Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halochromic coordination polymers based on a triarylmethane dye for reversible detection of acids†
Marina S.
Zavakhina
ab,
Irina V.
Yushina
a,
Denis G.
Samsonenko
ab,
Danil N.
Dybtsev
*ab,
Vladimir P.
Fedin
ab,
Stephen P.
Argent
c,
Alexander J.
Blake
 c and
Martin
Schröder
c and
Martin
Schröder
 ad
ad
aNikolaev Institute of Inorganic Chemistry, Russian Academy of Sciences, Siberian Branch, Acad. Lavrentiev Ave., 3, Novosibirsk, 630090, Russian Federation. E-mail: dan@niic.nsc.ru; Fax: +7 (383) 330 9489
bNovosibirsk State University, Pirogov str., 2, Novosibirsk, 630090, Russian Federation
cSchool of Chemistry, University of Nottingham, NG7 2RD Nottingham, UK
dSchool of Chemistry, University of Manchester, M13 9PL Manchester, UK
First published on 5th December 2016
Abstract
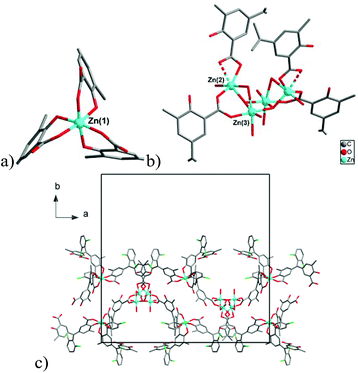
Chromeazurol B (Na2HL) is a pH-sensitive (halochromic) dye based on a hydroxytriarylmethane core and two carboxylate functional groups, which makes it suitable for the synthesis of coordination polymers. Two new coordination polymers [NaZn4(H2O)3(L)3]·3THF·3H2O (1) and [Zn3(H2O)3(μ2-OH2)(μ3-OH)(HL)2(H2L)]·2THF·3H2O (2) incorporating Chromeazurol B linkers have been prepared and characterised. The structure of 1 comprises pentanuclear heterometallic {Zn4Na} nodes linked by six L3− anions to give a layered structure with a honeycomb topology. 2 crystallizes as a double-chain ribbon (ladder) structure with two types of metal node: a mononuclear Zn(II) cation and tetranuclear {Zn(II)}4 cluster. Chromeazurol B anions link each tetranuclear cluster to four individual Zn(II) cations and each Zn(II) cation with four tetranuclear clusters. Both compounds show pH-sensitivity in water solution which can be observed visually, giving the first example of a halochromic coordination polymer. The halochromic properties of 1 towards HCl vapors were systematically investigated. As-synthesized violet-grey 1 reversibly changes color from orange to pink in the presence of vapors of 2 M and 7 M HCl, respectively. The coordination of the Chromeazurol B anion at each color stage was examined by diffuse reflectance spectroscopy and FT-IR measurements. The remarkable stability of 1 to acid and the observed reversible and reproducible color changes provide a new design for multifunctional sensor materials.
