 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the effect of fluorinated anions on the CO2/N2 separation of supported ionic liquid membranes†
Andreia S. L.
Gouveia
 ab,
Liliana C.
Tomé
ab,
Liliana C.
Tomé
 ab,
Elena I.
Lozinskaya
ab,
Elena I.
Lozinskaya
 c,
Alexander S.
Shaplov
c,
Alexander S.
Shaplov
 cd,
Yakov S.
Vygodskii
c and
Isabel M.
Marrucho
cd,
Yakov S.
Vygodskii
c and
Isabel M.
Marrucho
 *ab
*ab
aCentro de Química Estrutural, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, Avenida Rovisco Pais, 1049-001 Lisboa, Portugal. E-mail: isabel.marrucho@tecnico.ulisboa.pt
bInstituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Av. da República, 2780-157 Oeiras, Portugal
cA. N. Nesmeyanov Institute of Organoelement Compounds Russian academy of sciences (INEOS RAS), Vavilov St., 28, 119991 Moscow, Russia
dLuxembourg Institute of Science and Technology (LIST), 5 avenue des Hauts-Fourneaux, L-4362, Esch-sur-Alzette, Luxembourg
First published on 9th October 2017
Abstract
The CO2 and N2 permeation properties of ionic liquids (ILs) based on the 1-ethyl-3-methylimidazolium cation ([C2mim]+) and different fluorinated anions, namely 2,2,2-trifluoromethylsulfonyl-N-cyanoamide ([TFSAM]−), bis(fluorosulfonyl) imide ([FSI]−), nonafluorobutanesulfonate ([C4F9SO3]−), tris(pentafluoroethyl)trifluorophosphate ([FAP]−), and bis(pentafluoroethylsulfonyl)imide ([BETI]−) anions, were measured using supported ionic liquid membranes (SILMs). The results show that pure ILs containing [TFSAM]− and [FSI]− anions present the highest CO2 permeabilities, 753 and 843 Barrer, as well as the greatest CO2/N2 permselectivities of 43.9 and 46.1, respectively, with CO2/N2 separation performances on top of or above the Robeson 2008 upper bound. The re-design of the [TFSAM]− anion by structural unfolding was investigated through the use of IL mixtures. The gas transport and CO2/N2 separation properties through a pure [C2mim][TFSAM] SILM are compared to those of two different binary IL mixtures containing fluorinated and cyano-functionalized groups in the anions. Although the use of IL mixtures is a promising strategy to tailor gas permeation through SILMs, the pure [C2mim][TFSAM] SILM displays higher CO2 permeability, diffusivity and solubility than the selected IL mixtures. Nevertheless, both the prepared mixtures present CO2 separation performances that are on top of or above the Robeson plot.
Introduction
The development of supported ionic liquid membranes (SILMs) for CO2 separation has been widely investigated in recent years mainly due to their easy preparation and versatility.1–3 In contrast to traditional liquid membranes, which are produced by impregnating a porous membrane support with common organic solvents,4 SILMs use ionic liquids (ILs) and thus benefit from negligible displacement of the liquid phase from the membrane pores through evaporation,5,6 due to the low volatility of ILs.7 It should also be emphasized that within the CO2 separation context, the most important features of ILs are their high CO2 affinity over light gases8–10 and their inherent designer nature that enables the tailoring of IL properties by proper selection of cations and/or anions or via the addition of specific functional groups.Numerous works have investigated the effect of IL chemical structure on the gas permeation properties of SILMs. A broad diversity of cations, such as imidazolium,11 triazolium,12 thiazolium,13 pyridinium,14 cholinium,15 ammonium,16 and phosphonium,17 combined with halogens, sulfonates, carboxylates, fluorinated or cyano-functionalized anions, have been studied. Other works, mostly focusing on imidazolium-based ILs, have also explored the effect of alkyl,18 fluoroalkyl,19 etoxyalkyl,20 and aminoalkyl21-functionalized cations. Since IL anions have a stronger influence on the CO2 separation performance of SILMs than IL cations,1 they deserved from the start a closer look. The first studies on SILMs made use of fluorinated anions such as bis(trifluoromethylsulfonyl)imide [NTf2]−, tetrafluoroborate [BF4]−, and hexafluorophosphate [PF6]− and enabled drawing conclusions about the CO2-phylic behaviour and high CO2 permeabilities of these anions.22 More recently, low viscous ILs with cyano-functionalized anions, such as tricyanomethanide [C(CN)3]− and tetracyanoborate [B(CN)4]−,23–25 have been recognized as better candidates for the development of improved SILMs, because of their superior CO2 permeabilities and permselectivities when compared to the most used [NTf2]− anion. Task-specific ILs bearing amine groups, such as those containing amino acid anions,26–28 have also been proposed to prepare SILMs, since amine groups can chemically bond CO2 and act as carriers for CO2 facilitated transport through SILMs at low pressures. However, the high viscosity of these task-specific ILs is undoubtedly a key limitation, as CO2 diffusion is strongly compromised.
In an effort to improve the CO2 permeability and permselectivity properties of SILMs, our recent studies explored the use of IL mixtures by fixing the [C2mim]+ cation and researching on different anion chemical structures. Initially, SILMs based on IL mixtures combining anions with different CO2 solubility behaviours were investigated: thiocyanate ([SCN]−), dicyanamide ([N(CN)2]−) and bis(trifluoromethylsulfonyl)imide ([NTf2]−) that present physical solubility; acetate ([Ac]−) and lactate ([Lac]−), which additionally have chemical solubility.29 Afterwards, we focused on IL mixtures based on sulfate ([CH3SO4]−) and cyano-functionalized anions ([SCN]−, [N(CN)2]−, [C(CN)3]− and [B(CN)4]−).30 Moreover, we studied IL mixtures containing [C(CN)3]− and different amino acid anions, so that one IL component maintains the low viscosity, while the other provides the desired chemical characteristics for the active transport of CO2.31 The overall results of these studies showed that mixing anions with specific chemical features allows variations in IL viscosity and molar volume that significantly impact the gas transport through SILMs, and thus tailored permeabilities and permselectivities can be achieved.29–31
In the present work, the gas permeation properties and CO2/N2 separation performance of SILMs prepared with pure ILs bearing the [C2mim]+ cation and different less conventional fluorinated anions, namely [TFSAM]−, [FSI]−, [C4F9SO3]−, [FAP]− and [BETI]−, were evaluated and the effect of the fluorinated moieties in the IL anion was discussed. Despite the fact that several SILMs with common fluorinated anions have already been reported,1–3 the gas permeation properties of SILMs containing fluorinated anions, such as those selected herein, have still not been properly studied and discussed. Only three studies have reported SILMs that made use of [C4F9SO3]−, [FAP]− and [BETI]− anions. Pereiro et al.32 conducted single gas permeation experiments through a [C2C1py][C4F9SO3] SILM, at 294 K and 75 kPa, using CO2, N2, O2, hydrocarbon gases (CH4, C2H6, C3H8, C3H6) and perfluorocarbon gases (CF4, C2F6, C3F8). Scovazzo et al.22 determined ideal/mixed CO2/CH4 and CO2/N2 permselectivities in a [C4mim][BETI] SILM at 303 K and 200 kPa, while Althuluth et al.33 reported ideal/mixed CO2/CH4 permselectivities in a [C2mim][FAP] SILM at 313 K and 700 kPa. Nevertheless, the obtained results cannot be directly compared due to the different measurement conditions, as well as the use of diverse IL cation structures.
