Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emergence of novel hydrogen chlorides under high pressure†
Qingfeng
Zeng
*ab,
Shuyin
Yu
 ab,
Duan
Li
ab,
Artem R.
Oganov
bcd and
Gilles
Frapper
e
ab,
Duan
Li
ab,
Artem R.
Oganov
bcd and
Gilles
Frapper
e
aScience and Technology on Thermostructural Composite Materials Laboratory, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, P. R. China. E-mail: qfzeng@nwpu.edu.cn
bInternational Center for Materials Discovery, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, P. R. China
cDepartment of Geosciences, Center for Materials by Design, and Institute for Advanced Computational Science, State University of New York, Stony Brook, NY 11794-2100, USA
dMoscow Institute of Physics and Technology, Dolgoprudny, Moscow Region 141700, Russia
eIC2MP UMR 7285, Université de Poitiers, CNRS, 4, rue Michel Brunet, TSA 51106, 86073 Poitiers Cedex 9, France
First published on 1st March 2017
Abstract
HCl is a textbook example of a polar covalent molecule, and has a wide range of industrial applications. Inspired by the discovery of unexpected stable sodium and potassium chlorides, we performed systematic ab initio evolutionary searches for all stable compounds in the H–Cl system at pressures up to 400 GPa. Besides HCl, four new stoichiometries (H2Cl, H3Cl, H5Cl and H4Cl7) are found to be stable under pressure. Our predictions substantially differ from previous theoretical studies. We evidence a high significance of zero-point energy in determining phase stability. The newly discovered compounds display a rich variety of chemical bonding characteristics. At ambient pressure, H2, Cl2 and HCl molecular crystals are formed by weak intermolecular van der Waals interactions, and adjacent HCl molecules connect with each other to form asymmetric zigzag chains, which become symmetric under high pressure. In H5Cl, triangular H3+ cations are stabilized by electrostatic interactions with the anionic chloride network. Further increase of pressure drives H2 dimers to combine with H3+ cations to form H5+ units. We also found chlorine-based Kagomé layers which are intercalated with zigzag HCl chains in H4Cl7. These findings could help to understand how varied bonding features can co-exist and evolve in one compound under extreme conditions.
1 Introduction
In 1935, Wigner proposed an insulator-to-metal transition in solid hydrogen under pressure.1 The dissociation, rebonding and polymeriztion, and eventual metallization of molecular systems under high pressure provide a rich ground for novel physical and chemical phenomena. Chemical bonding is the key to understanding the structures and properties of materials, and impacts a broad range of fields including physics, chemistry, biology, and materials and planetary sciences. It is known that very unusual and unexpected compounds can become stable under pressure, accompanied by a variety of bizarre topologies, and their chemical nature is still not understood.2,3 For example, under pressure, the simple Na–Cl system contains stable compounds such as Na3Cl, Na2Cl, Na3Cl2, Na4Cl3, NaCl3 and NaCl7, as well as ‘normal’ NaCl.4,5While NaCl is a textbook example of an ionic crystal, HCl is an archetypal polar covalent molecule. One can expect large differences between the Na–Cl and H–Cl systems; however, we can imagine the H–Cl system to be even more diverse due to its increased variety of intermolecular and intramolecular interactions. In addition, hydrogen adds extra excitement: the high-pressure metallic phase of hydrogen has been predicted to possess a nearly room-temperature superconductivity (Tc ∼ 240 K),6 while superconducting metallic hydrogen remains elusive. In 2014, an unusual stoichiometry, H3S, was predicted with a high-Tc reaching ∼200 K at 200 GPa.7 Later, Eremets et al. verified that the phase responsible for high-Tc superconductivity (203 K) in the sulfur hydride system is H3S.8 It has been suggested that alloying hydrogen with other elements can greatly reduce the pressure required for stability of the superconducting state,9 and one may think not of only metal hydrides, but also of compounds with more electronegative atoms, such as the aforementioned H3S, or the HnCl compounds studied here.