Introduction
Metal–organic frameworks (MOFs) are hybrid materials assembled from metal ions or clusters and organic linkers,1 and their adaptable modular design results in various tailored properties for the resultant porous materials2 including gas storage and separation properties.3 The use of porous MOFs as sensors is another important direction and is receiving increasing attention.4 Compared to molecular sensors, porous MOFs have a number of advantages, such as potentially higher stability, and, most importantly, the microporous structure with extended surface area can ensure effective adsorption of analyte molecules from gas or liquid media into the pores, thus potentially improving detection limits. Also, by tuning the pore geometry and functionality, the crystalline MOF sensor can be adapted for the detection of a specific molecule within a set of complex mixtures. There are many interesting examples of MOFs used for the detection of explosives,5 hazardous organic substances,6 and metal cations.7 These properties are typically based on magnetic,8 electrochemical/electromechanical9 or luminescent10 response of the porous framework upon inclusion of the particular guest molecule. Although luminescence sensors possess good detection levels, an external UV light source is usually required for visualization. In contrast, colorimetric sensors featuring a change of color of the material by an external stimulus can potentially be monitored visually and are therefore simpler to use. To date, however, there are few reports on colorimetric sensing by MOFs in which different porphyrinic,11 diimide,12 or viologen-substituted ligands13 have been employed. In another example based on Co(II), a color change occurs when the metal cation changes coordination environment from octahedral to tetrahedral.14Halochromic compounds (pH indicators) are among the most commonly used sensors in chemistry. Such molecules often possess a system of conjugated bonds which are altered upon protonation or deprotonation. Hydroxytriarylmethane-based pH indicators are classic examples of such systems.15 These molecules, if incorporated into a coordination polymer, e.g., within the linkers of a MOF, could function as halochromic detectors within the resultant material. Strikingly, this idea, despite being rather simple and straightforward, has not to our knowledge been explored previously. Chromeazurol B (also known as Chrome Pure Blue BX, Eriochrome Azurol B, Mordant Blue 1, herein denoted as Na2HL) is a well-known pH-sensitive compound based on hydroxytriarylmethane core.16 Depending on its protonation level, it exists in one of four different forms, each having a distinct color, thus representing a one-component universal pH sensor (Fig. 1). It also bears two carboxylate groups in its structure suggesting its potential use as a bridging ligand within a MOF architecture. Although a few structurally characterized molecular coordination compounds based on hydroxyarylmethane ligands have been reported, their pH dependent halochromic behavior has not been investigated.17
In this work we present two new coordination polymers [NaZn4(H2O)3(L)3]·3THF·3H2O (1) and [Zn3(H2O)3(μ2-OH2)(μ3-OH)(HL)2(H2L)]·2THF·3H2O (2) based on the Chromeazurol B dye as a bridging linker, and report their optical properties for 1 as a function of pH. These compounds are the first examples of MOF materials that show halochromic behavior in aqueous solution as well as with acidic vapors. The observation of reversible color changes and the structural stability of 1 at low pH demonstrate its pH sensing capabilities and opens new perspectives for MOF materials in important sensing applications.
Results and discussion
Protonation levels of Chromeazurol B (Na2HL)
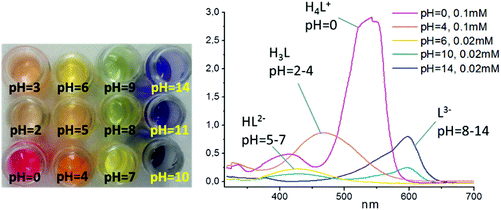
The dependence of Chromeazurol B color with pH was examined by spectrophotometry in solution for pH = 0–14. Four absorbance bands (550 nm, 480 nm, 430 nm, 600 nm) in the visible spectrum can be assigned to 4 different forms of the dye (Fig. 2).18 At pH = 0 the pink-red protonated form H4L+ is formed (ε(550 nm) = 2.8 × 104 M−1 cm−1), while at pH = 2–4 the less intense red-orange form H3L (ε(H3L, 480 nm) = 8.6 × 103 M−1 cm−1) is formed and at pH >4 a yellow-orange form HL2− (ε(430 nm) = 1.1 × 104 M−1 cm−1) exists. Finally, the fully deprotonated blue form of Chromeazurol B L3− (ε(600 nm) = 3.9 × 104 M−1 cm−1) is formed in highly basic solutions.15,16Synthesis
Compounds [NaZn4(H2O)3(L)3]·3THF·3H2O (1) and [Zn3(H2O)3(μ2-OH2)(μ3-OH)(HL)2(H2L)]·2THF·3H2O (2) were obtained by reaction of Chromeazurol B Na2(HL) with Zn(OAc)2 in water/THF solution. The solvent composition is critical, because compound 1 is obtained from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water
1 mixture of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF, while 2 can be isolated from the more polar 4
THF, while 2 can be isolated from the more polar 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water
1 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF solvent mixture. Both crystalline products are deeply colored and appear almost black. Crystals of the compound 1 adopt a rhombohedral shape with a dark golden hue, while 2 forms octahedral crystals with a greenish luster, red in transmitted light (Fig. S1†).