Additionally, this work investigates the impact on gas transport through SILMs of using a pure IL versus a structurally similar IL mixture as the liquid phase. Inspired by the fact that the [TFSAM]− anion has an unusual asymmetric chemical structure, which combines both fluorinated and cyano functionalities, the re-design of the chemical structure of a pure [C2mim][TFSAM] IL through the use of IL mixtures is explored. For that purpose, different pairs of ILs, based on the [C2mim]+ cation and anions containing fluorinated or cyano functionalities, were selected and their gas permeation properties were compared to those of the pure [C2mim][TFSAM] SILM. One of the IL mixtures contains [NTf2]− and [N(CN)2]− anions, whose gas permeation properties were previously determined,29 whereas the other IL mixture is based on [OTf]− and [SCN]− anions and its gas transport properties are reported here for the first time.
Results and discussion
Gas permeation through SILMs having fluorinated anions
The structures of the pure ILs bearing fluorinated anions are depicted in Fig. 1.The water content (wt%), molar mass (M), viscosity (η), density (ρ) and molar volume (Vm) values of the pure ILs used as liquid phases in the studied SILMs are summarized in Table 1. The thermophysical properties of the conventional [C2mim][NTf2] IL are also included for comparison.29 From Table 1, it can be observed that the IL containing the [C4F9SO3]− anion shows the highest viscosity, while [C2mim][FSI] presents the lowest viscosity. The IL viscosity values can be organized following the IL anion order: [C4F9SO3]− > [BETI]− > [FAP]− > [NTf2]− > [TFSAM]− > [FSI]−. A slightly different trend was observed for molar volumes, with the IL comprising the [FAP]− anion showing the highest molar volume, while [C2mim][FSI] exhibiting the lowest molar volume. These data will be used subsequently in the understanding of the gas permeation results.
| IL sample | H2O (wt%) | M (g mol−1) | η (mPa s) | ρ (g cm−3) | V m (cm3 mol−1) |
|---|---|---|---|---|---|
| a Viscosity (η) and density (ρ) measured at 293 K, except for [C2mim][C4F9SO3] IL (303 K). b Molar volume (Vm) obtained for 293 K, except for [C2mim][C4F9SO3] IL (303 K). c The values of [C2mim][NTf2] were taken from Tomé et al.29 d The density and viscosity values of [C2mim][FAP] were taken from Neves et al.52 | |||||
| [C2mim][TFSAM] | 0.02 | 284.26 | 23.7 | 1.3518 | 210.3 |
| [C2mim][FSI] | 0.09 | 291.30 | 22.4 | 1.4480 | 201.2 |
| [C2mim][NTf2]c | 0.02 | 391.31 | 39.1 | 1.5240 | 256.8 |
| [C2mim][FAP]d | — | 556.17 | 76.4 | 1.7081 | 325.6 |
| [C2mim][BETI] | 0.02 | 491.33 | 85.5 | 1.5989 | 307.3 |
| [C2mim][C4F9SO3] | 0.08 | 410.26 | 109.7 | 1.5420 | 266.1 |
The experimental gas permeability (P) values obtained through the prepared SILMs having ILs with fluorinated anions, measured at 293 K with a trans-membrane pressure differential of 100 kPa, are shown in Table 2. To the best of our knowledge, the CO2/N2 separation properties of [C2mim][TFSAM], [C2mim][FSI], [C2mim][FAP], [C2mim][BETI] and [C2mim][C4F9SO3] SILMs are reported here for the first time, while those of the [C2mim][NTf2] SILM were previously determined using the same experimental conditions.29 It is important to mention that in order to attain stable SILMs, both hydrophilic and hydrophobic supports were used according to the hydrophobicity of ILs, and the results are compared in this section, irrespective of the support used.
| SILM sample | P (CO2) | P (N2) | α (CO2/N2) |
|---|---|---|---|
| a Barrer (1 Barrer = 10−10 cm(STP)3 cm cm−2 s−1 cmHg−1). b The listed uncertainties represent the standard deviations, based on three experiments. c Values taken from Tomé et al.29 | |||
| [C2mim][TFSAM] | 753 ± 0.2 | 17 ± 0.1 | 43.9 ± 0.1 |
| [C2mim][FSI] | 843 ± 0.5 | 18 ± 0.2 | 46.1 ± 0.5 |
| [C2mim][NTf2]c | 589 ± 1.0 | 17 ± 0.1 | 35.5 ± 0.3 |
| [C2mim][FAP] | 624 ± 0.4 | 24 ± 0.1 | 26.0 ± 0.1 |
| [C2mim][BETI] | 437 ± 1.9 | 18 ± 0.1 | 24.8 ± 0.2 |
| [C2mim][C4F9SO3] | 32 ± 0.2 | 6 ± 0.1 | 5.5 ± 0.1 |
From Table 2, the same trend in gas permeability is valid for all the studied SILMs: PCO2 ≫ PN2, as expected. Regarding the influence of the fluorinated-based anions, SILMs having the [FSI]−, [TFSAM]− and [FAP]− anions present higher CO2 permeabilities of 843, 753 and 624 Barrer, respectively, than the SILM containing the [NTf2]− anion, which is well-known for its high CO2 permeability (589 Barrer).29 It should be noted that in spite of the similar structures of [NTf2]− and [FSI]− anions, in which the difference consists in two extra −CF3 groups in the [NTf2]− anion structure (Fig. 1), the CO2 permeability through the [C2mim][FSI] SILM is ∼1.5 times higher than that through the [C2mim][NTf2] SILM. Generally, CO2 permeabilities through the studied SILMs are found to decrease in the following IL anion order: [FSI]− > [TFSAM]− > [FAP]− > [NTf2]− > [BETI]− > [C4F9SO3]− (Table 2). Considering the IL anion viscosity trend, obtained at 293 K (Table 1), [FSI]− < [TFSAM]− < [NTf2]− < [FAP]− < [BETI]− < [C4F9SO3]−, it can be concluded that these experimental data are in agreement with the general trend usually observed in the literature, where ILs with high viscosities yield SILMs with low gas permeabilities.3,14,15,23,40 However, [C2mim][FAP] is the only exception since it presents a different behaviour (Table 2): despite its high viscosity (76.4 mPa s), it also exhibits high CO2 permeability (624 Barrer), higher than those of the [C2mim][NTf2] IL (39.1 mPa s and 589 Barrer). Notice that the [FAP]− anion has the most different chemical structure among all the IL anions studied in this work, consisting of a phosphorus atom surrounded by fluorine atoms, without sulfonyl functional groups (Fig. 1). Moreover, taking a closer look at the gas permeabilities obtained through SILMs immobilized with the remaining ILs, it can be seen that higher CO2 permeabilities are achieved for ILs with anions bearing a smaller number of fluorine elements, such as [TFSAM]− and [FSI]− anions (Table 2).
Gas diffusivity (D) is a mass transfer property that directly accounts for gas permeability (eqn (1)). Typically, the higher the gas diffusivity, the faster is the gas flux through the SILM. The experimental CO2 and N2 diffusivity values obtained through the prepared SILMs are presented in Table 3. The CO2 diffusivities of the SILMs with fluorinated anions can be ordered as follows: [FSI]− > [TFSAM]− > [FAP]− > [NTf2]− > [BETI]− > [C4F9SO3]−, which fully corresponds to the IL anion order observed for CO2 permeabilities (Table 2). As for N2 diffusivities the subsequent order is attained: ([FAP]− > [FSI]− > [TFSAM]− > [BETI]− > [NTf2]− > [C4F9SO3]−), which is nearly the same IL anion order observed for N2 permeabilities, but different from that obtained for CO2 diffusivities and permeabilities.
| SILM sample | Gas diffusivity (×1012) (m2 s−1) | |
|---|---|---|
| D (CO2) | D (N2) | |
| a The gas diffusivity values through the [C2mim][NTf2] SILM were taken from Tomé et al.29 | ||
| [C2mim][TFSAM] | 258 ± 1.1 | 291 ± 4.5 |
| [C2mim][FSI] | 315 ± 4.4 | 353 ± 7.5 |
| [C2mim][NTf2]a | 172 ± 1.7 | 203 ± 2.5 |
| [C2mim][FAP] | 214 ± 1.9 | 326 ± 0.2 |
| [C2mim][BETI] | 167 ± 2.1 | 216 ± 2.4 |
| [C2mim][C4F9SO3] | 55 ± 1.2 | 88 ± 2.0 |
The relationship between IL viscosity and gas diffusion is in fact the basis of the dependence of permeability on viscosity that we have shown above. A number of works have reported the inversely proportional relationship between gas diffusivity and IL viscosity.3,16,17,41 Along this line of thought, the relationship between experimental CO2 diffusivities and IL viscosity for the studied SILMs is depicted in Fig. 2.
In agreement to what was previously observed in the literature for other SILMs,4,21–23 the CO2 diffusivity through SILMs having fluorinated anions decreases as the IL viscosity increases. The SILMs with the lowest CO2 diffusivities are [C2mim][C4F9SO3] (55 × 10−12 m2 s−1) and [C2mim][BETI] (167 × 10−12 m2 s−1), which also have the lowest CO2 permeabilities (437 and 32 Barrer), depicting the highest viscosities (109.7 and 85.5 mPa). Similarly to what is mentioned above for CO2 permeabilities, again a deviant behaviour can be observed for the [C2mim][FAP] SILM, since its CO2 diffusivity is in between those of [C2mim][NTf2] and [C2mim][TFSAM] (Table 3), but its viscosity values (76.4 mPa s) are higher than those of [C2mim][NTf2] (39.1 mPa s) and [C2mim][TFSAM] (23.7 mPa s) (Fig. 2).
The gas solubility (S) values calculated using Eqn 1 are listed in Table 4. It can be seen that the [C2mim][C4F9SO3] SILM presents the lowest CO2 solubility (4 × 10−6 m(STP)3 m−3 Pa−1), while the previously reported [C2mim][NTf2] SILM has the highest CO2 solubility (26 × 10−6 m(STP)3 m−3 Pa−1). The CO2 solubility values of the remaining studied SILMs having different fluorinated anions are very similar (ranging from 20 × 10−6 to 22 × 10−6 m(STP)3 m−3 Pa−1) and can be ordered as follows: [TFSAM]− ≈ [FAP]− > [FSI]− ≈ [BETI]−. Regarding the N2 solubility, its values are always significantly lower (between 0.39 × 10−6 and 0.61 × 10−6 m(STP)3 m−3 Pa−1) than those of CO2 for all the SILMs studied.
| SILM sample | Gas solubility (×106) (m(STP)3 m−3 Pa−1) | |
|---|---|---|
| S (CO2) | S (N2) | |
| a The gas solubility values through the [C2mim][NTf2] SILM were taken from Tomé et al.29 | ||
| [C2mim][TFSAM] | 22 ± 0.09 | 0.44 ± 0.01 |
| [C2mim][FSI] | 20 ± 0.29 | 0.39 ± 0.01 |
| [C2mim][NTf2]a | 26 ± 0.28 | 0.61 ± 0.01 |
| [C2mim][FAP] | 22 ± 0.21 | 0.55 ± 0.001 |
| [C2mim][BETI] | 20 ± 0.17 | 0.61 ± 0.01 |
| [C2mim][C4F9SO3] | 4 ± 0.07 | 0.49 ± 0.002 |
Over the past few years, a number of correlations have been proposed with the intention of understanding the relationships between CO2 solubility and the intrinsic properties of ILs.42–44 The proposed models showed that CO2 solubility increases with increasing IL molecular weight, molar volume and free volume.10 Taking into consideration the gas solubility results obtained in this work (Table 4), as well as the range of molar volumes (from 201.2 up to 325.6 cm3 mol−1) and molecular weights (from 284.3 up to 556.2 g mol−1) of the ILs used, divergences from the abovementioned trends can be found for the studied SILMs having fluorinated anions. For example, the [C2mim][NTf2] SILM shows the highest CO2 solubility (26 × 10−6 m(STP)3 m−3 Pa−1), but it does not have the highest IL molar volume and molecular weight (Table 1). Likewise, the lowest CO2 solubility (4 × 10−6 m(STP)3 m−3 Pa−1) belongs to the [C2mim][C4F9SO3] SILM, although it does not present the lowest IL molar volume and molecular weight (Table 1). The effect of fluorination, either in the IL cation or anion, on CO2 solubility has been studied by different researchers.19,45–47 Tagiuri et al.48 explored the effect of cation on the CO2 solubility of three different ILs combining the [FSI]− anion. Moreover, Kroon et al.49 determined the CO2 solubility in the [C2mim][FAP] IL by measuring the bubble point pressures of the binary mixture of [C2mim][FAP] + CO2. The results showed that the CO2 solubility in [C2mim][FAP] is higher when compared to that of ILs having the same cation combined with other fluorinated anions such as [NTf2]−, [BF4]− and [PF6]−, due to the fact that [FAP]− has a large size and it is highly fluorinated. Although it has been recognized that introducing fluorination into the cation and/or anion can effectively improve CO2 solubility,46 it was recently reported after critical analysis that no special effect of the fluorination upon the CO2 solubility has been observed for both perfluorocarbon and heavily fluorinated ILs.50 In fact, the introduction of fluorination into the anions of the ILs studied in this work does not significantly affect the obtained gas solubility values (Table 4), except for the case of the [C2mim][C4F9SO3] IL that displays a very low CO2 solubility.