For the H–Cl system, HCl has been extensively studied in the past few decades.10–13 At very low temperatures, HCl adopts an ordered orthorhombic Cmc21 structure, which is also adopted by HBr and HF.11 With increasing pressure, the Cmc21 phase was suggested to transform into another orthorhombic Cmcm structure at 51 GPa with hydrogen bond symmetrization.14 The Cmcm phase is stable until 108 GPa,11 then a post-Cmcm phase with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) appears. However, at pressures higher than 230 GPa, the stability of HCl remains controversial. Duan et al.15 predicted that the P21/m structure would be the most stable one for HCl above 233 GPa, while a recent work by Chen et al.12 proposed C2/m and P4/nmm structures, which are more stable than P21/m above 250 GPa. Furthermore, the calculated enthalpy difference between C2/m and P4/nmm phases was negligibly small above 250 GPa. However, we know that zero-point energy (ZPE) usually plays a non-negligible role in the total energy of low-atomic-mass materials, and this effect was not considered in previous studies of the H–Cl system.
appears. However, at pressures higher than 230 GPa, the stability of HCl remains controversial. Duan et al.15 predicted that the P21/m structure would be the most stable one for HCl above 233 GPa, while a recent work by Chen et al.12 proposed C2/m and P4/nmm structures, which are more stable than P21/m above 250 GPa. Furthermore, the calculated enthalpy difference between C2/m and P4/nmm phases was negligibly small above 250 GPa. However, we know that zero-point energy (ZPE) usually plays a non-negligible role in the total energy of low-atomic-mass materials, and this effect was not considered in previous studies of the H–Cl system.
Besides HCl, several HxCly compounds have also been predicted.15,16 In 2015, Wang et al.16 studied the crystal structures and phase stabilities of the hydrogen-rich H2–HCl system by using a first-principles particle-swarm CALYPSO code. Between 100–300 GPa, two stable compounds with H5Cl and H2Cl stoichiometries were found. In H5Cl, they found the stabilization of cationic H3+ species. It becomes almost an equilateral triangle under very high pressure. Soon after, Duan et al.17 reexamined the phase stabilities of the H2–HCl system by using the same CALYPSO code. However, very different results were found in their study. Besides H5Cl and H2Cl, Duan et al. found that H3Cl and H7Cl could also be stabilized in the pressure range of 0–250 GPa, which is in contradiction with the results of Wang et al.,16 in which they found the very same two stoichiometries to be metastable up to 300 GPa. In the above two studies, their reactants are H2 and HCl, thus the stabilities of the chlorine-rich phases in the full H–Cl system are still unclear. The question of whether a chlorine-rich structure can be stabilized under high pressure is still unresolved. Here, we apply a powerful evolutionary algorithm, USPEX, to extensively explore the crystal structures and stoichiometries in the H–Cl system. We attempt to give an exhaustive picture of the H–Cl system under pressure.
2 Methods
Searches for stable compounds in the H-Cl system from ambient pressure up to 400 GPa were performed using the variable-composition ab initio evolutionary algorithm USPEX.18–20 In these searches, all HxCly compositions were allowed, under the constraint that the total number of atoms in the primitive cell could be up to 30 atoms. The first generation contained 100 candidate structures, while all subsequent generations contained 80 structures. In each new generation, 40% of the structures were produced by heredity, 20% by softmutation, 20% by transmutation and 20% were produced randomly.Local geometry relaxations were performed using density functional theory calculations with the use of VASP code.21 These calculations are based on the Perdew–Burke–Ernzerhof (PBE) functional,22 which belongs to the generalized gradient approximation (GGA) level of theory. We used the all-electron projector-augmented wave (PAW) method,23,24 with the outermost core radius of 1.1 a.u. for the H atom and 1.9 a.u. for the Cl atom. The plane-wave kinetic energy cutoff was set to 900 eV, and uniform Γ-centered k-meshes with a reciprocal space resolution of 2π × 0.03 Å−1 were used for sampling the Brillouin zone. These settings enabled excellent convergence of the energy differences, stress tensors, and structural parameters. Phonon spectra were calculated using the finite-displacement method as implemented in the PHONOPY package25 in order to probe the dynamical stability and explore the effects of ZPE on the thermodynamic stability of HxCly compounds (see the ESI†).
All molecular species were optimized at different levels of theory (B3PW91, PBE, MP2, CCSD). A triple-zeta basis set was employed for all atoms, which was increased using both polarized and diffuse functions, aug-cc-pVTZ.26,27 For each structure, the analytic Hessian was calculated to obtain the vibrational frequencies and determine the nature of the stationary point. These calculations were performed using the Gaussian package.28 As the results followed the same trend, we present here only the electrostatic potential results based on the B3PW91 functional. The surface potentials, Vs(r), were obtained using the Wave Function Analysis-Surface Analysis Suite (WFA).29
3 Results and discussion
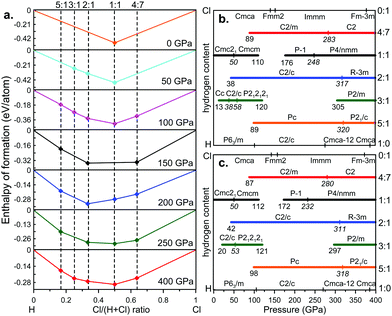
The calculated convex hulls and pressure–composition phase diagram taking into account the vibrational contributions (ZPE) of the H–Cl system are shown in Fig. 1. The enthalpies of formation of the HxCly phases have been calculated with respect to elemental hydrogen and chlorine in their most stable forms, i.e., P63/m, C2/c, Cmca![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2, Cmca and I41/amd structures for hydrogen,30 and Cmca, Fmm2, Immm and fcc structures for chlorine31 in their stability fields. The convex hull is a set of thermodynamically stable states, which are stable with respect to disproportion into other phases or pure elements. Any structure, for which the enthalpy of formation lies on the convex hull, is considered to be thermodynamically stable and in principle can be synthesized from any isochemical mixture.
2, Cmca and I41/amd structures for hydrogen,30 and Cmca, Fmm2, Immm and fcc structures for chlorine31 in their stability fields. The convex hull is a set of thermodynamically stable states, which are stable with respect to disproportion into other phases or pure elements. Any structure, for which the enthalpy of formation lies on the convex hull, is considered to be thermodynamically stable and in principle can be synthesized from any isochemical mixture.
Besides reproducing the well-known HCl, we also found the stable stoichiometries 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. It can be seen that HCl remains a stable compound in the pressure ranges of 0–112 GPa and 172–400 GPa, but between them, it will decompose into H2Cl and H4Cl7. HCl is predicted to adopt four phases with the space groups Cmc21 (0–50 GPa), Cmcm (50–112 GPa), P
7. It can be seen that HCl remains a stable compound in the pressure ranges of 0–112 GPa and 172–400 GPa, but between them, it will decompose into H2Cl and H4Cl7. HCl is predicted to adopt four phases with the space groups Cmc21 (0–50 GPa), Cmcm (50–112 GPa), P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (172–232 GPa) and P4/nmm (232–400 GPa). For the low-pressure Cmc21 and Cmcm phases, their enthalpies are indistinguishable above 50 GPa, due to the very similar structures. However, we find that hydrogen bond symmetrization occurs at around 50 GPa, consistent with previous studies.11,12,16,17 After considering ZPE correction, the P
(172–232 GPa) and P4/nmm (232–400 GPa). For the low-pressure Cmc21 and Cmcm phases, their enthalpies are indistinguishable above 50 GPa, due to the very similar structures. However, we find that hydrogen bond symmetrization occurs at around 50 GPa, consistent with previous studies.11,12,16,17 After considering ZPE correction, the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) phase transforms into the P4/nmm phase at 232 GPa instead of 250 GPa12 proposed by Chen et al.12 Besides, the P4/nmm phase has a slightly lower enthalpy than the C2/m structure.12
phase transforms into the P4/nmm phase at 232 GPa instead of 250 GPa12 proposed by Chen et al.12 Besides, the P4/nmm phase has a slightly lower enthalpy than the C2/m structure.12
For H7Cl discovered in the work of Duan et al.17 (see Fig. S2, ESI†), we found it is metastable with or without considering the ZPE correction. For H5Cl, our evolutionary searches revealed a new structure with the space group Pc at low pressures, which has a slightly lower energy than the previously proposed Cc structure16,17 (∼6 meV per atom at 150 GPa). At 318 GPa, the Pc phase transforms into a new structure with the space group P21/c. For H3Cl, it emerges on the phase diagram at 20 GPa and decomposes into H2Cl and H5Cl at 121 GPa and reappears at 297 GPa, which is consistent with the results of Duan et al.17 In their work, three phases with space groups Cc (12–40 GPa), C2/c (40–60 GPa), and P212121 (60–120 GPa)17 were predicted (see Fig. S2, ESI†). However, when ZPE is taken into account, the C2/c structure has a lower energy (∼7 meV per atom at 25 GPa) and becomes more stable than the Cc structure. The transition pressure from C2/c to P212121 becomes 53 GPa instead of 60 GPa. For H2Cl, the monoclinic C2/c structure appears at 42 GPa and transforms to the highly-symmetric R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure at 311 GPa. Remarkably, we found a new chlorine-rich compound H4Cl7, which becomes stable at 87 GPa and remains stable up to 400 GPa.
m structure at 311 GPa. Remarkably, we found a new chlorine-rich compound H4Cl7, which becomes stable at 87 GPa and remains stable up to 400 GPa.
Detailed lattice parameters and atomic Wyckoff positions of these thermodynamically stable HxCly phases are given in Table S1 (ESI†). Their calculated phonon spectra displayed in Fig. S3 (ESI†) confirm that all these compounds are dynamically stable with no imaginary phonon modes in the whole Brillouin zone. After analyzing their crystal structures, we identified a rich variety of structural features, such as asymmetric or symmetric zigzag HCl chains, host–guest inclusion compounds, chlorine-based Kagomé layers, hcp chlorine network with interstitial protons and stretched H2 units, one-dimensional (1D) hydrogen chains, bridging H3+ and H5+ units, interpenetrating graphene-like chlorine nets and others, which will be illustrated below.
3.1 HCl: a brief revisit of the pressure-induced symmetrization of the zigzag chains
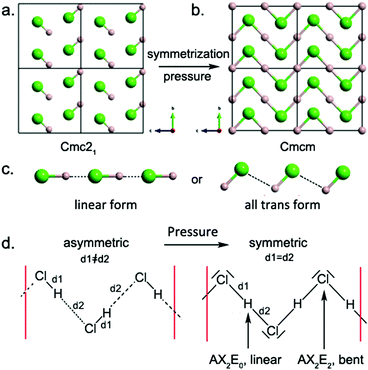
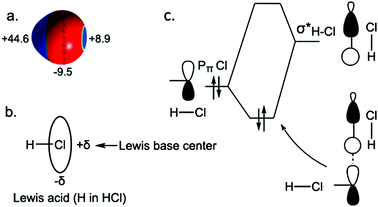
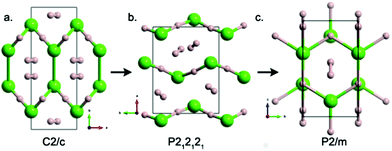
At ambient conditions, HCl adopts the orthorhombic Cmc21 structure, which remains stable up to 50 GPa (Fig. 2a). In this structure, HCl forms zigzag chains with a H–Cl distance of 1.313 Å, compared with the calculated H–Cl bond length of 1.311 Å in the gas phase (exp. 1.274 Å). The intermolecular H–Cl separations in Cmc21 are 2.219 Å, much less than the sum of the van der Waals radii of 2.950 Å32 and typical of H⋯Cl hydrogen bonds. In the next Cmcm phase, HCl⋯HCl contacts have an L-shaped geometry, with the H–Cl bond axis of one molecule perpendicular to the other one. A similar herringbone pattern is also observed in solid X2 (X, halogen).33,34 Why does the HCl chain possesses a zigzag shape (L-shaped geometry) rather than a linear or all-trans one (Fig. 2c)? Electrostatic arguments can be used to find the answer (Fig. 3).The computed electrostatic potential Vs(r) on the 0.001 a.u. surface of HCl is displayed in Fig. 3. A region of positive electrostatic potential – the so-called σ-hole35,36 – can be seen on the outer surface of the hydrogen, with a maximum positive Vs,max value of 44.6 kcal mol−1. Due to the two chlorine lone pairs of electrons in the 3px and 3py orbitals, the most negative values Vs,min are on the lateral rings of the chlorine negative potential surface, shown in red with a Vs,max of −9.5 kcal mol−1. Thus, the hydrogen atom may be considered as a Lewis acid site, and the halogen atom interacts as an electron donor through its π lone pairs, thus the angle for the H⋯Cl–H arrangement should be around 90°, therefore the linear form is rejected. Moreover, to understand why the L-shaped coordination (Cl⋯H–Cl = 180°) is preferred to the all-trans one (Cl⋯H–Cl = 90°), molecular orbital arguments can be used. As depicted in Fig. 3c, the bonding interaction between the filled pπ(Cl) orbital – a lone pair of Cl – of an HCl unit and the vacant σH–Cl* level of the other is optimized when the Cl⋯H–Cl angle is 180° (Fig. 3c). This two-electron stabilizing interaction and the anisotropy of the electron charge distribution of the halogen (Cl) atom explain why the HCl chain possesses an L-shaped geometry.
To summarize, this zigzag topology is closely related to the anisotropy of the electron charge distribution of the halogen (Cl) atom. A stabilizing interaction between two H–Cl units occurs in such a manner that a filled Cl-pπ orbital – a π lone pair of Cl – is oriented towards the H–Cl bond of the other. Eventually, symmetric hydrogen bonds appear. These symmetric HCl-based zigzag chains are also encountered in other phases at different compositions.11,14,37 In the Cmcm phase at 100 GPa, symmetric zigzag chains are stacked along the a-axis with an interchain spacing of 1.940 Å and a shortest Cl–Cl separation of 2.883 Å. Delocalized three-center two-electron bonding is expected along the σ H–Cl chains, which explains the increase in the covalent H–Cl bond length from 1.313 Å at 0 GPa to 1.442 Å at 100 GPa, and the decrease in the hydrogen-bond separation to 2.219 Å at 0 GPa. In agreement with the VSEPR model, here the zigzag chain contains chlorine atoms in a bent two-coordinate configuration (H–Cl–H angle of 84.2°) and two-coordinate almost linear hydrogen atoms (Fig. 2d and Fig. S4, ESI†).
The hydrogen bond symmetrization/desymmetrization process for HCl chains under high pressure is well characterized,38 and can be considered as a classical example of a second-order Peierls distortion in weakly 1D covalent dimer-based chains. The symmetric zigzag HCl chain displays Peierls-type instability of the σ-bands at the Fermi level, which induces a structural distortion along the σ H–Cl backbone – creation of short and long H–Cl bonds – when the pressure is reduced to ambient conditions. This dimerization process leads to the thermodynamically stable covalent HCl monomers. Finally, in addition to hydrogen bond symmetrization, reduction of the van der Waals volume occurs under compression, leading to stronger interchain Cl–Cl interactions. From 0 to 100 GPa, the interplane π stacking separation decreases drastically from 3.506 Å to 1.940 Å, respectively, while the interplane σ stacking Cl–Cl separation decreases from 4.024 Å to 2.514 Å. This effect leads to band gap closure upon compression (Fig. S5, ESI†).
For the high-pressure HCl phases, our structure searches revealed the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) structure as the most stable one at 150 GPa. The P
structure as the most stable one at 150 GPa. The P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) structure contains four H–Cl molecules in the unit cell and only possesses the space-inversion symmetry. Note that the zigzag H–Cl chains discovered in the Cmc21 and Cmcm phases collapse in the P
structure contains four H–Cl molecules in the unit cell and only possesses the space-inversion symmetry. Note that the zigzag H–Cl chains discovered in the Cmc21 and Cmcm phases collapse in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) phase instead of forming the intriguing distorted squares, where the chlorine atoms are connected by asymmetric hydrogen bonds. The tetragonal P4/nmm structure becomes stable at pressures above 232 GPa upon considering the ZPE effect. P4/nmm HCl possesses a layered mackinawite-type structure, and adjacent HCl-based zigzag chains get close to form 2D layers. The shortest interlayer Cl–Cl distance is 2.199 Å at 300 GPa. In each layer, HCl zigzag chains run along the a- and b-axes. Here, hydrogen atoms have a distorted tetrahedral coordination (Cl–H–Cl angle of 126.7°), while chlorine atoms have a four-fold umbrella coordination, using the interlayer space to contain lone electron pairs. The increased H–Cl bond length reflects the increase in the number of H–Cl bonds under compression – four in P4/nmm (1.574 Å at 300 GPa), two in symmetric Cmcm HCl (1.442 Å at 100 GPa), one in molecular Cmc21 (1.313 Å at 0 GPa) – reflecting electron delocalization in the covalent 2D layers of the highest-pressure P4/nmm phase.