THF solvent mixture. Both crystalline products are deeply colored and appear almost black. Crystals of the compound 1 adopt a rhombohedral shape with a dark golden hue, while 2 forms octahedral crystals with a greenish luster, red in transmitted light (Fig. S1†).
Structures
Sensing properties
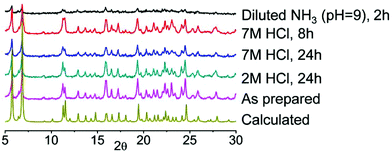
Successful synthesis of new coordination compounds incorporating Chromeazurol B prompted us to investigate the halochromic behavior of these materials. Preliminary experiments with thin layer samples of 1 and 2 in both water and dilute HCl solution (pH = 2) indicate that they have similar behavior. The color of crystals of both compounds changes reversibly from violet to orange by varying the pH of the media from neutral to acidic (Fig. S9†). Because of their similarity in pH behavior and the better crystallinity and quality for 1, we investigated the detailed halochromic properties of 1 only. The stability of 1 under a broad range of acidic and basic conditions was examined by powder X-ray diffraction analysis (Fig. 5). When exposed to vapors20 of an aqueous solution of 2 M HCl (partial pressure of HCl gas ca. 10−3 mmHg), 1 retains its crystallinity for at least 1 day. Moreover, under vapors of 7 M HCl (partial pressure of HCl gas ca. 0.64 mmHg) the crystals of 1 remain stable for at least 8 h and even after 1 day of exposure the quality of the diffraction pattern remains good. However, under vapors of aqueous NH3 solution at pH 9 1 decomposes rapidly and thus were only able to conduct sensing experiments under acidic atmospheres only.The halochromic properties of 1 were investigated by a number of methods. Because of the intense absorbance of 1, the crystals were ground and mixed with BaSO4 (5% of 1 in BaSO4 by weight). A thin layer of the corresponding powder mixture was exposed to vapors of solutions of HCl of different concentrations (2 M to 7 M). The violet-gray color of the as-synthesized 1@BaSO4 turned orange in 2 M and pink in 7 M HCl (Fig. 6a–c). When exposed to HCl vapors of intermediate concentration between 4 M and 6 M HCl the sample exhibited intermediate colors (Fig. S10†). In 7 M HCl the color of the sample changed over a few minutes, while in 2 M the transition takes few hours (Fig. S11†). It is important to note that all changes were completely reversible since pink and orange powders turned back to violet-gray after aeration, though the complete release of acid is a slower process than its uptake.
 | ||
| Fig. 6 Views of 1@BaSO4 (5% in BaSO4): (a) in air, (b) under 2 M HCl vapor and (c) under 7 M HCl vapor. | ||
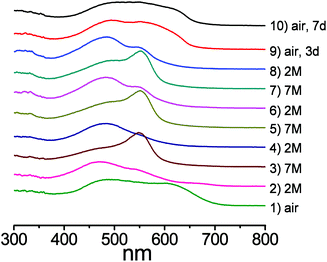
Diffuse reflectance spectroscopy (DRS) measurements clearly supported the visual observations as the spectra of the pelleted samples of 1@BaSO4 exposed to various media revealed remarkable changes depending on the acidity of the atmosphere (Fig. 7). For comparison we also carried out the DRS measurements of pellets of Na2HL@BaSO4, treated similarly to 1@BaSO4. The positions of the reflectance peaks for solid samples Na2HL@BaSO4 are similar to the adsorption bands in the UV-VIS spectra of Chromeazurol B in aqueous solutions at similar acidity. Also, the observed DRS peaks of 1@BaSO4 closely match those for the free ligand Na2HL@BaSO4. Therefore, coordination of Zn(II) to donor groups of the Chromeazurol B ligand does not seem to change the ability of the latter to accept the protons from the acidic environment. In other words, particular protonated forms of the pH-sensitive ligand exist in the coordination polymer 1 as in free Chromeazurol B under comparable conditions.
 | ||
| Fig. 7 Absorbance spectra and forms of the organic anion in 1: (a) under air, (b) under 2 M acid vapor and (c) under 7 M acid vapor. Black lines are for 1 and the red lines for Na2HL. | ||
The DRS data for as prepared Na2HL@BaSO4 show only the component HL2− at ca. 460 nm (ref. 17) while the spectrum of 1@BaSO4 consists of two absorption bands at ca. 480 and 620 nm (Fig. 7a), assigned to the two anionic forms L3− and HL2−, respectively, although only L3− is assumed in the crystal structure of 1 according to the requirement of charge-neutrality. The monoprotonated species HL2− probably exists in the crystalline compound as a result of the partial protonation of basic phenolate group by a solvent water and/or airborne moisture, which cannot be identified from the single-crystal X-ray diffraction data. During exposure of Na2HL@BaSO4 to 2 M HCl vapor the band at 460 nm undergoes a bathochromic shift to 470 nm, supporting the formation of the triply-protonated charge neutral form H3L (Fig. 7b). There is also a similar absorption band in the reflectance spectrum of 1@BaSO4 under 2 M HCl vapors so we can assume full protonation of the bound Chromeazurol B ligand in 1 under these conditions. Finally, when exposed to vapors of 7 M HCl the sample Na2HL@BaSO4 turned pink as does free Chromeazurol B in highly acidic solutions. This color and the major absorption band at ca. 550 nm are characteristic of the fully protonated H4L+ species (Fig. 7c). The diffuse reflectance spectrum of 1@BaSO4 exposed to 7 M HCl vapors has a similar pink color and, much like the free Chromeazurol B sample, displays the second component at ca. 475 nm, along with a major band at 550 nm. This suggests the simultaneous presence of H3L as well as H4L+ species in the samples. It should be noted that the DRS measurements and all required manipulations with the powder samples were carried out under air which may result in a partial reduction of the protonation level of the samples due to the release of volatile HCl. This explains the presence of the H3L component in the spectra of both samples Chromeazurol B and 1 exposed to the 7 M HCl vapors with the highest acid content. The obtained DRS data indicate that within the studied range of conditions the Chromeazurol B ligand undergoes the transition between three forms (HL2−, H3L and H4L+) while framework 1 between four forms (L3−, HL2−, H3L and H4L+), which makes the visual changes more characteristic and demonstrates the potential advantage of halochromic MOFs over molecular pH sensors.
Independent confirmation of the suggested protonated forms of Chromeazurol B ligand in crystalline 1 under different atmosphere (air, 2 M or 7 M HCl vapors) could be obtained by IR spectroscopy (Fig. 8 and S13†). Notable changes are observed for peaks at 735 and 1490 cm−1 assigned to vibrations of the Zn(II) bound carboxylate anion COO−, and for the distinct peak at 1620 cm−1 assigned to the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the protonated carboxylate group bound to Zn(II).21 Along with the increase in acidity of the media and hence the protonation level of 1, the peaks for Zn(II)-bound COO− gradually disappear while the corresponding peaks for Zn(II)-bound COOH emerge. Note, that the IR spectroscopic data support the structural models displayed in Fig. 7.
O stretching vibration of the protonated carboxylate group bound to Zn(II).21 Along with the increase in acidity of the media and hence the protonation level of 1, the peaks for Zn(II)-bound COO− gradually disappear while the corresponding peaks for Zn(II)-bound COOH emerge. Note, that the IR spectroscopic data support the structural models displayed in Fig. 7.
 | ||
| Fig. 8 IR spectra of 1 exposed in the air (black), in 2 M HCl vapors (red) and in 7 M HCl vapors (blue). | ||
We also estimated the level of protonation of 1 under vapors of 2 M or 7 M HCl after 1 h. The corresponding microcrystalline samples were dispersed in 3 ml of a distilled H2O and the acidities of the resultant solutions measured using a pH sensitive electrode. The amount of the acidic protons (degree of protonation), calculated from the pH values were found to be 2.8 and 3.9 per ligand L for the samples saturated at 2 M HCl and 7 M HCl, respectively (ESI Table S4†). These match very well to the theoretical protonation levels of the Chromeazurol B ligand (i.e., 3 and 4, respectively), proposed for the corresponding samples (Fig. 7b and c).
The fully reversible nature of the halochromic properties of 1 was demonstrated when 1@BaSO4 sample was alternately treated with 2 M HCl and 7 M HCl vapors several times (Fig. 9). The DRS data indicate reversible changes of the spectra supporting the corresponding visual changes of the sample within a few minutes or half an hour, depending on the HCl concentration (Fig. S14†). As noted above, the residual peaks at 550 nm in the compound exposed to 2 M HCl apparently result from the incomplete deprotonation of the sample. Note, however, that after these repeating experiments in the acidic atmosphere conditions the sample could be completely regenerated to its original color and spectra by aeration for few days.
Conclusions
The present work describes the synthesis and structural characterization of two coordination compounds based on Chromeazurol B dye. The products incorporate the pH-sensitive properties of the ligand resulting in coordination polymers with hitherto unprecedented halochromic properties. Various spectroscopic techniques provide the insights into the protonation levels of this pH-sensitive system. The marked stability of 1 and 2 from pH-neutral aqueous solutions to highly acidic HCl fumes, and the reversible and reproducible character of the corresponding color changes provide new opportunities and directions for MOFs in the design and development of multifunctional sensor materials.Acknowledgements
The work was supported by the Grant of the Government of the Russian Federation (Grant No. 14.Z50.31.0006). MS is grateful to EPSRC for support and to the ERC for an Advanced Grant.Notes and references
- (a) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed; (b) V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141 RSC; (c) S. Qiu and G. Zhu, Coord. Chem. Rev., 2009, 253, 2891 CrossRef CAS.
- (a) B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106 CrossRef CAS; (b) P. Peluso, V. Mamane and S. Cossu, J. Chromatogr., A, 2014, 1363, 11 CrossRef CAS PubMed; (c) H. Wu, Q. Gong, D. H. Olson and J. Li, Chem. Rev., 2012, 112, 836 CrossRef CAS PubMed; (d) V. Stavila, A. A. Talin and M. D. Allendorf, Chem. Soc. Rev., 2014, 43, 5994 RSC.
- (a) Z. Zhang, Z.-Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868 RSC; (b) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed; (c) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (d) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed; (e) X. Lin, N. R. Champness and M. Schröder, Top. Curr. Chem., 2010, 293, 35 CrossRef CAS PubMed.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC; (c) D. Liu, K. Lu, C. Poon and W. Lin, Inorg. Chem., 2014, 53, 1916 CrossRef CAS PubMed; (d) B. Liu, J. Mater. Chem., 2012, 22, 10094 RSC; (e) P. Kumar, A. Deep and K.-H. Kim, Trends Anal. Chem., 2015, 73, 39 CrossRef CAS.
- (a) C. Zhang, L. Sun, Y. Yan, J. Li, X. Song, Y. Liu and Z. Liang, Dalton Trans., 2015, 44, 230 RSC; (b) X.-G. Liu, H. Wang, B. Chen, Y. Zou, Z.-G. Gu, Z. Zhao and L. Shen, Chem. Commun., 2015, 51, 1677 RSC.
- N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 244–245, 444 CrossRef CAS PubMed.
- (a) R.-M. Wen, S.-D. Han, G.-J. Ren, Z. Chang, Y.-W. Li and X.-H. Bu, Dalton Trans., 2015, 44, 10914 RSC; (b) B. Liu, L. Hou, W.-P. Wu, A.-N. Dou and Y.-Y. Wang, Dalton Trans., 2015, 44, 4423 RSC.
- (a) C.-B. Tian, R.-P. Chen, C. He, W.-J. Li, Q. Wei, X.-D. Zhang and S.-W. Du, Chem. Commun., 2014, 50, 1915 RSC; (b) M. C. Muñoz, A. B. Gaspar, A. Galet and J. A. Real, Inorg. Chem., 2007, 46, 8182 CrossRef PubMed; (c) G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS PubMed.
- (a) Y. Wang, H. Ge, Y. Wu, G. Ye, H. Chen and X. Hu, Talanta, 2014, 129, 100 CrossRef CAS PubMed; (b) S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574 CrossRef CAS PubMed; (c) M. D. Allendorf, R. J. T. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall and P. J. Hesketh, J. Am. Chem. Soc., 2008, 130, 14404 CrossRef CAS PubMed.
- (a) J. Aguilera-Sigalat and D. Bradshaw, Chem. Commun., 2014, 50, 4711 RSC; (b) Y. Lu and B. Yan, Chem. Commun., 2014, 50, 13323 RSC; (c) H.-L. Jiang, D. Feng, K. Wang, Z.-Y. Gu, Z. Wei, Y.-P. Chen and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934 CrossRef CAS PubMed; (d) B. V. Harbuzaru, A. Corma, F. Rey, J. L. Jord, D. Ananias, L. D. Carlos and J. Rocha, Angew. Chem., Int. Ed., 2009, 48, 6476 CrossRef CAS PubMed; (e) Y.-H. Han, C.-B. Tian, Q.-H. Lia and S.-W. Du, J. Mater. Chem. C, 2014, 2, 8065 RSC.
- B. J. Deibert and J. Li, Chem. Commun., 2014, 50, 9636 RSC.
- A. Mallick, B. Garai, M. A. Addicoat, P. St. Petkov, T. Heinec and R. Banerjee, Chem. Sci., 2015, 6, 1420 RSC.
- (a) B. Tan, C. Chen, L.-X. Cai, Y.-J. Zhang, X.-Y. Huang and J. Zhang, Inorg. Chem., 2015, 54, 3456 CrossRef CAS PubMed; (b) J. Wu, C. Tao, Y. Li, J. Li and J. Yu, Chem. Sci., 2015, 6, 2922 RSC; (c) H.-Y. Li, Y.-L. Wei, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Mater., 2015, 27, 1327 CrossRef CAS.
- Q. Chen, Z. Chang, W.-C. Song, H. Song, H.-B. Song, T.-L. Hu and X.-H. Bu, Angew. Chem., Int. Ed., 2013, 52, 11550 CrossRef CAS PubMed.
- P. F. Gordon and P. Gregory, Organic Chemistry in Colour, Springer-Verlag, 1987 Search PubMed.
- N. Pollaková-Mouková, D. Gotzmannová, V. Kubáň and L. Sommer, Collect. Czech. Chem. Commun., 1981, 46, 354 CrossRef.
- (a) S. C. Burdette, G. K. Walkup, B. Spingler, R. Y. Tsien and S. J. Lippard, J. Am. Chem. Soc., 2001, 123, 7831 CrossRef CAS PubMed; (b) D. Dong, X. Jing, X. Zhang, X. Hu, Y. Wu and C. Duan, Tetrahedron, 2012, 68, 306 CrossRef CAS; (c) A. P. Meacham, K. L. Druce, Z. R. Bell, M. D. Ward, J. B. Keister and A. B. P. Lever, Inorg. Chem., 2003, 42, 7887 CrossRef CAS PubMed; (d) D. A. Shultz, S. H. Bodnar and J. W. Kampf, Chem. Commun., 2001, 93 RSC; (e) L. J. McCormick, B. F. Abrahams, B. A. Boughton, M. J. Grannas, T. A. Hudson and R. Robson, Inorg. Chem., 2014, 53, 1721 CrossRef CAS PubMed; (f) D. A. Shultz, S. H. Bodnar, R. K. Kumar and J. W. Kampf, J. Am. Chem. Soc., 1999, 121, 10664 CrossRef CAS.
- Deprotonation of both carboxylic groups of Chromeazurol B takes place at similar pH, therefore, the H2L– intermediate cannot be isolated as a dominating form in solution.
- A. L. Spek, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 9 CrossRef CAS PubMed.
- P. E. Liley, G. H. Thomson, D. G. Friend, T. E. Daubert and E. Buck, in Perry's Chemical Engineers’ Handbook, ed. R. H. Perry, D. W. Green and J. O. Maloney, McGraw-Hill, New York, 7th edn, 1999, section 2, p. 76 Search PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 4th edn, 1986 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and analytical methods, crystallographic information, IR spectra, TGA plots and additional figures and photographs. CCDC 1504745 and 1504746. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt03969c |
| This journal is © The Royal Society of Chemistry 2017 |