Re-designing the [TFSAM]− anion by structural unfolding: effect on gas permeation
Taking into account that the [TFSAM]− anion has an unconventional and asymmetric chemical structure, combining both fluorinated and cyano functionalities, which have both been recognized to be responsible for high CO2 separation performance, we explore here the effect of structural unfolding of the pure [C2mim][TFSAM] IL on gas permeation properties of SILMs using IL mixtures. Thus, two equimolar IL mixtures were used for this purpose: [C2mim][SCN][OTf] (Fig. 3), which is studied here for the first time, and [C2mim][N(CN)2][NTf2], whose gas permeation and thermophysical properties were previously determined by us.29 Both these mixtures have IL anions that show structural similarities to the [TFSAM]− anion (Fig. 3). The composition description, water content (wt%), molar mass (M), viscosity (η), density (ρ) and molar volume (Vm) values of the pure ILs, [C2mim][TFSAM], [C2mim][SCN], [C2mim][OTf], [C2mim][N(CN)2], [C2mim][NTf2], and the selected IL mixtures are listed in Table 5, while their gas permeability, diffusivity and solubility values are depicted in Fig. 4(a)–(c), respectively.| IL sample | Composition description (mole fraction) | H2O (wt%) | M (g mol−1) | η (mPa s) | ρ (g cm−3) | V m (cm3 mol−1) |
|---|---|---|---|---|---|---|
| a Viscosity (η) and density (ρ) measured at 293 K. b Molar volume (Vm) obtained at 293 K. c The values of [C2mim][N(CN)2][NTf2] were taken from Tomé et al.29 | ||||||
| [C2mim][TFSAM] | Pure | 0.02 | 284.26 | 23.7 | 1.3518 | 210.3 |
| [C2mim][SCN]c | Pure | 0.09 | 169.25 | 27.9 | 1.1190 | 151.2 |
| [C2mim][SCN][OTf] | 0.5 [C2mim][SCN] + 0.5 [C2mim][OTf] | 0.16 | 214.74 | 36.4 | 1.2638 | 169.9 |
| [C2mim][OTf] | Pure | 0.10 | 260.23 | 49.5 | 1.3850 | 187.9 |
| [C2mim][N(CN)2]c | Pure | 0.09 | 177.21 | 18.0 | 1.1060 | 160.2 |
| [C2mim][N(CN)2][NTf2]c | 0.5[C2mim][N(CN)2] + 0.5 [C2mim][NTf2] | 0.12 | 284.26 | 29.2 | 1.3620 | 208.7 |
| [C2mim][NTf2]c | Pure | 0.02 | 391.31 | 39.1 | 1.5240 | 256.8 |
 | ||
| Fig. 4 (a) Gas permeability, (b) CO2 diffusivity as a function of IL viscosity and (c) CO2 solubility as a function of IL molar volume for the studied SILMs. Error bars represent standard deviations based on three experimental replicas. The gas permeability, diffusivity and solubility values of [C2mim][N(CN)2][NTf2] and [C2mim][NTf2] were taken from Tomé et al.29 | ||
Fig. 4(a) and (b) show that the pure [C2mim][TFSAM] SILM exhibits higher gas permeabilities and diffusivities than SILMs composed of both [C2mim][SCN][OTf] and [C2mim][N(CN)2][NTf2] IL mixtures. Moreover, the addition of 0.5 mole fraction of [C2mim][SCN] did not significantly affect CO2 permeability but decreased to almost half the N2 permeability compared to those of the pure [C2mim][OTf] SILM. From Fig. 4(b), where the relationship between CO2 diffusivity and IL viscosity is illustrated, it can also be seen that both [C2mim][N(CN)2][NTf2] and [C2mim][SCN][OTf] IL mixtures present slightly higher viscosities (Table 5) and lower CO2 diffusivities than those of the pure [C2mim][TFSAM] SILM. Also, the presence of 0.5 mole fraction of [C2mim][SCN] leads to a decrease in IL viscosity and an increase in CO2 diffusivity. The same behaviour was found for [C2mim][N(CN)2][NTf2] with the addition of 0.5 mole fraction of [C2mim][N(CN)2]. These results are in accordance with the general trend observed in the literature: the CO2 diffusivity decreases with the increase in IL viscosity.4,21–23
Concerning gas solubility, from Fig. 4(c), it can be observed that CO2 solubilities of both IL mixtures are in between those of the individual IL components. Moreover, the presence of 0.5 mole fraction of [C2mim][OTf] or [C2mim][NTf2] in the corresponding mixtures leads to an increase in the CO2 solubilities, probably due to the fact that they present higher molar volume compared to the pure [C2mim][SCN] or [C2mim][N(CN)2], respectively. Furthermore, the [C2mim][N(CN)2][NTf2] mixture displays a CO2 solubility (20 × 10−6 m(STP)3 m−3 Pa−1) closer to that of the pure [C2mim][TFSAM] SILM (22 × 10−6 m(STP)3 m−3 Pa−1), probably due to the fact that these IL phases have very close molar volumes (208.7 and 210.3 cm3 mol−1, respectively) and the same molecular weight (284.3 g mol−1). Conversely, the [C2mim][SCN][OTf] mixture has lower CO2 solubility (15 × 10−6 m(STP)3 m−3 Pa−1) than that observed for the pure [C2mim][TFSAM] SILM, which is in agreement with the lower molar volume (169.9 cm3 mol−1) and molecular weight (214.7 cm3 mol−1) presented by this IL mixture containing the [SCN]− and [OTf]− anions. Despite the fact that it is possible to obtain similar viscosities and molar volumes of the pure [C2mim][TFSAM] IL by mixing the [C2mim][N(CN)2] and [C2mim][NTf2] ILs, improved gas permeabilities, diffusivities and solubilities were obtained through the pure [C2mim][TFSAM] SILM.
Comparison of CO2/N2 separation performance
The gas permeabilities and CO2/N2 permselectivities of all the studied SILMs are listed in Table 6. Amongst the pure SILMs with fluorinated anions, the [C2mim][FSI] and [C2mim][TFSAM] SILMs not only show the highest gas permeabilities (843 and 753 Barrer, respectively), but also have the largest CO2/N2 permselectivities (43.9 and 46.1, respectively). In contrast, the lowest gas permeabilities and CO2/N2 permselectivity belong to the [C2mim][C4F9SO3] SILM. The CO2/N2 permselectivities of the pure SILMs decrease as the fluorinated chain increases in the IL anion: [FSI]− > [TFSAM]− > [NTf2]− > [BETI]−, the [C2mim][FAP] and [C2mim][C4F9SO3] SILMs being the only exceptions.| SILM sample | P (CO2) | P (N2) | α (CO2/N2) |
|---|---|---|---|
| a Barrer (1 Barrer = 10−10 cm(STP)3 cm cm−2 s−1 cmHg−1). b The listed uncertainties represent the standard deviations, based on three experiments. c Values taken from Tomé et al.29 | |||
| [C2mim][TFSAM] | 753 ± 0.2 | 17 ± 0.1 | 43.9 ± 0.1 |
| [C2mim][FSI] | 843 ± 0.5 | 18 ± 0.2 | 46.1 ± 0.5 |
| [C2mim][FAP] | 624 ± 0.4 | 24 ± 0.1 | 26.0 ± 0.1 |
| [C2mim][BETI] | 437 ± 1.9 | 18 ± 0.1 | 24.8 ± 0.2 |
| [C2mim][C4F9SO3] | 32 ± 0.2 | 6 ± 0.1 | 5.5 ± 0.1 |
| [C2mim][SCN]c | 263 ± 0.6 | 5 ± 0.2 | 56.6 ± 1.9 |
| [C2mim][SCN][OTf] | 428 ± 0.5 | 7 ± 0.1 | 57.6 ± 0.4 |
| [C2mim][OTf] | 479 ± 0.6 | 12 ± 0.1 | 41.4 ± 0.3 |
| [C2mim][N(CN)2]c | 476 ± 0.8 | 7 ± 0.1 | 67.8 ± 0.6 |
| [C2mim][N(CN)2][NTf2]c | 589 ± 1.9 | 14 ± 0.2 | 41.8 ± 0.7 |
| [C2mim][NTf2]c | 589 ± 1.0 | 17 ± 0.1 | 35.5 ± 0.3 |
Concerning the effect of the structural unfolding of the [TFSAM]− anion, and as previously discussed, the pure [C2mim][TFSAM] SILM presents higher gas permeabilities compared to its structurally similar IL mixtures (Table 6). Nevertheless, the greatest CO2/N2 permselectivity (57.6) was achieved for the SILM containing the [C2mim][SCN][OTf] IL mixture.
With the purpose of comparing the performance results obtained in this work to those reported in the literature for other SILMs, Fig. 5 displays the Robeson plot for CO2/N2 separation, where the CO2/N2 permselectivity is plotted against CO2 permeability and the solid black line represents the empirical 2008 upper bound for this gas pair.51 It can be seen that among the SILMs immobilized with the pure IL having fluorinated anions, both the [C2mim][TFSAM] and [C2mim][FSI] SILMs fall or exceed the Robeson 2008 upper bound, meaning that these two ILs are the most promising candidates for CO2/N2 separation processes. Comparing the results of the pure [C2mim][TFSAM] SILM with those of selected IL mixtures, Fig. 5 clearly shows that the CO2/N2 separation performance of the SILM immobilized with the [C2mim][SCN][OTf] IL mixture surpasses the upper bound, primarily due to its higher CO2/N2 permselectivity (57.6). On the other hand, the CO2/N2 separation efficiency of the [C2mim][N(CN)2][NTf2] SILM is on top of the upper bound, since it presents lower permselectivity (41.8), despite its high CO2 permeability (589 Barrer) in comparison to that of the [C2mim][SCN][OTf] SILM (428 Barrer). Actually, it is the [C2mim][N(CN)2][NTf2] SILM that discloses the most similar CO2/N2 separation performance results to the pure synthesized [C2mim][TFSAM] IL (Fig. 5).
 | ||
| Fig. 5 CO2/N2 separation performance of all the studied SILMs. The experimental error is within the data points. Data are plotted on a log–log scale and the upper bound is adapted from Robeson.51 The literature data previously reported for other SILMs (□)15,17,22,24,29,30,32,53 are also illustrated for comparison. The values of [C2mim][N(CN)2][NTf2] and [C2mim][NTf2] SILMs were taken from Tomé et al.29 | ||
Experimental
Materials
Lithium bis(pentafluoroethylsulfonyl)imide (Li(CF3CF2SO2)2N, LiBETI, 98%, Chameleon Reagent) and lithium nonafluoro-1-butanesulfonate (LiC4F9SO3, > 95%, TCI Chemicals) were used without purification. Reagent-grade dichloromethane, acetonitrile, hexane and ethyl acetate were obtained from Aldrich or Merck and were dried by vacuum distillation over P2O5. N-Methylimidazole (98%, Aldrich) and bromoethane (98%, Acros) were distilled under an inert atmosphere over CaH2.1-Ethyl-3-methylimidazolium bis(fluorosulfonyl)imide ([C2mim][FSI], 99.5 wt%, Solvionic), 1-ethyl-3-methylimidazolium tris(pentafluoro-ethyl)trifluorophosphate ([C2mim][FAP], 98 wt%, Merck), 1-ethyl-3-methylimidazolium thiocyanate ([C2mim][SCN], > 98 wt%, IoLiTec) and 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ([C2mim][OTf], ≥ 98 wt%, Aldrich) were obtained from the specified suppliers. To reduce the content of water and other volatile substances, the pure ILs were dried at approximately 1 Pa and 318 K for at least 4 days.
IL synthesis and characterization
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.84 (s, 3H, CH3), 1.42 (t, 3H, CH2
CH3), 3.84 (s, 3H, CH3), 1.42 (t, 3H, CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) , JHH = 7.5 Hz); 13C NMR (100.6 MHz, DMSO-d6): 136.1, 125.0–115.4 (q, 1JCF = 325 Hz), 123.4, 121.8, 44.1, 35.5, 14.8; 19F NMR (282.4 MHz, DMSO-d6): −77.8; IR (KBr pellet): 3158 (m, νC–H), 3117 (m, νC–H), 2192 (vs, νC–N), 1573 (m), 1468 (w), 1333 (vs, νasSO2), 1236 (s), 1217 (vs, νCF), 1170 (vs, νsSO2), 1119 (s, νCF), 832 (s), 752 (w), 639 (m), 595 (s), 479 (m) cm−1.
, JHH = 7.5 Hz); 13C NMR (100.6 MHz, DMSO-d6): 136.1, 125.0–115.4 (q, 1JCF = 325 Hz), 123.4, 121.8, 44.1, 35.5, 14.8; 19F NMR (282.4 MHz, DMSO-d6): −77.8; IR (KBr pellet): 3158 (m, νC–H), 3117 (m, νC–H), 2192 (vs, νC–N), 1573 (m), 1468 (w), 1333 (vs, νasSO2), 1236 (s), 1217 (vs, νCF), 1170 (vs, νsSO2), 1119 (s, νCF), 832 (s), 752 (w), 639 (m), 595 (s), 479 (m) cm−1.
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.85 (s, 3H, CH3), 1.43–1.39 (m, 3H, CH2
CH3), 3.85 (s, 3H, CH3), 1.43–1.39 (m, 3H, CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ); 13C NMR (100.6 MHz, DMSO-d6): 136.2, 123.5, 121.9, 117.1 (qt, –CF2–
); 13C NMR (100.6 MHz, DMSO-d6): 136.2, 123.5, 121.9, 117.1 (qt, –CF2–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F3, 1J = 288 Hz, 2J = 34 Hz), 113.4 (tt, (–)O3S-
F3, 1J = 288 Hz, 2J = 34 Hz), 113.4 (tt, (–)O3S-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F2–, 1J = 288 Hz, 2J = 34 Hz), 110.4 (tp, (–)O3S-CF2–
F2–, 1J = 288 Hz, 2J = 34 Hz), 110.4 (tp, (–)O3S-CF2–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F2–, 1J = 266, 2J = 33 Hz), 109.8 (qq, –
F2–, 1J = 266, 2J = 33 Hz), 109.8 (qq, –![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F2–CF3, 1J = 268 Hz, 2J = 38 Hz), 44.1, 35.6, 14.9; 19F NMR (282.4 MHz, DMSO-d6): −80.8, −114.9, −121.6, −125.9; IR (KBr pellet): 3156 (s, νC–H), 3117 (s, νC–H), 2993 (m, νC–H), 1574 (s), 1462 (m), 1432 (w), 1393 (w), 1353 (s), 1261 (vs, νasSO2), 1236 (vs), 1214 (vs, νCF), 1170 (vs, νsSO2), 1134 (s, νCF), 1057 (vs), 1119 (m), 1006 (m), 988 (w), 870 (m), 845 (m), 802 (m), 736 (m), 699 (m), 679 (w), 656 (s), 620 (s), 596 (m), 564 (m), 532 (s) cm−1.
F2–CF3, 1J = 268 Hz, 2J = 38 Hz), 44.1, 35.6, 14.9; 19F NMR (282.4 MHz, DMSO-d6): −80.8, −114.9, −121.6, −125.9; IR (KBr pellet): 3156 (s, νC–H), 3117 (s, νC–H), 2993 (m, νC–H), 1574 (s), 1462 (m), 1432 (w), 1393 (w), 1353 (s), 1261 (vs, νasSO2), 1236 (vs), 1214 (vs, νCF), 1170 (vs, νsSO2), 1134 (s, νCF), 1057 (vs), 1119 (m), 1006 (m), 988 (w), 870 (m), 845 (m), 802 (m), 736 (m), 699 (m), 679 (w), 656 (s), 620 (s), 596 (m), 564 (m), 532 (s) cm−1.
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) CH3), 3.84 (s, 3H, CH3), 1.43–1.40 (m, 3H, CH2
CH3), 3.84 (s, 3H, CH3), 1.43–1.40 (m, 3H, CH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ); 13C NMR (100.6 MHz, DMSO-d6): 136.7, 123.9, 122.4, 117.7 (qt, –CF2–
); 13C NMR (100.6 MHz, DMSO-d6): 136.7, 123.9, 122.4, 117.7 (qt, –CF2–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F3, 1J = 287 Hz, 2J = 34 Hz), 110.1 (tq, –
F3, 1J = 287 Hz, 2J = 34 Hz), 110.1 (tq, –![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F2–CF3, 1J = 293 Hz, 2J = 37 Hz), 44.6, 36.0, 15.4; 19F NMR (282.4 MHz, DMSO-d6): −78.8, −117.6; IR (KBr pellet): 3160 (m, νC–H), 3124 (m, νC–H), 2993 (w, νC–H), 1574 (m), 1472 (w), 1432 (w), 1355 (vs, νasSO2), 1331 (vs, νCF), 1223 (vs, νCF), 1172 (vs, νsSO2), 1087 (s, νCF), 978 (s), 824 (w), 775 (w), 755 (m), 742 (m), 701 (w), 644 (m), 616 (s), 536 (m), 525 (m) cm−1.
F2–CF3, 1J = 293 Hz, 2J = 37 Hz), 44.6, 36.0, 15.4; 19F NMR (282.4 MHz, DMSO-d6): −78.8, −117.6; IR (KBr pellet): 3160 (m, νC–H), 3124 (m, νC–H), 2993 (w, νC–H), 1574 (m), 1472 (w), 1432 (w), 1355 (vs, νasSO2), 1331 (vs, νCF), 1223 (vs, νCF), 1172 (vs, νsSO2), 1087 (s, νCF), 978 (s), 824 (w), 775 (w), 755 (m), 742 (m), 701 (w), 644 (m), 616 (s), 536 (m), 525 (m) cm−1.
NMR spectra were recorded on an AMX-400 spectrometer (Bruker) at 298 K in the indicated deuterated solvent and are listed in ppm. The signal corresponding to the residual protons of the deuterated solvent was used as an internal standard for 1H and 13C NMR, while for 19F NMR CHCl2F was used as an external standard. IR spectra were acquired on a Nicolet Magna-750 Fourier IR-spectrometer using KBr pellets (128 scans, resolution was 2 cm−1).
Ideal gas permeabilities and diffusivities through the prepared SILMs were measured using a time-lag apparatus.36 First, each SILM was degassed under vacuum inside the permeation cell for 12 h. Then, CO2 and N2 permeation experiments were carried out at 293 K with a trans-membrane pressure differential of 100 kPa. All the permeation data were measured at least in triplicate on a single SILM sample. The highest relative standard uncertainty registered for gas permeability measurements was 0.03. The permeation cell and lines were evacuated until the pressure was below 0.1 kPa before each run. No residual IL was found inside the permeation cell at the end of the experiments. The thickness of the SILMs was assumed to be equivalent to the membrane filter thickness.
Gas transport through the prepared SILMs was assumed to follow a solution–diffusion mass transfer mechanism,37 where the permeability (P) is related to diffusivity (D) and solubility (S) as follows:
| P = D × S | (1) |
The permeate flux of each gas (Ji) was determined experimentally using eqn (2),38 where Vp is the permeate volume, Δpd is the variation of downstream pressure, A is the effective membrane surface area, t is the experimental time, R is the gas constant and T is the temperature.
 | (2) |
Ideal gas permeability (Pi) was then determined from the steady-state gas flux (Ji), the membrane thickness (![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) ) and the trans-membrane pressure difference (Δpi), as shown in eqn (3).38
) and the trans-membrane pressure difference (Δpi), as shown in eqn (3).38
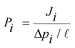 | (3) |
Gas diffusivity (Di) was determined according to eqn (4). The time-lag parameter (θ) was calculated by extrapolating the slope of the linear portion of the pdvs. t curve back to the time axis, where the intercept was equal to θ.39
 | (4) |
After Pi and Di were known, the gas solubility (Si) was calculated using the relationship shown in eqn (1). The ideal permeability selectivity (or permselectivity), αi/j, was obtained by dividing the permeability of the more permeable species i to the permeability of the less permeable species j. The permselectivity can also be expressed as the product of the diffusivity selectivity and the solubility selectivity:
 | (5) |
Conclusions
In this work, ILs containing a common cation ([C2mim]+) and different fluorinated anions ([TFSAM]−, [FSI]−, [C4F9SO3]−, [BETI]−, [FAP]−) were synthesized and used as liquid phases to prepare SILMs for flue gas separation (CO2/N2). The single CO2 and N2 permeation properties through the prepared SILMs were determined. The viscosity and density of the IL phases were also evaluated. The results showed that CO2 permeabilities and diffusivities through the studied SILMs follow the same fluorinated anion order: [FSI]− > [TFSAM]− > [FAP]− > [NTf2]− > [BETI]− > [C4F9SO3]−, which is inversely related to IL viscosity, with the only outlier being [C2mim][FAP]. Conversely, the introduction of fluorination in the IL anions did not significantly affect gas solubility, except for the case of the [C2mim][C4F9SO3] SILM displaying a very low CO2 solubility. Among the pure SILMs, it is worth noting that the best separation performances were achieved for [C2mim][TFSAM] and [C2mim][FSI] SILMs that fall on top of or surpassed the Robeson 2008 upper bound, with CO2 permeabilities of 753 and 843 Barrer and CO2/N2 permselectivities of 43.9 and 46.1, respectively.Furthermore, the effect of structural unfolding of the [TFSAM]− anion on gas permeation properties of SILMs was investigated using IL mixtures comprising both fluorinated and cyano functionalities in the anions. The pure [C2mim][TFSAM] IL provided a membrane with improved CO2 permeabilities, diffusivities and solubilities compared to those of the SILMs based on the selected [C2mim][SCN][OTf] and [C2mim][N(CN)2][NTf2] IL mixtures. Overall, and despite the fact that the [C2mim][SCN][OTf] SILM revealed better CO2/N2 separation performance essentially due to its higher CO2/N2 permselectivity (57.6), the [C2mim][N(CN)2][NTf2] IL mixture disclosed the most similar results to the pure synthesized [C2mim][TFSAM] IL, not only in terms of thermophysical properties, but also regarding gas transport and CO2/N2 separation performance.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Andreia S. L. Gouveia and Liliana C. Tomé are grateful to FCT (Fundação para a Ciência e a Tecnologia) for their Doctoral (SFRH/BD/116600/2016) and Post-doctoral research grants (SFRH/BPD/101793/2014), respectively, Isabel M. Marrucho acknowledges FCT/MCTES (Portugal) for a contract under Investigador FCT 2012. This work was partially supported by FCT through the project PTDC/CTM-POL/2676/2014, R&D unit UID/Multi/04551/2013 (GreenIT), R&D unit UID/QUI/00100/2013 (CQE) and through the grant of President of the Russian Federation ‘‘For Young Outstanding Professors” (project no. MD-2371.2017.3).Notes and references
- L. C. Tomé and I. M. Marrucho, Chem. Soc. Rev., 2016, 45, 2785–2824 RSC.
- Z. Dai, R. D. Noble, D. L. Gin, X. Zhang and L. Deng, J. Membr. Sci., 2016, 497, 1–20 CrossRef CAS.
- P. Scovazzo, J. Membr. Sci., 2009, 343, 199–211 CrossRef CAS.
- P. K. Parhi, J. Chem., 2013, 11 Search PubMed.
- L. J. Lozano, C. Godínez, A. P. de los Ríos, F. J. Hernández-Fernández, S. Sánchez-Segado and F. J. Alguacil, J. Membr. Sci., 2011, 376, 1–14 CrossRef CAS.
- P. Luis, T. Van Gerven and B. Van der Bruggen, Prog. Energy Combust. Sci., 2012, 38, 419–448 CrossRef CAS.
- M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS PubMed.
- C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke and E. J. Maginn, J. Am. Chem. Soc., 2004, 126, 5300–5308 CrossRef CAS PubMed.
- M. Hasib-ur-Rahman, M. Siaj and F. Larachi, Chem. Eng. Process., 2010, 49, 313–322 CrossRef CAS.
- M. Ramdin, T. W. de Loos and T. J. H. Vlugt, Ind. Eng. Chem. Res., 2012, 51, 8149–8177 CrossRef CAS.
- L. A. Neves, J. G. Crespo and I. M. Coelhoso, J. Membr. Sci., 2010, 357, 160–170 CrossRef CAS.
- P. C. Hillesheim, J. A. Singh, S. M. Mahurin, P. F. Fulvio, Y. Oyola, X. Zhu, D.-e. Jiang and S. Dai, RSC Adv., 2013, 3, 3981–3989 RSC.
- P. C. Hillesheim, S. M. Mahurin, P. F. Fulvio, J. S. Yeary, Y. Oyola, D.-e. Jiang and S. Dai, Ind. Eng. Chem. Res., 2012, 51, 11530–11537 CrossRef CAS.
- S. M. Mahurin, T. Dai, J. S. Yeary, H. Luo and S. Dai, Ind. Eng. Chem. Res., 2011, 50, 14061–14069 CrossRef CAS.
- L. C. Tomé, D. J. S. Patinha, R. Ferreira, H. Garcia, C. Silva Pereira, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, ChemSusChem, 2014, 7, 110–113 CrossRef PubMed.
- R. Condemarin and P. Scovazzo, Chem. Eng. J., 2009, 147, 51–57 CrossRef CAS.
- L. Ferguson and P. Scovazzo, Ind. Eng. Chem. Res., 2007, 46, 1369–1374 CrossRef CAS.
- J. J. Close, K. Farmer, S. S. Moganty and R. E. Baltus, J. Membr. Sci., 2012, 390–391, 201–210 CrossRef CAS.
- J. E. Bara, C. J. Gabriel, T. K. Carlisle, D. E. Camper, A. Finotello, D. L. Gin and R. D. Noble, Chem. Eng. J., 2009, 147, 43–50 CrossRef CAS.
- P. Cserjési, N. Nemestóthy, A. Vass, Z. Csanádi and K. Bélafi-Bakó, Desalination, 2009, 245, 743–747 CrossRef.
- C. Myers, H. Pennline, D. Luebke, J. Ilconich, J. K. Dixon, E. J. Maginn and J. F. Brennecke, J. Membr. Sci., 2008, 322, 28–31 CrossRef CAS.
- P. Scovazzo, D. Havard, M. McShea, S. Mixon and D. Morgan, J. Membr. Sci., 2009, 327, 41–48 CrossRef CAS.
- S. M. Mahurin, J. S. Lee, G. A. Baker, H. Luo and S. Dai, J. Membr. Sci., 2010, 353, 177–183 CrossRef CAS.
- S. M. Mahurin, P. C. Hillesheim, J. S. Yeary, D.-e. Jiang and S. Dai, RSC Adv., 2012, 2, 11813–11819 RSC.
- S. M. Mahurin, J. S. Yeary, S. N. Baker, D.-e. Jiang, S. Dai and G. A. Baker, J. Membr. Sci., 2012, 401–402, 61–67 CrossRef CAS.
- E. Kamio, T. Matsuki, S. Kasahara and H. Matsuyama, Sep. Sci. Technol., 2017, 52, 209–220 CrossRef CAS.
- S. Kasahara, E. Kamio, T. Ishigami and H. Matsuyama, J. Membr. Sci., 2012, 415–416, 168–175 CrossRef CAS.
- S. Kasahara, E. Kamio, T. Ishigami and H. Matsuyama, Chem. Commun., 2012, 48, 6903–6905 RSC.
- L. C. Tomé, D. J. S. Patinha, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, RSC Adv., 2013, 3, 12220–12229 RSC.
- L. C. Tomé, C. Florindo, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, Phys. Chem. Chem. Phys., 2014, 16, 17172–17182 RSC.
- A. S. L. Gouveia, L. C. Tomé and I. M. Marrucho, J. Membr. Sci., 2016, 510, 174–181 CrossRef CAS.
- A. B. Pereiro, L. C. Tomé, S. Martinho, L. P. N. Rebelo and I. M. Marrucho, Ind. Eng. Chem. Res., 2013, 52, 4994–5001 CrossRef CAS.
- M. Althuluth, J. P. Overbeek, H. J. van Wees, L. F. Zubeir, W. G. Haije, A. Berrouk, C. J. Peters and M. C. Kroon, J. Membr. Sci., 2015, 484, 80–86 CrossRef CAS.
- Y. U. Paulechka, G. J. Kabo, A. V. Blokhin, A. S. Shaplov, E. I. Lozinskaya and Y. S. Vygodskii, J. Chem. Thermodyn., 2007, 39, 158–166 CrossRef CAS.
- A. S. Shaplov, E. I. Lozinskaya, P. S. Vlasov, S. M. Morozova, D. Y. Antonov, P. H. Aubert, M. Armand and Y. S. Vygodskii, Electrochim. Acta, 2015, 175, 254–260 CrossRef CAS.
- L. C. Tomé, D. Mecerreyes, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, J. Membr. Sci., 2013, 428, 260–266 CrossRef.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- S. Matteucci, Y. Yampolskii, B. D. Freeman and I. Pinnau, Materials Science of Membranes for Gas and Vapor Separation, John Wiley & Sons, Ltd, 2006, pp. 1–47 Search PubMed.
- S. W. Rutherford and D. D. Do, Adsorption, 1997, 3, 283–312 CrossRef CAS.
- E. Santos, J. Albo, C. I. Daniel, C. A. M. Portugal, J. G. Crespo and A. Irabien, J. Membr. Sci., 2013, 430, 56–61 CrossRef CAS.
- D. Morgan, L. Ferguson and P. Scovazzo, Ind. Eng. Chem. Res., 2005, 44, 4815–4823 CrossRef CAS.
- D. Camper, J. Bara, C. Koval and R. Noble, Ind. Eng. Chem. Res., 2006, 45, 6279–6283 CrossRef CAS.
- P. J. Carvalho and J. A. P. Coutinho, J. Phys. Chem. Lett., 2010, 1, 774–780 CrossRef CAS.
- M. S. Shannon, J. M. Tedstone, S. P. O. Danielsen, M. S. Hindman, A. C. Irvin and J. E. Bara, Ind. Eng. Chem. Res., 2012, 51, 5565–5576 CrossRef CAS.
- J. L. Anderson, J. K. Dixon and J. F. Brennecke, Acc. Chem. Res., 2007, 40, 1208–1216 CrossRef CAS PubMed.
- M. J. Muldoon, S. N. V. K. Aki, J. L. Anderson, J. K. Dixon and J. F. Brennecke, J. Phys. Chem. B, 2007, 111, 9001–9009 CrossRef CAS PubMed.
- Y.-F. Hu, Z.-C. Liu, C.-M. Xu and X.-M. Zhang, Chem. Soc. Rev., 2011, 40, 3802–3823 RSC.
- A. Tagiuri, K. Z. Sumon, A. Henni, K. Zanganeh and A. Shafeen, Fluid Phase Equilib., 2014, 375, 324–331 CrossRef CAS.
- M. Althuluth, M. T. Mota-Martinez, M. C. Kroon and C. J. Peters, J. Chem. Eng. Data, 2012, 57, 3422–3425 CrossRef CAS.
- P. J. Carvalho, K. A. Kurnia and J. A. P. Coutinho, Phys. Chem. Chem. Phys., 2016, 18, 14757–14771 RSC.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- C. M. S. S. Neves, K. A. Kurnia, K. Shimizu, I. M. Marrucho, L. P. N. Rebelo, J. A. P. Coutinho, M. G. Freire and J. N. Canongia Lopes, Phys. Chem. Chem. Phys., 2014, 16, 21340–21348 RSC.
- P. Cserjési, N. Nemestóthy and K. Bélafi-Bakó, J. Membr. Sci., 2010, 349, 6–11 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp06297d |
| This journal is © the Owner Societies 2017 |



