |