Open Access Article
Open Access ArticleElectrochemical study of the promoting effect of Fe on oxygen evolution at thin ‘NiFe–Bi’ films and the inhibiting effect of Al in borate electrolyte†
Remi
Fayad
 ,
Jihan
Dhainy
,
Hiba
Ghandour
and
Lara
Halaoui
,
Jihan
Dhainy
,
Hiba
Ghandour
and
Lara
Halaoui
 *
*
Department of Chemistry, American University of Beirut, Beirut, Lebanon 110236. E-mail: Lara.Halaoui@aub.edu.lb
First published on 15th August 2017
Abstract
In this study, we investigated the electrochemical effects of Fe co-deposition in thin Ni oxo/hydroxo films in borate, termed ‘NiFe–Bi’, electrodeposited from a Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio of 9
Fe ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 4
4, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in solution. The NiFe–Bi films were investigated as-deposited and after anodic conditioning, and compared to Ni–Bi without intentional Fe doping at Ni(OH)2 loading from a submonolayer to 10 layers. Fe co-deposition enhanced the oxygen evolution reaction (OER) of as-deposited NiFe–Bi films relative to as-deposited Ni–Bi; however, anodic biasing was still required to maximize catalysis. The Ni(OH)2/NiOOH redox peaks of NiFe–Bi deposited from 6
6 in solution. The NiFe–Bi films were investigated as-deposited and after anodic conditioning, and compared to Ni–Bi without intentional Fe doping at Ni(OH)2 loading from a submonolayer to 10 layers. Fe co-deposition enhanced the oxygen evolution reaction (OER) of as-deposited NiFe–Bi films relative to as-deposited Ni–Bi; however, anodic biasing was still required to maximize catalysis. The Ni(OH)2/NiOOH redox peaks of NiFe–Bi deposited from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 4
4 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 Ni
6 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe were more cathodic and more reversible than Ni–Bi peaks. Anodic conditioning caused the Ni(OH)2/NiOOH redox peaks of Ni–Bi and NiFe–Bi to shift anodically but without narrowing of peak separation, different from the effects of Fe co-deposition. After anodic conditioning, the turnover frequency (TOF) for OER per Ni center for Ni–Bi and NiFe–Bi was more proportional to the Ni content within the first linear Tafel region, with a promoting effect of Fe, and the films exhibited similar Tafel slopes of 37–42 mV dec−1. With increasing overpotential however, the TOF increased more significantly at high Fe
Fe were more cathodic and more reversible than Ni–Bi peaks. Anodic conditioning caused the Ni(OH)2/NiOOH redox peaks of Ni–Bi and NiFe–Bi to shift anodically but without narrowing of peak separation, different from the effects of Fe co-deposition. After anodic conditioning, the turnover frequency (TOF) for OER per Ni center for Ni–Bi and NiFe–Bi was more proportional to the Ni content within the first linear Tafel region, with a promoting effect of Fe, and the films exhibited similar Tafel slopes of 37–42 mV dec−1. With increasing overpotential however, the TOF increased more significantly at high Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio and was not proportional to the Ni content, and the Tafel slopes varied while notably decreasing for some NiFe–Bi films. Electrochemical results can support a Ni active site at low overpotential, but point to possibly different roles of Fe at low versus high potential. In addition, the effect of adding Fe3+ and Al3+ to the electrolyte was studied after deposition of Ni–Bi. Adding Fe resulted in reaching almost maximum activity in a single potential scan, confirming the promoting role of Fe in Ni–Bi and providing a quick alternative to applying anodic bias for hours to increase activity, while Al in the electrolyte poisoned OER catalysis at Ni–Bi and at NiFe–Bi. In the presence of both Fe and Al ions in solution however, with their opposite effects on the OER, the Ni(OH)2/NiOOH redox peaks shifted anodically with potential scanning.
Ni ratio and was not proportional to the Ni content, and the Tafel slopes varied while notably decreasing for some NiFe–Bi films. Electrochemical results can support a Ni active site at low overpotential, but point to possibly different roles of Fe at low versus high potential. In addition, the effect of adding Fe3+ and Al3+ to the electrolyte was studied after deposition of Ni–Bi. Adding Fe resulted in reaching almost maximum activity in a single potential scan, confirming the promoting role of Fe in Ni–Bi and providing a quick alternative to applying anodic bias for hours to increase activity, while Al in the electrolyte poisoned OER catalysis at Ni–Bi and at NiFe–Bi. In the presence of both Fe and Al ions in solution however, with their opposite effects on the OER, the Ni(OH)2/NiOOH redox peaks shifted anodically with potential scanning.
Introduction
Hydrogen produced by splitting water using sunlight on a semiconductor electrode1–3 can offer a continuous and clean energy source to meet the global energy need and limit the reliance on fossil fuel, when other renewables such as solar and wind vary with weather and the diurnal cycle. This has long been considered as a ‘holy grail’ in chemistry, and research into finding efficient and stable photoelectrodes and multi-electron catalysts for solar splitting of water1,2 has continued for four decades since it was first reported on a TiO2 photoanode.3Water splitting is comprised of two half-reactions: the hydrogen evolution reaction (HER), and the oxygen evolution reaction (OER). The OER, as 4OH− → O2 + 4e + 2H2O in alkaline solution or 2H2O → O2 + 4H+ + 4e in acidic solution, is a four-electron four-proton process producing one dioxygen from two water molecules.1,2 It is thus kinetically sluggish and requires a catalyst. Rare metal oxides RuO2 and IrO2 are the most active catalysts for this reaction,1,4 but extensive current research is directed to finding stable and efficient catalysts from non-precious first row metals. Reported catalysts include spinels such as Co3O4 and NiCo2O4, perovskites, and pyrochlores.1 Ni-based oxides remain as some of the best non-noble OER catalysts in alkaline solution, and a synergistic promoting effect has been observed for their bimetallic and trimetallic oxides with metals such as Co4–10 and Fe.10–15 In early studies, spinel NiCo2O4 was found to be more active than NiO and Co2O4,16 and Fe impurities were found to enhance Ni-oxide activity.17 Ni0.9Fe0.1Ox was reported11 to have greater activity than IrO2 and exhibit a similar activity to the best catalyst in alkaline solution (Ba0.5Sr0.5Co0.8Fe0.2O3−δ).18 Promotion of NiOx activity by other metals, including Al with Fe, has been reported in combinatorial studies.12,19
The rational design of OER catalysts necessitates understanding of structure–activity relations and the role of guest metals. Because electrodes for the OER may also need to operate at high overpotential – the exchange current density for HER catalysts such as Pt and Pd is on the order of 10−3 A cm−2 while that of OER catalysts such as RuO2 is about 10−5 to 10−6 A cm−2 –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 catalyst activity and stability need to be determined at low and high current densities. This is particularly essential for OER electrodes that undergo structural changes in the cell, such as NiOx electrodes.
1 catalyst activity and stability need to be determined at low and high current densities. This is particularly essential for OER electrodes that undergo structural changes in the cell, such as NiOx electrodes.
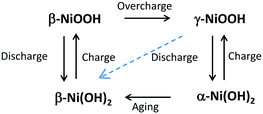
The transformations of NiOx in the electrochemical medium are summarized in the following Bode diagram:20,21
The mechanism by which Fe promotes the OER in Ni(Fe)Ox remains the subject of discussions.22–28 This has revolved around whether Ni or Fe is the active site, the role of Fe in increasing NiOx activity, and the most active phase. Within the hypothesis of a Ni active site, Fe was proposed to exert an electronic effect on Ni;24 Raman studies showed that Fe affects the Ni-oxide electronic environment,14 and electronic and structural changes have been observed using Mössbauer spectroscopy.28 Fe was also suggested to increase the conductivity of tetravalent nickel oxide or to change the mechanism.17 It has also been proposed that Fe is the active site; a study by Bell and co-workers reported that OER intermediates adsorb too weakly on γ-NiOOH and too strongly on γ-FeOOH, while Fe sites surrounded by Ni next-nearest neighbors in γ-NiOOH had near-optimal adsorption energy.22 Bard and co-worker reported SECM results supporting an Fe active site.27 Stahl and co-workers suggested that an Fe4+ species at edges and corners could be more kinetically active for OER.25 Regarding the most active phase, Li and Selloni determined using density functional theory that Fe-doped β-NiOOH has the lowest overpotential for OER, followed by NiFe2O4, Fe-doped γ-NiOOH, and then γ-NiOOH.26
High OER activity was reported by Nocera and co-workers for electrodeposited amorphous Co-29 and Ni-30–32(oxo)/hydroxo films in phosphate or borate (Bi) buffers, respectively, and these catalysts attracted interest after coupling to solar cells in what was termed an ‘artificial leaf’.33,34 The maximum OER activity of the Ni-based catalyst in borate, termed Ni–Bi, was reached after anodic biasing.31 Bediako et al. reported that at water oxidation potentials the Ni oxidation state in ‘anodized’ Ni–Bi was 3.6 and resembled that in γ-NiOOH, while the oxidation state before conditioning was 3.16 and EXAFS spectroscopy showed a Jahn–Teller distorted Ni(III) phase as that in β-NiOOH.31 The authors concluded that β-NiOOH transformed to γ-NiOOH with anodic bias and, since the OER activity increased, that γ-NiOOH is more active for OER than β-NiOOH, opposite of the views in the literature that β-NiOOH is more active.31
The inherent OER activity of Ni–Bi has come into question in the following work by Boettcher and co-workers that showed that the activity did not increase when the borate electrolyte was purified from Fe, and that incidental Fe doping occurs from traces of Fe.35 This was consistent with the effect of incidental inclusion in NiOx of Fe from KOH reported by the same group24 and earlier by Corrigan.17
In this study, we investigated the electrochemical behavior of electrodeposited ultra-thin Ni–Bi films with Fe co-precipitated from Fe3+ and Ni2+ nitrates in potassium borate at Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratios of 1
Ni ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 4
9, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 6
6, and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The electrochemical behavior (OER activity and redox potentials) of the films, termed NiFe–Bi, was investigated before and after anodic conditioning compared to that of Ni–Bi without intentional Fe deposition at Ni(OH)2 loading from a submonolayer to 10 layers. Fe co-deposition enhanced OER activity of the as-deposited NiFe–Bi films, however anodic biasing was still needed to maximize activity. The Ni(OH)2/NiOOH redox peaks of NiFe–Bi deposited from 6
4. The electrochemical behavior (OER activity and redox potentials) of the films, termed NiFe–Bi, was investigated before and after anodic conditioning compared to that of Ni–Bi without intentional Fe deposition at Ni(OH)2 loading from a submonolayer to 10 layers. Fe co-deposition enhanced OER activity of the as-deposited NiFe–Bi films, however anodic biasing was still needed to maximize activity. The Ni(OH)2/NiOOH redox peaks of NiFe–Bi deposited from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 4
4 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 Ni
6 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe were more cathodic and more reversible than Ni–Bi peaks; while anodic conditioning caused the redox peaks of Ni–Bi and NiFe–Bi to shift anodically but without narrowing of peak separation, different from the effects of Fe co-deposition. After anodic conditioning, the apparent turnover frequency (TOF) for OER per Ni center at Ni–Bi and NiFe–Bi was more proportional to the Ni content within a first linear Tafel region at current densities below 1 mA cm−2, with a promoting effect of Fe, and NiFe–Bi and Ni–Bi films had similar Tafel slopes of ∼37–42 mV dec−1. With increasing potential however, the apparent TOF per Ni center increased more significantly at high Fe
Fe were more cathodic and more reversible than Ni–Bi peaks; while anodic conditioning caused the redox peaks of Ni–Bi and NiFe–Bi to shift anodically but without narrowing of peak separation, different from the effects of Fe co-deposition. After anodic conditioning, the apparent turnover frequency (TOF) for OER per Ni center at Ni–Bi and NiFe–Bi was more proportional to the Ni content within a first linear Tafel region at current densities below 1 mA cm−2, with a promoting effect of Fe, and NiFe–Bi and Ni–Bi films had similar Tafel slopes of ∼37–42 mV dec−1. With increasing potential however, the apparent TOF per Ni center increased more significantly at high Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio and was no longer proportional to the Ni content, and the Tafel slopes varied, and notably decreased for some NiFe–Bi films. Electrochemical results can support a Ni active site at low overpotential, but point to possibly different roles of Fe at low versus high potential. In addition, we studied the effect of adding Fe3+versus Al3+ to the electrolyte after deposition of Ni–Bi, as these two ions are known to stabilize α-Ni(OH)2/γ-NiOOH, and we report that Al3+ in the electrolyte had a poisoning effect on the OER for Ni–Bi, and also for co-deposited NiFe–Bi, while addition of Fe3+ to the electrolyte resulted in reaching almost maximum activity in a single potential scan, confirming the role of Fe3+ in enhancing catalysis and providing a quicker alternative to applying anodic bias for hours. In the presence of both ions however, with their opposite effects on OER activity, the redox peaks shifted anodically with potential scanning.
Ni ratio and was no longer proportional to the Ni content, and the Tafel slopes varied, and notably decreased for some NiFe–Bi films. Electrochemical results can support a Ni active site at low overpotential, but point to possibly different roles of Fe at low versus high potential. In addition, we studied the effect of adding Fe3+versus Al3+ to the electrolyte after deposition of Ni–Bi, as these two ions are known to stabilize α-Ni(OH)2/γ-NiOOH, and we report that Al3+ in the electrolyte had a poisoning effect on the OER for Ni–Bi, and also for co-deposited NiFe–Bi, while addition of Fe3+ to the electrolyte resulted in reaching almost maximum activity in a single potential scan, confirming the role of Fe3+ in enhancing catalysis and providing a quicker alternative to applying anodic bias for hours. In the presence of both ions however, with their opposite effects on OER activity, the redox peaks shifted anodically with potential scanning.
Results and discussion
I. NiFe–Bi as-deposited films: electrochemical characterization and OER activity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios of 9
Fe ratios of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 4
4, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, at 1 mC cm−2. Films are termed relative to the Ni
6, at 1 mC cm−2. Films are termed relative to the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio in the electrodeposition medium and/or the electrodeposition charge density.
Fe ratio in the electrodeposition medium and/or the electrodeposition charge density.
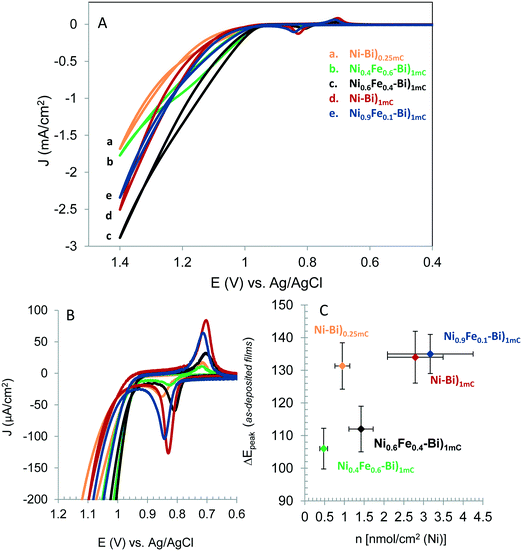
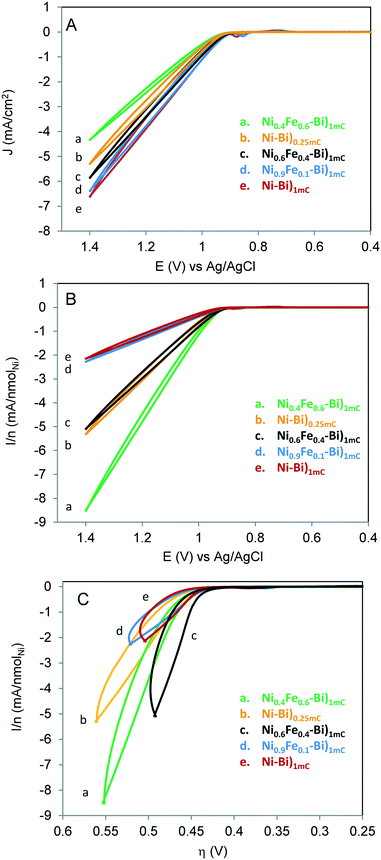
Fig. 1A shows the first CV scans acquired for Ni–Bi)1mC, Ni–Bi)0.25mC, Ni0.9Fe0.1–Bi)1mC, Ni0.6Fe0.4–Bi)1mC and Ni0.4Fe0.6–Bi)1mC at 10 mV s−1 in 1 M KBi pH 9.16. The CVs feature the well-reported quasi-reversible Ni(OH)2/NiOOH redox behavior, whose redox peak potentials and peak separation depend on the phase and guest metal cation.20,21 The Ni(OH)2/NiOOH cathodic charge of Ni–Bi)1mC was on average 0.4 ± 0.1 mC cm−2, thus only a fraction of the charge went to film deposition and the rest results from double layer charging and oxygen evolution which becomes more significant as films become thicker or with Fe included (vide infra). The ratio of Ni in Ni–Bi)1mC to Ni–Bi)0.25mC was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is similar to the ratio of the charges; but became on average ca. 3.6 in Ni–Bi)10mC to Ni–Bi)1mC. The peak charges and potentials depended on the %Fe in the deposition solution. Co-deposition from 40% and 60% Fe caused considerably less Ni deposition which is indicative of Fe co-precipitation since the same charge was passed, and is consistent with a report of Fe co-deposition decreasing the amount of Ni.23 The ratio of Ni in Ni0.6Fe0.4–Bi)1mC to Ni–Bi)1mC was ca. 0.5 compared to the 0.6 ratio in solution, and 0.2 in Ni40Fe60–Bi)1mC to Ni–Bi)1mC compared to the 0.4 solution ratio. This is attributed to increased OER activity with more Fe co-deposited, decreasing further Ni deposition. On the other hand, 10% Fe did not reduce Ni(OH)2 precipitation on average compared to Ni–Bi)1mC (Table 1).
1 which is similar to the ratio of the charges; but became on average ca. 3.6 in Ni–Bi)10mC to Ni–Bi)1mC. The peak charges and potentials depended on the %Fe in the deposition solution. Co-deposition from 40% and 60% Fe caused considerably less Ni deposition which is indicative of Fe co-precipitation since the same charge was passed, and is consistent with a report of Fe co-deposition decreasing the amount of Ni.23 The ratio of Ni in Ni0.6Fe0.4–Bi)1mC to Ni–Bi)1mC was ca. 0.5 compared to the 0.6 ratio in solution, and 0.2 in Ni40Fe60–Bi)1mC to Ni–Bi)1mC compared to the 0.4 solution ratio. This is attributed to increased OER activity with more Fe co-deposited, decreasing further Ni deposition. On the other hand, 10% Fe did not reduce Ni(OH)2 precipitation on average compared to Ni–Bi)1mC (Table 1).
| Films | nmolNi cm−2 (based on 1.6 e per Ni) | Thickness (nm) | Monolayereq [Ni(OH2)] | TOFapp,Ni,@η450mV (s−1) | TOFapp,Ni,@η500mV (s−1) | TOFapp,Ni,@η650mV (s−1) |
|---|---|---|---|---|---|---|
| Ni–Bi)1mC | 2.79 ± 0.70 | 2.07 ± 0.52 | 2.59 ± 0.65 | 0.025 ± 0.011 | 0.052 ± 0.023 | 0.676 ± 0.207 |
| Ni0.9Fe0.1–Bi)1mC | 3.17 ± 1.08 | 2.35 ± 0.80 | 2.94 ± 1.00 | 0.022 ± 0.003 | 0.054 ± 0.027 | 0.813 ± 0.381 |
| Ni0.6Fe0.4–Bi)1mC | 1.42 ± 0.31 | 1.05 ± 0.23 | 1.32 ± 0.28 | 0.044 ± 0.014 | 0.195 ± 0.090 | 2.156 ± 0.785 |
| Ni0.4Fe0.6–Bi)1mC | 0.48 ± 0.10 | 0.35 ± 0.07 | 0.44 ± 0.09 | 0.090 ± 0.013 | 0.323 ± 0.072 | 3.698 ± 0.994 |
| Ni–Bi)250μC | 0.95 ± 0.19 | 0.70 ± 0.14 | 0.88 ± 0.17 | 0.053 ± 0.017 | 0.088 ± 0.022 | 1.059 ± 0.344 |
| Ni–Bi)10mC | 10.59 ± 1.47 | 7.85 ± 1.09 | 9.82 ± 1.36 | 0.014 ± 0.001 | 0.032 ± 0.010 | 0.617 ± 0.116 |
Fig. 1B shows the Ni(OH)2/NiOOH redox peaks of the films in Fig. 1A. Peak positions were measured at N = 3 films each. Ep,a for Ni–Bi)1mC occurred at 0.836 ± 0.008 V and Ep,c at 0.702 ± 0.000 V. The anodic peak was shifted cathodically and the cathodic peak generally was shifted anodically (5 out of 6 films, and 1 film with no change in Ep,c) for Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi relative to Ni–Bi)1mC. Their respective half-wave potential E1/2 equaled 0.764 ± 0.009 V and 0.772 ± 0.004 V, therefore there was no measurable shift from E1/2 of Ni–Bi)1mC (0.769 ± 0.004 V) and Ni0.9Fe0.1–Bi (0.772 ± 0.009 V). A plot of ΔEpeakversus nmol cm−2 of Ni is presented in Fig. 1C, and shows narrowing in peak separation for 40% and 60% Fe in the deposition medium (N = 3 each). ΔEp equaled 112 ± 7 mV for Ni0.6Fe0.4–Bi and 106 ± 6 mV for Ni0.4Fe0.6–Bi compared to 134 ± 8 mV for Ni–Bi)1mC and 135 ± 6 mV for Ni0.9Fe0.1–Bi. The increase in reversibility was not caused by lower Ni content (or in thinner films) as ΔEp equaled 131 ± 7 mV for Ni–Bi)0.25mC that has Ni coverage intermediate between Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi. A decrease in peak separation from 101 mV to 80 mV17 or from 150 mV to 125 mV36 was reported by Corrigan with Fe inclusion in NiOx, which also occurred with other ions,36 and is another indication of Fe incorporation in Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi.
The inclusion of Fe therefore did not cause a thermodynamic shift in the Ni(OH)2/NiOOH redox potential, but caused a kinetic facility for Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi. The absence of an anodic shift with Fe in NiOx contradicts several reported observations,17,24,25,28,35,36 from which there emerged in the literature what appears to be a consensus that Fe causes an anodic shift in the Ni(OH)2/NiOOH oxidation due to an electronic effect, as discussed below.24,25 We did not observe an anodic shift in NiFe–Bi also as we used a second source of nickel nitrate. Reviewing published work also showed that an anodic shift was not observed in every report of Fe in NiOx.23,27 For instance, inspection of the CVs in Fig. 1 of ref. 23 by Swierk et al. shows narrowing in ΔEp and no anodic shift with Fe,23 and the CV in Fig. 1B in the work by Scherson and co-workers does not show an anodic shift but possibly a slight cathodic shift.37 It is not evident what causes the variability between results, but it may be due to the differences in the deposition conditions, electrolyte composition and the resulting initial film structure, since the redox peaks depend on both the phase and the guest metal cations.20,21,36
| Films | nmolNi cm−2 | Thickness (nm) | Monolayereq [Ni(OH2)] | TOFSS (s−1) (@η ∼ 409–414 mV) | TOFSS (s−1) (@η ∼ 430mV) | TOFSS (s−1) (@η ∼ 450mV) | TOFSS (s−1) (@η ∼ 460mV) |
|---|---|---|---|---|---|---|---|
| a The numbers are the TOF of 2 out of the 3 films, since the highest potential at which the measurements were performed did not yield an overpotential of 460 mV for the third film; the corresponding number of moles is in parenthesis. | |||||||
| Ni–Bi)1mC | 2.77 ± 0.99 | 2.05 ± 0.73 | 2.57 ± 0.91 | 0.131 ± 0.031 (409 ± 2) | 0.53 ± 0.15 (430 ± 1) | 2.09 ± 0.82 (450 ± 1) | 2.74 ± 1.62 (457 ± 3) (N = 2,a 2.37 ± 1.00 nmol cm−2) |
| Ni0.9Fe0.1–Bi)1mC | 3.27 ± 1.09 | 2.42 ± 0.80 | 3.03 ± 1.01 | 0.147 ± 0.052 (411 ± 3) | 0.494 ± 0.09 (429 ± 2) | 1.97 ± 0.68 (449 ± 2) | 2.35 ± 0.01 (460 ± 0) (N = 2,a 2.65 ± 0.22 nmol cm−2) |
| Ni0.6Fe0.4–Bi)1mC | 1.10 ± 0.42 | 0.81 ± 0.31 | 1.02 ± 0.39 | 0.225 ± 0.079 (411 ± 1) | 0.56 ± 0.26 (429 ± 3) | 2.26 ± 0.81 (449 ± 1) | 5.08 ± 0.73 (460 ± 0) |
| Ni0.4Fe0.6–Bi)1mC | 0.57 ± 0.070 | 0.42 ± 0.05 | 0.53 ± 0.065 | 0.225 ± 0.041 (414 ± 1) | 0.493 ± 0.049 (430 ± 1) | 1.73 ± 0.09 (452 ± 2) | 3.22 ± 0.45 (461 ± 1) |
| Ni–Bi)250μC | 0.89 ± 0.092 | 0.66 ± 0.07 | 0.83 ± 0.085 | 0.161 ± 0.05 (414 ± 2) | 0.231 ± 0.14 (429 ± 1) | 0.96 ± 0.26 (450 ± 4) | 1.48 ± 0.27 (461 ± 2) |
| Ni–Bi)10mC | 10.45 ± 0.22 | 7.75 ± 0.16 | 9.69 ± 0.20 | 0.162 ± 0.069 (414 ± 1) | 0.45 ± 0.057 (430 ± 1) | — | — |
Ni–Bi films deposited by passing a charge of 1 mC cm−2 thus contained on average ca. 2.8 nmol cm−2 of Ni and were ca. 2 nm thick (Tables 1 and 2, before and after conditioning) as calculated from the integrated cathodic peaks. The ultra-thin Ni–Bi and NiFe–Bi film structure on FTO could not be discerned in the SEM images above the larger nanostructures of the FTO surface (Fig. SI.1†). The SEM images of Ni–Bi)10mC (Fig. SI.2†), that contained on average 10.6 nmol cm−2 of Ni and had a calculated thickness of ca. 8 nm, and of Ni0.4Fe0.6–Bi)10mC (Fig. SI.3†) show the films' nanoscale features grown onto the FTO nanostructures. A nanostructured film growth was seen in the SEM images of thicker films Ni–Bi)400mC and Ni0.6Fe0.4–Bi)400mC. The SEM images of these films deposited at 400 mC cm−2 before and after anodization (vide infra) (Fig. SI.4 and SI.5†) revealed nanostructured fractal-like growth with very thin walls. Interestingly, the SEM images of Ni0.6Fe0.4–Bi)400mC show a difference in surface morphology with smaller nanostructures and pores compared to that of Ni–Bi)400mC. Nocera and co-workers reported SEM images of Ni–Bi film deposited at 10 C cm−2 (thickness of 3 μm) and the nanostructure observed here cannot be seen in the SEM images presented for these thick films.34 The EDX spectra revealed Ni in Ni–Bi and both Ni and Fe in NiFe–Bi films (Fig. SI.6†).
Ni–Bi)1mC, Ni0.9Fe0.1–Bi)1mC, Ni0.6Fe0.4–Bi)1mC, Ni0.4Fe0.6–Bi)1mC, and Ni–Bi)0.25mC in Fig. 1 contained respectively 3.02, 2.70, 1.37, 0.40 and 0.75 nmol cm−2 of Ni, and their equivalent thickness and Ni(OH)2 coverage were: 2.24 nm and 2.8 monolayers for Ni–Bi)1mC, 0.56 nm and 0.70 monolayer for Ni–Bi)0.25mC, and 2.5 equivalent monolayers for Ni0.9Fe0.1–Bi, 1.3 for Ni0.6Fe0.4–Bi, and 0.37 for Ni0.4Fe0.6–Bi. Fig. 1 shows greater OER current density for Ni0.6Fe0.4–Bi)1mC than for Ni–Bi)1mC despite its two times smaller Ni content, and a greater current density for Ni0.4Fe0.6–Bi with θNi ∼ 0.4 than for Ni–Bi)0.25mC with θNi ∼ 0.7. Fig. 2 presents the currents normalized to nmol of Ni versus potential (E) and overpotential (η), and shows that starting from the foot of the anodic peak, currents were the largest per Ni site for Ni0.4Fe0.6–Bi, followed by Ni0.6Fe0.4–Bi, and were the lowest for Ni–Bi)1mC and Ni0.9Fe0.1–Bi. To determine their dependence on Fe content and thickness, the apparent TOF per electroactive Ni center at different overpotentials was calculated from the forward sweeps of the first CVs at 10 mV s−1 without stirring – to minimize structural changes and incidental Fe incorporation – and is presented in Table 1 and Fig. 2. At η = 450 mV at low current densities of ∼13–64 μA cm−2 the average TOF was the highest for Ni0.4Fe0.6–Bi equaling 0.09 s−1, and decreased with increasing Ni content to 0.053 s−1 for Ni–Bi)0.25mC, 0.022–0.025 s−1 for Ni–Bi)1mC and Ni0.9Fe0.1–Bi, and 0.014 s−1 for Ni–Bi)10mC. At η = 500 mV, at 30–159 μA cm−2, the promoting effect of Fe increased, with an average TOF of 0.32 s−1 for Ni0.4Fe0.6–Bi and 0.20 s−1 for Ni0.6Fe0.4–Bi compared to 0.09 s−1 for Ni–Bi)0.25mC, 0.05 s−1 for Ni–Bi)1mC and Ni0.9Fe0.1–Bi, and 0.03 s−1 for Ni–Bi)10mC. At η = 650 mV, or current densities of 0.3–1.3 mA cm−2 for thinner films or 2.2–3.5 mA cm−2 for Ni–Bi)10mC, the TOF was ca. 3.7 s−1 for Ni0.4Fe0.6–Bi)1mC, 2.2 s−1 for Ni0.6Fe0.4–Bi)1mC, 1.1 s−1 for Ni–Bi)0.25mC, 0.7 s−1 for Ni–Bi)1mC, and 0.62 s−1 for Ni–Bi)10mC.
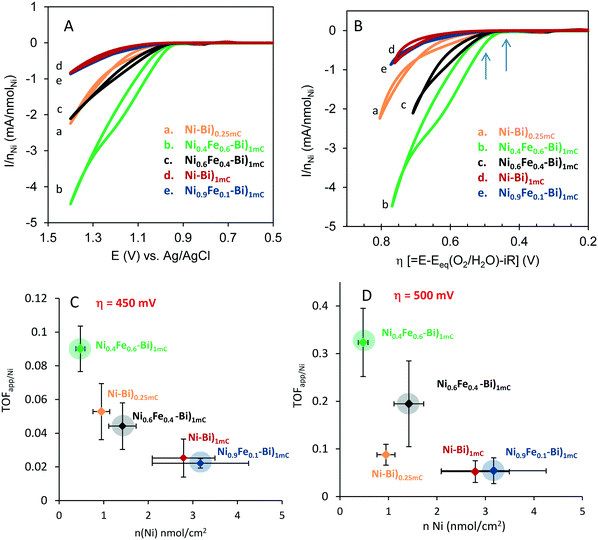 | ||
| Fig. 2 Current divided per nNiversus potential (CVs in Fig. 1) (A) and versus overpotential η (B) for as-deposited Ni–Bi)0.25mC (a) Ni0.4Fe0.6–Bi)1mC (b), Ni0.6Fe0.4–Bi)1mC (c), Ni–Bi)1mC (d) and Ni0.9Fe0.1–Bi)1mC (e). Apparent turnover frequency per Ni center (TOFapp/Ni) versus nmol cm−2 of Ni measured at 3 films each of Ni–Bi)0.25mC, Ni0.4Fe0.6–Bi)1mC, Ni0.6Fe0.4–Bi)1mC, Ni0.9Fe0.1–Bi)1mC, and Ni–Bi)1mC at η = 450 mV (C) and η = 500 mV (D); the points for films with co-deposited Fe have shaded circles for emphasis. The arrows in panel B refer to the overpotentials for the TOF in (C) and (D). | ||
OER activity thus decreased with increasing Ni content in as-deposited Ni–Bi and NiFe–Bi at low potential. This can indicate that only some sites – possibly at the surface – are initially catalytically active in as-deposited films even in the presence of Fe. With increasing potential, as-deposited NiFe–Bi from 60% and 40% Fe exhibited greater promotion by Fe, an effect also observed for anodically-conditioned films, and the TOF at Ni–Bi from a submonolayer to 10 layers became more proportional to the Ni content.
II. NiFe–Bi anodically-conditioned films: electrochemical characterization and OER activity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio during co-deposition.
Ni ratio during co-deposition.
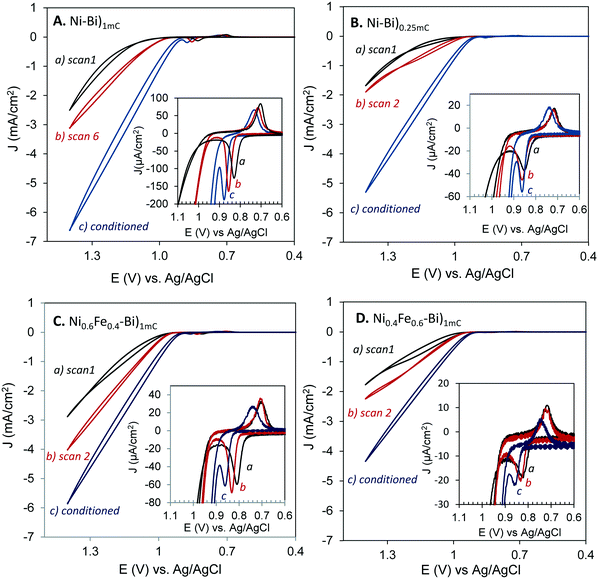
The Ni(OH)2/NiOOH anodic and cathodic peaks of Ni–Bi and NiFe–Bi shifted anodically with anodic conditioning, and the reversibility of the redox couple remained unchanged. For instance, Ep,a of Ni–Bi)1mC shifted to 0.871 ± 0.005 V from 0.836 ± 0.008 V and Ep,a of Ni0.6Fe0.4–Bi)1mC shifted to 0.862 ± 0.016 V from 0.820 ± 0.011 V; with E1/2 shifting by 33 ± 9 mV and 40 ± 8 mV, respectively (N = 3 each). For comparison, Bediako et al. reported Ep,a of Ni–Bi in KBi at 1.05 V and ∼1.025 V vs. NHE before and after anodic conditioning, respectively,31 while Boettcher and co-workers reported an anodic shift that was related to Fe inclusion.24,35 ΔEp of anodically-conditioned Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi equaled 116 ± 5 mV and 106 ± 7 mV, respectively, and ΔEp of Ni–Bi)1mC, Ni0.9Fe0.1–Bi)1mC, and Ni–Bi)0.25mC equaled 139 ± 8, 136 ± 6, and 128 ± 6 mV, respectively, which are all similar to those before conditioning. Fe3+ addition to KBi that immediately increased the OER activity of Ni–Bi also did not decrease ΔEp (vide infra).
Electrochemical precipitation of Ni(OH)2 results in α-Ni(OH)2, β-Ni(OH)2, or α/β-Ni(OH)2 mixed phases depending on the conditions and co-precipitation of metal cations.21 Upon aging α-Ni(OH)2 transforms into β-Ni(OH)2 by loss of intercalated water and anions between sheets via dissolution and re-deposition or a ‘zipping’ mechanism.21 Nocera and co-workers observed that as-deposited Ni–Bi is similar to β-NiOOH at water oxidation potential31 which translates according to the Bode diagram into as-deposited reduced Ni–Bi to be similar to dehydrated β(II) (or deposited as α-Ni(OH)2 that transformed to β-Ni(OH)2). Co-deposition of Fe2+ or Fe3+ has been reported to lead to Fe3+ occupying Ni2+ sites in α-Ni(OH)2.38 Fe(III), Co(III) and Al(III) reportedly stabilize α-Ni(OH)2 and the α/γ couple, possibly because their greater charge increases anion bonding between sheets and hinders transformation to β-Ni(OH)2.39–43 A positive shift in the Ni(OH)2/NiOOH peaks and greater reversibility have been reported with Fe incorporation, and the Ni(OH)2/NiOOH peaks also depend on the phase with α/γ reported to be more reversible and β(II)/β(III) to occur at more positive potentials (although some studies could have involved Fe inclusion in the electrolyte).17,20,24,36
Fe is thus pictured to substitute for Ni sites in Ni–Bi during co-deposition resulting in Fe-α-Ni(OH)2. To explain the increase of OER activity with anodic bias at Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi, it could be thought that deposition from high %Fe in solution leads to segregated Fe-α-Ni(OH)2, β-Ni(OH)2 and Fe(OH)2 regions, though the presence of one redox peak indicates that films behave as a single phase, possibly as a mixed α/β.20,21 Dissolution and re-deposition with anodic biasing could then lead to a more uniform Fe-α-Ni(OH)2/Fe-γ-NiOOH increasing activity. Dissolution and re-deposition must have led to the smoothing in the nanostructured morphology observed in the SEM images of Ni–Bi)400mC and Ni0.6Fe0.4–Bi)400mC after conditioning (Fig. SI.4 and SI.5†), which showed the same general nanostructured fractal morphology like those of as-deposited films but with smoother structures. Assuming Fe-γ-NiOOH as the active catalyst would agree with anodized Ni–Bi resembling γ-NiOOH31 and the necessity of Fe inclusion to enhance OER at Ni–Bi.24,35 An Fe-α-Ni(OH)2/Fe-γ-NiOOH structure in anodized Ni0.4Fe0.6–Bi and Ni0.6Fe0.4–Bi agrees with the smaller peak separation reported with Fe co-precipitation and for the α/γ couple.17,20,36 However, there would remain inconsistencies that need to be explained in this picture; the first is that the peak separation is as narrow as that in as-deposited NiFe–Bi after conditioning, and the second is that the redox couple in anodized Ni–Bi does not become more reversible as OER activity increases when Fe inclusion was reported to take place.24,35
The electrochemical results can be more likely explained by hypothesizing that anodic biasing causes a change in the surface-active sites while Fe is included at the surface of the films, possibly of nanocrystalline domains,31 rather than in the internal structure. Ni–Bi was reported by Bediako et al. to consist of nanocrystalline oxides with the smallest ordered domains of 2–3 nm diameter.31 Incidental Fe inclusion can occur from traces of Fe in the electrolyte, and 14% Fe inclusion has been measured by Boettcher et al. into NiBi (deposited at 10 mC cm−2) from borate,35 and is therefore accordingly assumed to occur as well in Ni–Bi)1mC – and also possibly NiFe–Bi)1mC – from traces in the electrolyte as reported35 (EDX in our study cannot be used to assess the presence of Fe in Ni–Bi, note on EDX in ESI,† Fig. SI.6). It could be that Fe is modifying Ni active sites at the surface structurally or electronically or creates different active surface sites. It has been proposed for instance by Stahl and co-workers that Fe(IV) at edges and corners in NiOx can be kinetically more active.25 The hypothesis of surface active sites modification with including Fe explains or is not inconsistent with the following observations: 1) the presence of high %Fe in solution during co-deposition causes a smaller Ni(OH)2/NiOOH redox peak separation and therefore greater kinetic facility, however subjecting Ni–Bi to anodic bias in KBi did not decrease ΔEp even though OER activity increased, and anodic conditioning was shown to incorporate Fe in the films from the electrolyte.35 Notably, OER activity of Ni–Bi increased immediately by addition of 0.16 mM Fe3+ to the electrolyte (vide infra) but this also did not decrease ΔEp. The greater reversibility is therefore attributed to inclusion of Fe in the bulk of the films, but this process must be different from the one increasing OER activity with anodic bias which did not narrow ΔEp. A greater Fe to Ni ratio could be needed inside the films (versus the surface) for greater reversibility during deposition of NiFe–Bi from 40% or 60% Fe. 2) The presence of high %Fe in solution during electrodeposition caused a cathodic shift while anodic conditioning caused an anodic shift in the redox peaks, pointing to the presence of two different processes. 3) ΔEp was the same for Ni0.4Fe0.6–Bi and Ni0.6Fe0.4–Bi before and after conditioning, even as the activity increased and E1/2 positively shifted with anodic bias application. 4) The similar OER activity per Ni site for as-deposited Ni–Bi)1mC and Ni–Bi)10mC in the first CV scan with increasing bias may indicate a structural or electronic change in surface exposed sites possibly at nanocrystalline domains,31 rather than in sites buried in the bulk; otherwise, it would be that similar inclusion of Fe occurs in the bulk of multilayers from the first scan independent of the thickness which is a less plausible picture. Therefore, it is possible that anodic conditioning causes restructuring with inclusion of Fe to form the active catalytic surface sites, but this process does not extend fully to the bulk.
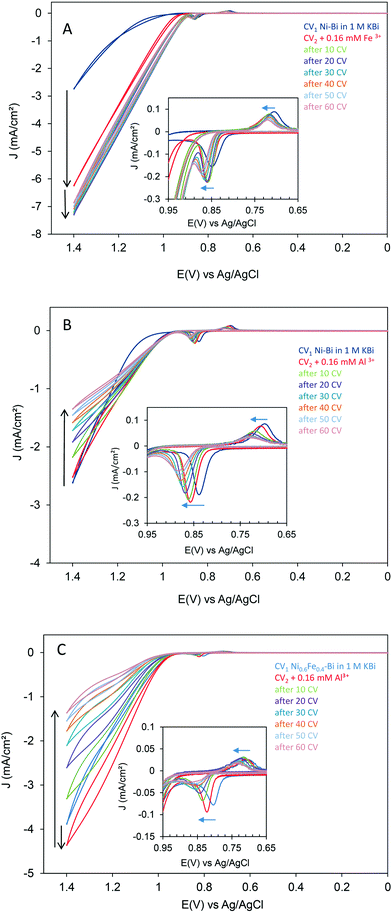
In addition, the effect of adding 0.16 mM Al3+ (99.999%) to 1 M KBi on OER for co-deposited Ni0.6Fe0.4–Bi)1mC was investigated. Fig. 4C shows a first scan CV of Ni0.6Fe0.4–Bi)1mC at 10 mV s−1 before and after addition of Al3+ to 1 M KBi, then after acquiring 10 CVs at 100 mV s−1 – repeated six times. The first scan CV after addition of Al3+ showed an increase in OER activity compared to the CV in 1 M KBi – possibly indicating a restructuring of the surface. However, subsequent potential scanning resulted in a decrease in OER activity. The Ni(OH)2 redox peaks also decreased in magnitude and positively shifted. There was a difference however in the behavior of Ni–Bi)1mC and NiFe–Bi)1mC that is worth noting. While in the case of Ni–Bi)1mC, the OER activity only decreased at the high potential and the CVs are similar at the beginning of the OER wave (Fig. 4B), in the case of Ni0.6Fe0.4–Bi)1mC the currents were initially larger as expected but the subsequent decrease in the presence of Al3+ was observed at both low and high potential which could be caused by Al replacing Fe in NiFe–Bi, and an inherent activity that is independent of Al inclusion in Ni–Bi at low potential and these differences require further study. We also investigated the competition between Al3+ and Fe3+ for co-inclusion in the films in an experiment where equal amounts of Fe3+ and Al3+ (0.08 mM each) were added to the electrolyte after deposition of Ni–Bi)1mC (Fig. SI.12†). The first CV in the presence of both ions showed a significant increase in OER activity resembling the immediate increase that was observed (Fig. 4A) when adding 0.16 mM Fe3+ albeit with smaller OER currents. However, OER currents decreased with subsequent scanning, attributed to the poisoning effect of Al3+ at high potential which must be co-included or competitively included in the the presence of a similar amount of Fe3+ in solution.
The poisoning effect of Al could be attributed to reducing the number of active sites if Fe is assumed as the active site, or to inhibiting promotion by Fe, and was observed for Ni–Bi)1mC or when Fe is co-deposited in NiFe–Bi or when Al and Fe are present in equal amounts in the electrolyte. In the initial experiments, a similar poisoning effect was not observed when Al was added to the electrodeposition medium of Ni–Bi at a ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al of 6
Al of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the effect of Al co-inclusion in Ni–Bi films is under current study. The role of the guest metal may depend on pre-catalyst preparation and structure, as AlNiOx was reported to be as active as FeNiOx,19 and AlFeNiO4 was reported to have the greatest activity amongst several bimetallic and trimetallic oxides.12,19 (Another example is inclusion of Co that was reported to not increase OER activity of Ni-oxide by blocking its structural transformation to the active catalyst,11 while a synergistic effect of Co in NiOx has been observed16).
4, and the effect of Al co-inclusion in Ni–Bi films is under current study. The role of the guest metal may depend on pre-catalyst preparation and structure, as AlNiOx was reported to be as active as FeNiOx,19 and AlFeNiO4 was reported to have the greatest activity amongst several bimetallic and trimetallic oxides.12,19 (Another example is inclusion of Co that was reported to not increase OER activity of Ni-oxide by blocking its structural transformation to the active catalyst,11 while a synergistic effect of Co in NiOx has been observed16).
The promoting effect of Fe on OER activity of NiFe-oxide has been linked to a partial charge transfer effect, and this was reasoned to reconcile with the anodic shift in the redox peaks attributed to inclusion of Fe.35,25 It is noted and requires further investigation that an anodic shift in the Ni(OH)2/NiOOH redox peaks was observed with potential scanning in the presence of both ions, but was uncorrelated in this case with their opposite effects on OER activity: adding Fe3+ to the electrolyte caused a fast increase in OER activity with potential scanning, while adding Al3+ poisoned OER catalysis.
Tafel slopes at low and high current density. Fig. 5A and B present Tafel plots (η versus log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J) of Ni–Bi)1mC and Ni–Bi)0.25mC (A), and of Ni0.4Fe0.6–Bi)1mC and Ni0.9Fe0.1–Bi)1mC (B) films (for films with CVs in Fig. 1 after conditioning); and Fig. 5C presents Tafel plots of three Ni0.6Fe0.4–Bi)1mC films. Two linear Tafel regions were identified, the first at current densities lower than ∼1 mA cm−2 and the second at current densities of ∼1–3 mA cm−2. The Tafel slopes of anodically-conditioned Ni–Bi and NiFe–Bi (N = 3 each) are presented Table 3. The slope in the first region was not dependent on %Fe during deposition, and equaled 37 ± 2 mV dec−1 for Ni–Bi)1mC and Ni0.9Fe0.1–Bi, 37 ± 1 mV dec−1 for Ni0.6Fe0.4–Bi, 38 ± 3 mV dec−1 for Ni–Bi)10mC, 42 ± 2 mV dec−1 for Ni0.6Fe0.4–Bi and 51 ± 6 mV dec−1 for Ni–Bi)0.25mC. The larger slope of NiBi)0.25mC could be due to a limited active area. On the other hand, the Tafel slopes were not reproducible in the second linear region. The Tafel slopes of Ni–Bi)1mC and Ni–Bi)10mC at current densities greater than ∼1 mA cm−2 either increased or in some cases were unchanged or slightly decreased, which may be due to variations in Fe uptake from solution. The Tafel slopes of Ni0.9Fe0.1–Bi, Ni–Bi)0.25mC and of two of three Ni0.4Fe0.6–Bi films increased at high overpotential but with different magnitudes, and the Tafel slope decreased for the third Ni0.4Fe0.6–Bi film. Three Ni0.6Fe0.4–Bi films exhibited an unusual decrease in their Tafel slope, from 38 mV dec−1 to 30 mV dec−1, from 37 mV dec−1 to 31 mV dec−1, and from 36 to 18 mV dec−1 (Table 3 and Fig. 5C). With the variation, a trend could be seen to emerge regarding the effect of Fe in preventing the increase in the Tafel slope at high current density.
J) of Ni–Bi)1mC and Ni–Bi)0.25mC (A), and of Ni0.4Fe0.6–Bi)1mC and Ni0.9Fe0.1–Bi)1mC (B) films (for films with CVs in Fig. 1 after conditioning); and Fig. 5C presents Tafel plots of three Ni0.6Fe0.4–Bi)1mC films. Two linear Tafel regions were identified, the first at current densities lower than ∼1 mA cm−2 and the second at current densities of ∼1–3 mA cm−2. The Tafel slopes of anodically-conditioned Ni–Bi and NiFe–Bi (N = 3 each) are presented Table 3. The slope in the first region was not dependent on %Fe during deposition, and equaled 37 ± 2 mV dec−1 for Ni–Bi)1mC and Ni0.9Fe0.1–Bi, 37 ± 1 mV dec−1 for Ni0.6Fe0.4–Bi, 38 ± 3 mV dec−1 for Ni–Bi)10mC, 42 ± 2 mV dec−1 for Ni0.6Fe0.4–Bi and 51 ± 6 mV dec−1 for Ni–Bi)0.25mC. The larger slope of NiBi)0.25mC could be due to a limited active area. On the other hand, the Tafel slopes were not reproducible in the second linear region. The Tafel slopes of Ni–Bi)1mC and Ni–Bi)10mC at current densities greater than ∼1 mA cm−2 either increased or in some cases were unchanged or slightly decreased, which may be due to variations in Fe uptake from solution. The Tafel slopes of Ni0.9Fe0.1–Bi, Ni–Bi)0.25mC and of two of three Ni0.4Fe0.6–Bi films increased at high overpotential but with different magnitudes, and the Tafel slope decreased for the third Ni0.4Fe0.6–Bi film. Three Ni0.6Fe0.4–Bi films exhibited an unusual decrease in their Tafel slope, from 38 mV dec−1 to 30 mV dec−1, from 37 mV dec−1 to 31 mV dec−1, and from 36 to 18 mV dec−1 (Table 3 and Fig. 5C). With the variation, a trend could be seen to emerge regarding the effect of Fe in preventing the increase in the Tafel slope at high current density.
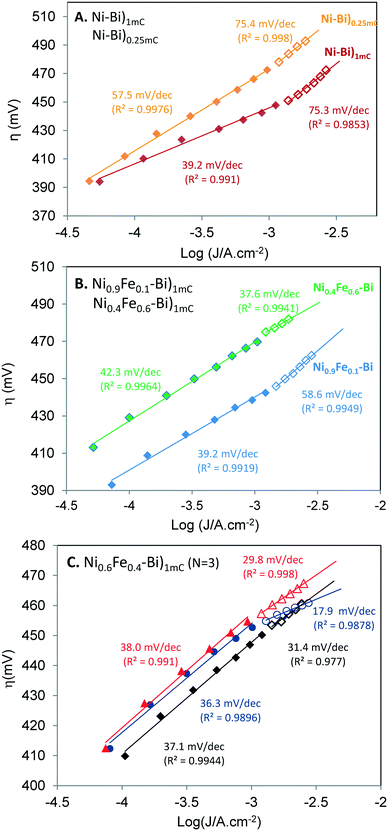 | ||
| Fig. 5 Tafel plots for Ni–Bi)1mC and Ni–Bi)0.25mC (A) and for Ni0.9Fe0.1–Bi)1mC and Ni0.4Fe0.6–Bi)1mC (B), for films with CVs in Fig. 1 after anodic conditioning. (C) Tafel plots for 3 independently prepared Ni0.6Fe0.4–Bi)1mC anodically-conditioned films. The Tafel slope and the correlation coefficients for the best fit line are shown in the two linear regions. | ||
| Films (N = 3 or 2) | nmolNi cm−2 | Monolayereq [Ni(OH2)] | Slope α1 (mV dec−1) (up to ∼1 mA cm−2) | Slope α2 (mV dec−1) (≳1 to 2–3 mA cm−2) | (one slope α mV dec−1) |
|---|---|---|---|---|---|
| Ni–Bi)1mC | 2.77 ± 0.99 | 2.57 ± 0.91 | 35 | 32 | (34) |
| 39 | 75 | ||||
| 38 | 35 | (36) | |||
| 37 ± 2 | |||||
| Ni0.9Fe0.1–Bi)1mC | 3.27 ± 1.09 | 3.03 ± 1.01 | 39 | 59 | |
| 36 | 45 | ||||
| 36 | 48 | ||||
| 37 ± 2 | |||||
| Ni0.6Fe0.4–Bi)1mC | 1.10 ± 0.42 | 1.02 ± 0.39 | 38 | 30 | (34) |
| 37 | 31 | (36) | |||
| 36 | 18 | ||||
| 37 ± 1 | |||||
| Ni0.4Fe0.6–Bi)1mC | 0.57 ± 0.07 | 0.53 ± 0.07 | 42 | 38 | (43) |
| 40 | 48 | (41) | |||
| 43 | 88 | ||||
| 42 ± 2 | |||||
| Ni–Bi)250μC | 0.89 ± 0.09 | 0.83 ± 0.09 | 57 | 75 | |
| 46 | 92 | ||||
| 50 | 141 | ||||
| 51 ± 6 | |||||
| Ni–Bi)10mC | 10.45 ± 0.22 | 9.69 ± 0.20 | 36 | 32 | (34) |
| 40 | 60 | ||||
| 38 ± 3 | |||||
Slopes between 30 and 40 mV dec−1 have been reported for NiOx, and larger or smaller Tafel slopes were measured with increasing thickness or Fe content, respectively. Corrigan reported a slope of 50 mV dec−1 for NiOx in KOH in the presence of 1 ppm iron and 40 mV dec−1 for thinner films, 25 mV dec−1 with 10% co-precipitated Fe, ca. 20 mV dec−1 at 50–75% Fe down to as low as 15 mV dec−1 at 75% Fe.17 30 mV dec−1 was measured by Nocera and co-workers for thin Ni–Bi32 and 60 mV dec−1 for thicker films.30 46 mV dec−1 was reported by Boettcher and co-workers for Ni–Bi deposited at 10 mC cm−2.35 The Tafel slopes of Ni-oxides and other electrodes have been reported to increase at high current density.11,17,44–46 For instance, the Tafel slope of NiOx in KOH (in the absence of Fe) reported by Corrigan increased from 50 to 70 mV dec−1 at currents greater than 1 mA cm−2,17 and so did the Tafel slope of NiCoOx.11 Another example is Pt in alkaline solution with an increase from 46 mV dec−1 to 148 mV dec−1.44,45 An increase in the Tafel slope can be caused by a different rate determining step in the same pathway since different reactions have different potential dependencies, a variation of intermediate coverage which would manifest in linear regions with lower slopes at low overpotential and higher slopes at high overpotentials,45,46 uncompensated resistance, and degradation or structural changes.1 The similar behaviors of Ni–Bi)1mC and Ni–Bi)10mC, and the increase in the slope for Ni–Bi)0.25mC do not support that resistance is causing the higher slope at high potential. Catalyst degradation can also be ruled out as a cause of the increased slope since measurements were conducted from high to low current.
On the other hand, a decrease in the Tafel slope has not been reported to our knowledge for NiOx. However, Corrigan observed that 1 ppm Fe in solution prevented the increase in the Tafel slope of NiOx in KOH at high overpotential, as seen in Fig. 4 of ref. 17. The Tafel behavior of Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi was different from that of Ni–Bi)0.25mC, which exhibited a greater increase in the slope at high current density despite comparable Ni content, which could point to the role of Fe in preventing the same slope increase or causing its decrease. A decrease in the Tafel slope was reported for OER on Pt in sulfuric acid (from 117 mV dec−1 to 57 mV dec−1 at high potential) and was attributed by Schultze and Haga to ‘resonance tunneling’,47 and by Conway and Liu to redox mediation involving higher oxidation states of Pt proposing these ideas as equivalents.48
Effect of Fe on OER rate at low versus high overpotential. The apparent turnover frequency for OER per Ni center was calculated from steady-state currents (TOFss) at different overpotentials for anodically-conditioned Ni–Bi and NiFe–Bi measured from high to low potential (Table 2). Fig. 6 shows TOFssversus the number of moles of Ni (nNi) at average η = 409–414 mV (A), ∼430 mV (B), and at ∼450 and ∼460 mV (C). The average TOFss in the average range 409–414 mV equaled 0.23 s−1 for Ni60Fe40–Bi and Ni40Fe60–Bi, 0.13–0.16 s−1 for Ni–Bi)1mC, Ni–Bi)10mC and Ni0.9Fe0.1–Bi)1mC, and 0.16 s−1 for Ni–Bi)0.25mC (Table 2). The average TOFss at ∼430 mV equaled 0.45–0.56 s−1 for Ni–Bi)1mC, Ni–Bi)10mC and NiFe–Bi with the higher average (0.56 s−1) for Ni0.6Fe0.4–Bi, and was lower (0.2 s−1) for Ni–Bi)0.25mC. The average TOFss for Ni0.6Fe0.4–Bi reached 5.08 ± 0.73 s−1 at η = 460 ± 0 mV (N = 3), greater than that for Ni0.9Fe0.1–Bi (2.35 ± 0.01 s−1 at 460 ± 0 mV, N = 2) and Ni–Bi)1mC (2.74 ± 1.62 s−1 at η = 457 mV ±3, N = 2). The ratio of the average TOF at 460 mV for Ni0.6Fe0.4–Bi (N = 3) to Ni–Bi)1mC (N = 2) and to Ni0.9Fe0.1–Bi (N = 2) is 1.85 and 2.16, respectively, compared to ratios of 1.06 and 1.13 at 430 mV (N = 3 each), and ratios of 1.08 and 1.15 at 450 mV (N = 3 each). The promoting effect of Fe in Ni0.6Fe0.4–Bi was therefore greater at higher overpotential. The difference was not as apparent when compared to the lowest overpotential range measured (at the beginning of the Tafel plot at ∼409–414 mV), where these ratios were 1.7 and 1.5, respectively (cf.Table 2). At ∼460 mV, the TOF of Ni0.4Fe0.6–Bi (3.22 ± 0.45 s−1 at 461 ± 1 mV) was 2.2-fold larger than that of Ni–Bi)0.25mC (1.48 ± 0.27 s−1 at 461 ± 2 mV) with its higher Ni content in a submonolayer coverage.
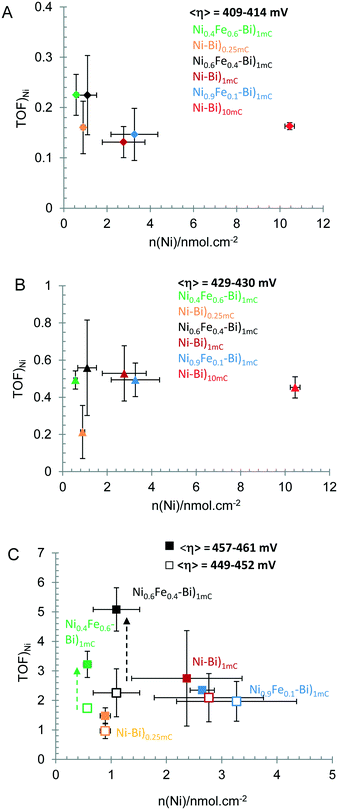 | ||
| Fig. 6 Steady-state turnover frequency per Ni (TOFSS/Ni) versus nmol cm−2 of Ni measured at anodically-conditioned Ni–Bi)0.25mC, Ni0.6Fe0.4–Bi)1mC, Ni0.9Fe0.1–Bi)1mC, Ni–Bi)1mC, and Ni0.4Fe0.6–Bi)1mC, at average η = 409–414 mV (A); <η> = 429–430 mV (B); <η> = 449–452 mV (□) and 457–461 mV (■) (C). All measurements are for N = 3 films except for Ni–Bi)1mC and Ni0.9Fe0.1–Bi)1mC at overpotentials of 457–461 mV and for Ni–Bi)10mC where N = 2. Details are in Table 2. | ||
A similar general trend could also be observed from CVs at 10 mV s−1 without stirring but with lower TOFs. Fig. 7 shows CVs (A) and normalized current to nNiversus E (B) and η (C) plots for anodically-conditioned Ni–Bi)1mC, Ni0.9Fe0.1–Bi)1mC, Ni0.6Fe0.4–Bi)1mC Ni0.4Fe0.6–Bi)1mC and Ni–Bi)0.25mC. Fig. 7B shows greater normalized currents versus potential for Ni0.4Fe0.6–Bi with the smallest Ni content, followed by Ni0.6Fe0.4–Bi and Ni–Bi)0.25mC. When the normalized currents are plotted versus overpotential in Fig. 7C promotion by Fe is seen more clearly with increasing overpotential for Ni0.6Fe0.4–Bi followed by Ni0.4Fe0.6–Bi. We note however that there was larger variability from CVs of films at higher η. At η = 420 mV, the average TOF from the CV currents was 0.142 ± 0.047 s−1 and 0.156 ± 0.018 for Ni–Bi)1mC and Ni–Bi)10mC, respectively, and 0.27 s−1 ± 0.027 s−1 and 0.189 ± 0.020 s−1 for Ni0.6Fe0.4–Bi)1mC and Ni0.4Fe0.6–Bi)1mC, respectively, for 3 films each. At η = 430 mV, the average TOF equaled 0.260 ± 0.073 s−1 for Ni–Bi)1mC, compared to 0.403 ± 0.043 s−1 for Ni0.6Fe0.4–Bi)1mC. At 460 and 480 mV, the average TOFs were 1.92-fold and 2.2-fold greater, respectively, of Ni0.6Fe0.4–Bi)1mC (3.065 ± 1.675 s−1 and 6.39 ± 3.41 s−1, respectively, N = 3 each) compared to those of Ni–Bi)1mC (1.599 ± 0.357 s−1 and 2.93 ± 0.71 s−1, respectively, N = 3 each), greater than the 1.55 ratio at 430 mV, but comparable on average to the 1.9 ratio at 420 mV; but with a larger variation around the mean.
A comparison of OER activity (the TOF and the overpotential needed to deliver 1 mA cm−2) to reported thin Ni–Bi films32,34,35 and nanostructured 3D Ni–Bi films49–54 and the affecting factors are presented in detail in the ESI.† In general, the apparent steady-state TOF for the ultra-thin films in this study at low potentials is lower than that reported for Ni–Bi films, and the onset of the linear Tafel region started at higher overpotentials but shifted to lower values with increasing coverage from Ni–Bi)1mC to Ni–Bi)10mC, and then to Ni–Bi)100mC and the overpotential needed to drive 1 mA cm−2 decreased with increasing coverage. A current density of 1 mA cm−2 was measured at steady-state at 430–440 mV for Ni–Bi)1mC, at ca. 420 mV for Ni–Bi)10mC, and at 399–404 mV for Ni–Bi)100mC (N = 3). By comparison, Dincã et al. reported 1 mA cm−2 at 425 mV for Ni–Bi deposited at 300 mC cm−2 in 1 M KBi,34 while Bediako et al. reported 1 mA cm−2 at ∼400 mV for thin Ni–Bi at 1 mC cm−2 (from Tafel plots presented) which contained ca. 5.9 nmol cm−2 Ni – the latter with a TOF of 0.9 s−1 at 400 mV32 which is greater than that observed in this work. Boettcher et al. reported for Ni–Bi deposited by passing 10 mC cm−2 a TOF of 0.38 s−1 at 400 mV after conditioning, but only 0.03 s−1 in Fe-free electrolyte at 400 mV; meanwhile, they reported significantly greater TOFs for films with co-deposited Fe (with 1.1 e per Ni).35 It is possible that experimental variations leading to differences in inclusion of Fe, the impurities, resistance and structure of the substrate and electrolyte concentration, could have affected the amount deposited and film coverage and therefore the catalytic activity, the onset of the Tafel region and TOF at low η. On the other hand, 3D NiBi, Ni–Co–Pi, and NiFe–Bi nanoarrays with greater catalyst loading were recently reported to yield significantly greater currents at lower overpotential, but lower TOFs and larger Tafel slopes (100–200 mV dec−1).49–54 For instance, the TOF at 600 mV for 3D Ni–Bi–Pi was 0.2 s−1 at 600 mV for 0.77 μmol cm−2 Ni,51 and a bimetallic Ni-substituted Co–Bi on carbon cloth (3.8 μm thick) with a loading of 2.1 mg cm−2 that required only 388 mV to deliver 10 mA cm−2 was reported to have a TOF of 0.33 s−1 at 500 mV, and 0.2 s−1 at 450 mV.49 The ultra-thin Ni–Bi)1mC film with ∼2.8 nmol cm−2 yielded an apparent TOFss per Ni of 2.74 ± 1.62 s−1 at η = 457 mV ±3 (N = 2), while the apparent TOFss at Ni0.6Fe0.4–Bi with ∼1 nmol cm−2 Ni reached 5.08 ± 0.73 s−1 at η = 460 ± 0 mV (N = 3).
The mechanism by which OER activity for NiOx is enhanced by inclusion of Fe, and whether the active site is a Ni site or an Fe site are not settled questions for NiOx electrodes.22–28 There are two hypotheses: one hypothesis assumes a Ni active site promoted by Fe – with possible electronic effects14,24,28 – and the other assumes an Fe active site.22,27 Bell and co-workers showed that Fe sites surrounded by Ni nearest neighbors in γ-NiOOH have near-optimal adsorption energy for OER intermediates.27 The electrochemical results at low and high overpotential are discussed within these different hypotheses.
In this work, for ultra-thin Ni–Bi and NiFe–Bi films, the general proportionality of the calculated TOF per Ni center to the electrochemically accessible Ni centers at overpotentials within the first Tafel region for conditioned Ni–Bi between 2–3 layers and ∼10 layers and for NiFe–Bi (at 430 mV, Fig. 6B) is a result that can be more consistent with the first hypothesis of a Ni active site with the greatest promotion by Fe at optimal doping. The effect of Fe in promoting the activity of Ni and its effect on the redox behavior of Ni(OH)2 films has been discussed in the literature. Boettcher et al. showed that while Fe increased the conductivity of NiOx,24 as indicated earlier by Corrigan,17 this did not account for the increase in OER activity.24 They proposed that the promoting role of Fe in Ni active site occurs via partial charge transfer24 – likened to the effect of the more electronegative Au support in increasing OER activity of Co-oxide by pulling electron density towards it55,56 – and this explained the anodic shift in the Ni(OH)2/NiOOH redox peak with Fe inclusion.24 In this regard, Stahl and co-workers also presented an explanation for the anodic shift by reasoning that Fe3+ in the second coordination sphere will result in the oxide/hydroxide bridging ligands to have a less e-donating ability, which destabilizes Ni3+ and increases the redox potential.25 Corrigan et al. on the other hand earlier reported in situ Mössbauer spectral changes that showed electronic and structural rearrangements with Fe in NiOx, with a highly oxidized iron species as a result of partial transfer of electron densities away from Fe3+ sites, which could occur when the NiII sites are oxidized in Fe–Ni(OH)2.28 We observed in this work that anodic conditioning results in an anodic shift in the redox peaks, similar to the observation by Boettcher and co-workers; however Fe at 40% or 60% during co-deposition with Ni resulted in a cathodic shift in Ep,a without a shift in E1/2. We observed also an anodic shift in the Ni–Bi redox peaks in the presence of Al3+ even though OER activity was poisoned. These differences still need to be reconciled for a complete understanding of the role of Fe in the OER activity of NiOx films, and if there is a promotion effect of a Ni site by Fe linked to the effect on the redox behavior.
On the other hand, with increasing overpotential, the apparent TOF calculated per electrochemically active Ni center was no longer on average proportional to the number of Ni centers and instead a more significant increase in TOF was measured on average for Ni0.6Fe0.4–Bi)1mC relative to Ni–Bi)1mC than at the lower potentials (Fig. 6C). In addition, the apparent TOF of ultrathin Ni–Bi)0.25mC which was more comparable at low overpotential (409–414 mV) to Ni0.6Fe0.4–Bi and Ni0.4Fe0.6–Bi with the comparable Ni(OH)2 coverage becoming significantly lower with increasing potential. The greater increase in OER activity with Fe observed at higher overpotential for NiFe–Bi appears to be similar to the results of Corrigan for Fe-free NiOx and NiOx with 1 ppm Fe in KOH solution, where currents are seen to be equal at low overpotential but become higher with Fe at high overpotential.17 These results could be pointing to a possibly different role of Fe at high overpotential, and could be consistent with a hypothesis of Fe active site with greater activity at these potentials. The effect of Al in decreasing the current of Ni–Bi)1mC at high potential but not at low potential can also indicate the existence of different mechanisms depending on the potential. The effect of Al in poisoning the OER activity in NiFe–Bi can support the picture of Al replacing Fe lowering activity at high potential if Fe is the active site. In this case, however, Al also poisoned OER at low potential therefore decreasing the initial promotion by Fe in as-deposited NiFe–Bi. The role of Al in Ni–Bi and NiFe–Bi and the effect of the inclusion method on the role it plays in OER are under further investigation.
Conclusions
We investigated the effect of co-precipitation of Fe with Ni in NiFe-oxo/hydroxo ultra-thin films in borate on OER catalysis and Ni(OH)2/NiOOH redox behavior compared to that of Ni–Bi films with coverage from a submonolayer to multilayers. The NiFe–Bi and Ni–Bi films were investigated as-deposited and after application of anodic bias. In addition, we studied the effects of adding Fe3+ and Al3+ to the electrolyte after Ni–Bi film deposition and the effect of adding Al3+ after co-deposition of NiFe–Bi. The main observations and conclusions drawn from this study are summarized here: 1) as-deposited NiFe–Bi films are more active for oxygen evolution per Ni site than Ni–Bi; however they still required, similar to Ni–Bi, application of anodic bias to reach the maximum activity even with an initial Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio of 40
Fe ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 in solution, indicating the need of a transformation to form the most active catalyst. 2) The effects of Fe co-deposition which caused more cathodic and more reversible Ni(OH)2/NiOOH redox peaks were different from the effects of applying anodic bias, which caused an anodic shift and no change in peak separation of Ni–Bi and NiFe–Bi, leading to the conclusion that the process that increases OER activity with anodic bias – though it has been shown to lead to Fe inclusion – results in a different structure than when Fe is co-precipitated with Ni, consistent with the observation in (1). A possible hypothesis that can reconcile these results is that surface site modification with Fe inclusion rather than its bulk inclusion is the cause of the increase in OER activity with anodic conditioning. Furthermore, we observed that addition of Fe3+ to the electrolyte after deposition of Ni–Bi causes a fast increase in OER activity (and therefore 3 h anodic conditioning can be replaced by this process) without narrowing the redox peaks separation, also supporting this hypothesis of surface change, while addition of Al3+ to the electrolyte poisoned OER catalysis for both Ni–Bi and NiFe–Bi. Noteworthy is that the redox peaks shifted anodically in the presence of both ions, with their opposite effects on catalysis. 3) The apparent TOF per Ni for OER of anodically-conditioned Ni–Bi and NiFe–Bi showed proportionality to the Ni content within the first Tafel region, which could be consistent with a Ni active site, but increased more significantly with co-deposited Fe at optimal loading at high current density. The Tafel slopes of conditioned Ni–Bi and NiFe–Bi were similar at low current density equaling 37–42 mV dec−1, but variations were measured at high current density depending on the Fe content. These observations pointed to the possibility of different roles of Fe at low and high potential, which could be an Fe active site at high potential, requiring further study. Important questions thus remain with regard to the nature of the active site, and the specific structural or electronic role Fe plays in the internal or surface site catalytic activity as a function of potential and film thickness. The role of other ions such as Al3+ and the dependence of their effect on the mode of incorporation are also future questions for investigation. The relation between the anodic shift observed with anodic biasing and in the presence of some ions and the OER activity also requires further examination.
60 in solution, indicating the need of a transformation to form the most active catalyst. 2) The effects of Fe co-deposition which caused more cathodic and more reversible Ni(OH)2/NiOOH redox peaks were different from the effects of applying anodic bias, which caused an anodic shift and no change in peak separation of Ni–Bi and NiFe–Bi, leading to the conclusion that the process that increases OER activity with anodic bias – though it has been shown to lead to Fe inclusion – results in a different structure than when Fe is co-precipitated with Ni, consistent with the observation in (1). A possible hypothesis that can reconcile these results is that surface site modification with Fe inclusion rather than its bulk inclusion is the cause of the increase in OER activity with anodic conditioning. Furthermore, we observed that addition of Fe3+ to the electrolyte after deposition of Ni–Bi causes a fast increase in OER activity (and therefore 3 h anodic conditioning can be replaced by this process) without narrowing the redox peaks separation, also supporting this hypothesis of surface change, while addition of Al3+ to the electrolyte poisoned OER catalysis for both Ni–Bi and NiFe–Bi. Noteworthy is that the redox peaks shifted anodically in the presence of both ions, with their opposite effects on catalysis. 3) The apparent TOF per Ni for OER of anodically-conditioned Ni–Bi and NiFe–Bi showed proportionality to the Ni content within the first Tafel region, which could be consistent with a Ni active site, but increased more significantly with co-deposited Fe at optimal loading at high current density. The Tafel slopes of conditioned Ni–Bi and NiFe–Bi were similar at low current density equaling 37–42 mV dec−1, but variations were measured at high current density depending on the Fe content. These observations pointed to the possibility of different roles of Fe at low and high potential, which could be an Fe active site at high potential, requiring further study. Important questions thus remain with regard to the nature of the active site, and the specific structural or electronic role Fe plays in the internal or surface site catalytic activity as a function of potential and film thickness. The role of other ions such as Al3+ and the dependence of their effect on the mode of incorporation are also future questions for investigation. The relation between the anodic shift observed with anodic biasing and in the presence of some ions and the OER activity also requires further examination.
Experimental methods
Materials
Nickel nitrate pentahydrate (Ni(NO3)2·5H2O, 99.999%, trace metal analysis, Aldrich) was used for all films except for the experiments examining the effect of addition of Fe3+ and Al3+ to the potassium borate electrolyte and in the absence of added metal cations, and as the second source for testing the absence of anodic shifts with Fe co-deposition where nickel(II) nitrate hexahydrate (99.9985%, metals basis, Alfa Aesar) was used. Ferric nitrate nonahydrate (Fe(NO3)3·9H2O, 98%, Aldrich); aluminum nitrate nonahydrate (99.999%, trace metal basis, Acros Organics; termed source 1), aluminum nitrate nonahydrate (98% Al by EDTA titration by manufacturer, contains Fe at 0.001% by manufacturer; Baker Chemical Co.), boric acid (H3BO3, 99.5%, Aldrich), and potassium hydroxide (KOH, Aldrich) were used in this study. All films were electrodeposited on fluorine-doped tin oxide coated glass (FTO, R = 15 Ω sq−1, except experiment in Fig. 4CR = 7 Ω sq−1, Solaronix). Deionized water (resistivity 18 μΩ cm, Nanopure Diamond) was used for solution preparation.Electrodeposition of Ni–Bi and NiFe–Bi films
FTO electrodes were cleaned by ultrasonication in isopropanol for 30 min and rinsed with water, followed by ultrasonication in water for 10–15 min, and air drying. Ni–Bi and NiFe–Bi films were electrodeposited on FTO in a 3-electrode electrochemical cell with Ag/AgCl (in saturated KCl) as the reference electrode and a 2 mm diameter Pt wire as the counter electrode. Electrodeposition was conducted from a 0.4 mM Ni(NO3)3 or 0.4 mM total concentration of Ni(NO3)3 and Fe(NO3)3 in 0.1 M KBi (aq) solution pH ∼9.2 at Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratios of 9
Fe ratios of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 4
4, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Films were electrodeposited by applying an anodic potential of 0.953 V vs. Ag/AgCl, and the amount deposited was controlled by halting the experiment when the charge reached a certain value. Ni–Bi and NiFe–Bi films were deposited by passing a charge of 1 mC cm−2, while thicker Ni–Bi films were electrodeposited at 10 mC cm−2 and thinner films were deposited by passing a charge of 250 μC cm−2. These ultra-thin Ni(OH)2 films prepared by passing a charge of 250 μC cm−2 were intended to have a low number of Ni-sites as films obtained at 6
6. Films were electrodeposited by applying an anodic potential of 0.953 V vs. Ag/AgCl, and the amount deposited was controlled by halting the experiment when the charge reached a certain value. Ni–Bi and NiFe–Bi films were deposited by passing a charge of 1 mC cm−2, while thicker Ni–Bi films were electrodeposited at 10 mC cm−2 and thinner films were deposited by passing a charge of 250 μC cm−2. These ultra-thin Ni(OH)2 films prepared by passing a charge of 250 μC cm−2 were intended to have a low number of Ni-sites as films obtained at 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or 4
4 or 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratios of Ni
6 ratios of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe at 1 mC cm−2 charge. Electrode areas were defined with insulating epoxy between 1.0 and 1.5 cm2. Current densities are reported relative to the geometric area of the electrode.
Fe at 1 mC cm−2 charge. Electrode areas were defined with insulating epoxy between 1.0 and 1.5 cm2. Current densities are reported relative to the geometric area of the electrode.
Electrochemical measurements
Electrochemical measurements were performed in a 3-electrode electrochemical cell using a CHI Model 630A electrochemical workstation with Ag/AgCl (saturated KCl) as the reference electrode, either home-made or from Bioanalytical Systems (BAS), and a 2 mm diameter Pt wire as the counter electrode in 1 M KBi of pH ∼9.2–9.4. Cyclic voltammograms were acquired by scanning the potential between −0.6 V and 1.4 V vs. Ag/AgCl first in the positive direction. Films were either examined as-deposited (as a first CV scan without aging) or following a procedure of anodic conditioning. Anodic conditioning consisted of holding the potential at 0.903 V vs. Ag/AgCl in 1 M KBi for 3 h with stirring. Cyclic voltammograms were acquired in unstirred solutions. Tafel slope measurements were performed by carrying out controlled potential electrolysis in a solution of 1 M KBi electrolyte at pH 9.2–9.4. Prior to data collection, the resistance of the solution at open circuit potential was measured using an iR test function to correct for ohmic potential losses. Steady-state currents were measured at different applied potentials using amperometry (i–t curves) while the solution was stirred at 600 rpm. Films required 400 to 600 s to reach a steady state. Currents were collected at potentials ranging between 1.12 V to 0.84 V vs. Ag/AgCl (at 20 mV increments, from high potential to low potential). Charges under the cathodic peaks were calculated using the CHI instrument's 760 software using the Gaussian peak definition. The overpotential was calculated using η = E − Eeq(O2/H2O) − iR, where EeqO2/H2O at the specific solution pH measured (in the measurements shown either 9.16 or 9.41) is calculated as follows: Eeq (V) = 1.23 − (0.059 × pH) + (0.059 × log(0.209)/4) − 0.197. The turnover frequency per Ni TOF was calculated as i/4FnNi, where i is the current in A, F is Faraday's constant, 4 is the number of electrons per O2 and nNi is the number of moles of Ni.Effect of addition of Fe3+ and Al3+ to the electrolyte
Ni–Bi and Ni0.6Fe0.4–Bi films were electrodeposited on FTO at 0.953 V vs. Ag/AgCl by passing a charge of 1 mC cm−2. Films were rinsed and moved to 20 mL of 1.0 M KBi pH ∼9.2. A first CV scan was acquired at 10 mV s−1, then 8 μL of either 0.4 M Fe3+ (for case of Ni–Bi) or 0.4 M Al3+ (for case of Ni–Bi or Ni0.6Fe0.4–Bi) was added to the electrolyte and mixed, or 4 μL each of Al3+ and Fe3+ in one experiment, and CV was acquired at 10 mV s−1 immediately after addition of the metal cations. Ten CVs were then acquired at 100 mV s−1, followed by acquiring a CV at 10 mV s−1. This was repeated 6 or 7 times. In one experiment, three CVs were acquired for Ni–Bi at 10 mV s−1, 20 mV s−1 and 50 mV s−1 in 1 M KBi, which increased the film OER activity, before adding the 8 μL of Al3+, and the experiment was continued as above.SEM imaging and EDX
SEM images were acquired using a Tescan MIRA 3 LMU, FEG SEM, equipped with a SE detector, and an IN-Beam SE detector, and EDX spectra were acquired using an Oxford Instruments X-Max 20 EDX detector, running with Oxford INCA software.Acknowledgements
We thank the University Research Board (URB) of the American University of Beirut for financial support of this research (Award 103186). LIH thanks Prof. D. Nocera and his group for hosting her in his laboratory at the MIT during a sabbatical in 2012, where she first became exposed and interested in Ni–Bi films. We also thank Ms. Nour Beydoun and Mr. Rida Farhat for the assistance they provided in some film preparation for SEM imaging and EDX and Mr Joan Younes (Central Research Science Laboratory, AUB) for the assistance he provided while acquiring SEM imaging and EDX analysis.References
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- A. J. Bard, J. Am. Chem. Soc., 2010, 132, 7559 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- S. Trasatti, J. Electroanal. Chem., 1980, 111, 125 CrossRef CAS.
- G. Wu, N. Li, D. R. Zhou, K. Mitsuo and B. Q. Xu, J. Solid State Chem., 2004, 177, 3682 CrossRef CAS.
- M. R. G. de Chialvo and A. C. Chialvo, Electrochim. Acta, 1993, 38, 2247 CrossRef.
- B. Cui, H. Lin, J. B. Li, X. Li, J. Yang and J. Tao, Adv. Funct. Mater., 2008, 18, 1440 CrossRef CAS.
- S. K. Tiwari, S. Samuel, R. N. Singh, G. Poillerat, J. F. Koenig and P. Chartier, Int. J. Hydrogen Energy, 1995, 20, 9 CrossRef CAS.
- R. N. Singh, M. Hamdani, J. F. Koenig, G. Poillerat, J. L. Gautier and P. Chartier, J. Appl. Electrochem., 1990, 20, 442 CrossRef CAS.
- S. Klaus, Y. Cai, M. W. Louie, L. Trotochaud and A. T. Bell, J. Phys. Chem. C, 2015, 119, 7243–7254 CAS.
- L. Trotochaud, J. K. Ranney, K. N. Williams and S. W. Boettcher, J. Am. Chem. Soc., 2012, 134, 17253 CrossRef CAS PubMed.
- J. B. Gerken, S. E. Shaner, R. C. Masse, N. J. Porubsky and S. S. Stahl, Energy Environ. Sci., 2014, 7, 2376 CAS.
- R. D. L. Smith, M. S. Prevot, R. D. Fagan, S. Trudel and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 11580 CrossRef CAS PubMed.
- M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329 CrossRef CAS PubMed.
- M. K. Bates, Q. Jia, H. Doan, W. Liang and S. Mukerjee, ACS Catal., 2016, 6, 155 CrossRef CAS.
- S. M. Jasem and A. C. C. Tseung, J. Electrochem. Soc., 1979, 126, 1353 CrossRef CAS.
- D. A. Corrigan, J. Electrochem. Soc., 1987, 134, 377 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383 CrossRef CAS PubMed.
- J. Y. C. Chen, J. T. Miller, J. B. Gerken and S. S. Stahl, Energy Environ. Sci., 2014, 7, 1382 CAS.
- M. Wehrens-Dijksma and P. H. L. Notten, Electrochim. Acta, 2006, 51, 3609 CrossRef CAS.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Proc. R. Soc. A, 2015, 471, 20140792 CrossRef PubMed.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M. J. Cheng, D. Sokaras, T. C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Norskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305 CrossRef CAS PubMed.
- J. R. Swierk, S. Klaus, L. Trotochaud, A. T. Bell and T. D. Tilley, J. Phys. Chem. C, 2015, 119, 19022 CAS.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744 CrossRef CAS PubMed.
- J. Y. C. Chen, L. N. Dang, H. F. Liang, W. L. Bi, J. B. Gerken, S. Jin, E. E. Alp and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 15090 CrossRef CAS PubMed.
- Y.-F. Li and A. Selloni, ACS Catal., 2014, 4, 1148 CrossRef CAS.
- H. S. Ahn and A. J. Bard, J. Am. Chem. Soc., 2016, 138, 313 CrossRef CAS PubMed.
- D. A. Corrigan, R. S. Conell, C. A. Fierro and D. A. Scherson, J. Phys. Chem., 1987, 91, 5009 CrossRef CAS.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072 CrossRef CAS PubMed.
- Y. Surendranath, M. W. Kanan and D. G. Nocera, J. Am. Chem. Soc., 2010, 132, 16501 CrossRef CAS PubMed.
- D. K. Bediako, B. Lassalle-Kaiser, Y. Surendranath, J. Yano, V. K. Yachandra and D. G. Nocera, J. Am. Chem. Soc., 2012, 134, 6801 CrossRef CAS PubMed.
- D. K. Bediako, Y. Surendranath and D. G. Nocera, J. Am. Chem. Soc., 2013, 135, 3662 CrossRef CAS PubMed.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767 CrossRef CAS PubMed.
- M. Dincã, Y. Surendranath and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10337 CrossRef PubMed.
- A. M. Smith, L. Trotochaud, M. S. Burke and S. W. Boettcher, Chem. Commun., 2015, 51, 526 RSC.
- D. A. Corrigan and R. M. Bendert, J. Electrochem. Soc., 1989, 136, 723 CrossRef CAS.
- S. H. Kim, D. A. Tryk, M. R. Antonio, R. Carr and D. Scherson, J. Phys. Chem., 1994, 98, 10269 CrossRef CAS.
- M. Balasubramanian, C. A. Melendres and S. Mini, J. Phys. Chem. B, 2000, 104, 4300 CrossRef CAS.
- P. Axmann and O. Glemser, J. Alloys Compd., 1997, 246, 232 CrossRef CAS.
- B. C. Cornilsen, X. Shan and P. L. Loyselle, in Nickel Hydroxide Electrodes, ed. D. A. Corrigan and A. H. Zimmerman, The Electrochemical Society Proceedings Series, Pennington, NJ, 1990, PV 90-4, pp. 82–96 Search PubMed.
- C. Delmas, J. J. Braconnier, Y. Borthomieu and P. Hagenmuller, Mater. Res. Bull., 1987, 22, 741 CrossRef CAS.
- C. Delmas, C. Faure, L. Gautier, L. GuerlouDemourgues and A. Rougier, Philos. Trans. R. Soc., A, 1996, 354, 1545 CrossRef CAS.
- L. Demourguesguerlou, L. Fournes and C. Delmas, J. Solid State Chem., 1995, 114, 6 CrossRef CAS.
- B. E. Conway and T. C. Liu, Langmuir, 1990, 6, 268 CrossRef CAS.
- A. Damjanovic, A. Dey and J. M. Bockris, Electrochim. Acta, 1966, 11, 791–814 CrossRef CAS.
- E. Gileadi, Electrode kinetics for chemists, chemical engineers, and materials scientists, Capstone, 1993 Search PubMed.
- J. Schultze and M. Haga, Z. Phys. Chem., 1977, 104, 73 CrossRef CAS.
- B. Conway and T. Liu, Mater. Chem. Phys., 1989, 22, 163 CrossRef CAS.
- M. Ma, F. Qu, X. Ji, D. Liu, S. Hao, G. Du, A. Asiri, Y. Yao, L. Chen and X. Sun, Small, 2017, 1700394 CrossRef PubMed.
- L. Yang, L. Xie, R. Ge, R. Kong, Z. Liu, G. Du, A. M. Asiri, Y. Yao and Y. Luo, ACS Appl. Mater. Interfaces, 2017, 9, 19502 CAS.
- M. Ma, D. Liu, S. Hao, R. Kong, G. Du, A. M. Asiri, Y. Yao and X. Sun, Inorg. Chem. Front., 2017, 4, 840 RSC.
- L. Xie, F. Qu, Z. Liu, X. Ren, S. Hao, R. Ge, G. Du, A. M. Asiri, X. Sun and L. Chen, J. Mater. Chem. A, 2017, 5, 7806 CAS.
- R. Ge, X. Ren, F. Qu, D. Liu, M. Ma, S. Hao, G. Du, A. M. Asiri, L. Chen and X. Sun, Chem. – Eur. J., 2017, 23, 6959 CrossRef CAS PubMed.
- X. Ji, L. Ciu, D. Liu, S. Hao, J. Liu, F. Qu, Y. Ma, G. Du, A. M. Asiri and X. Sun, Chem. Commun., 2017, 53, 3070 RSC.
- B. S. Yeo and A. T. Bell, J. Am. Chem. Soc., 2011, 133, 5587 CrossRef CAS PubMed.
- B. S. Yeo and A. T. Bell, J. Phys. Chem. C, 2012, 116, 8394 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of as-deposited Ni–Bi)1mC and Ni0.6Fe40–Bi)1mC, Ni–Bi)10mC and Ni0.4Fe0.6–Bi)10mC films, and Ni–Bi)400mC and Ni0.6Fe0.4–Bi)400mC as-deposited and after conditioning, and EDX spectra of Ni–Bi and NiFe–Bi films. First scan CV, an intermediate scan, and a CV after anodic conditioning for Ni0.9Fe0.1Ox)1mC; multiple CV scans of Ni–Bi)1mC in 1 M KBi showing the gradual increase in OER activity; cyclic voltammograms of the Ni–Bi)1mC film as a first scan in 1 M KBi before and immediately after addition of 0.16 mM Al3+ (source 2: 98% Al, 0.001% Fe) then after multiple CVs were acquired showing the poisoning effect of Al; multiple CV scans of Ni–Bi)1mC in 1 M KBi showing the increase in OER activity followed by CVs after addition of 0.16 mM Al3+ to KBi from two Al sources showing the decrease in OER activity after addition of the metal cation, and similarly after addition of 0.08 mM each of Al3+ and Fe3+. Comparison of OER activity of these ultra-thin films to those of literature reports on Ni–Bi. See DOI: 10.1039/c7cy00873b |
| This journal is © The Royal Society of Chemistry 2017 |