phase instead of forming the intriguing distorted squares, where the chlorine atoms are connected by asymmetric hydrogen bonds. The tetragonal P4/nmm structure becomes stable at pressures above 232 GPa upon considering the ZPE effect. P4/nmm HCl possesses a layered mackinawite-type structure, and adjacent HCl-based zigzag chains get close to form 2D layers. The shortest interlayer Cl–Cl distance is 2.199 Å at 300 GPa. In each layer, HCl zigzag chains run along the a- and b-axes. Here, hydrogen atoms have a distorted tetrahedral coordination (Cl–H–Cl angle of 126.7°), while chlorine atoms have a four-fold umbrella coordination, using the interlayer space to contain lone electron pairs. The increased H–Cl bond length reflects the increase in the number of H–Cl bonds under compression – four in P4/nmm (1.574 Å at 300 GPa), two in symmetric Cmcm HCl (1.442 Å at 100 GPa), one in molecular Cmc21 (1.313 Å at 0 GPa) – reflecting electron delocalization in the covalent 2D layers of the highest-pressure P4/nmm phase.
3.2 H2Cl and H3Cl: HCl zigzag chains + H2 units
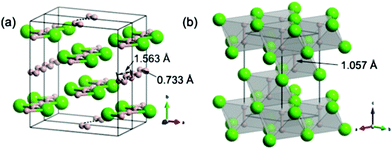
The hydrogen-rich compound H2Cl crystallizes in the monoclinic C2/c structure (Fig. 4a). This structure is formed by planar symmetric zigzag HCl chains and H2 dimers. H2 dimers also form planar zigzag chains with alternating short intramolecular (0.733 Å at 100 GPa) and long intermolecular (1.563 Å at 100 GPa) distances. As expected from band theory (Peierls distortion), this encapsulated (H2)n chain is insulating and presents yet another classical textbook hydrogen chain model in an experimentally synthesizable material. Pairs of stacked HCl chains are observed in the ac planes. A shortest Cl–Cl separation of 2.496 Å is found, compared to the van der Waals Cl–Cl separation of 3.50 Å at 0 GPa. Thus, some attractive interactions are at work in these paired chains, a signature of the chain-pairing effect.At 311 GPa, the low-pressure C2/c structure transforms to a previously omitted16 rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure (Fig. 4b), which has a cubic close packing of Cl atoms in which all octahedral voids are filled by H2 groups. The H–H distance (1.057 Å) is much longer than that of the H2 molecule (0.74 Å), though still bonding, which indicates weakening due to depletion of the σ-bonding orbital that donates part of its electron to the Cl framework. The band structure of the R
m structure (Fig. 4b), which has a cubic close packing of Cl atoms in which all octahedral voids are filled by H2 groups. The H–H distance (1.057 Å) is much longer than that of the H2 molecule (0.74 Å), though still bonding, which indicates weakening due to depletion of the σ-bonding orbital that donates part of its electron to the Cl framework. The band structure of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase displays a ‘flat band-steep band’ character39 (Fig. S6, ESI†), suggesting potential superconductivity. The electron–phonon coupling (EPC) calculation shows that the EPC constant λ is 0.66 with an ωlog of 1368 K at 400 GPa. By using the Allen–Dynes modified McMillan equation,40 we obtained the superconducting transition temperature (Tc) of 43.9–44.8 K (μ* = 0.1–0.13). Also, we found that coupling of the electrons to hydrogen vibrations in the frequency region of 33–60 THz contributes ∼64.1% of the total λ.
m phase displays a ‘flat band-steep band’ character39 (Fig. S6, ESI†), suggesting potential superconductivity. The electron–phonon coupling (EPC) calculation shows that the EPC constant λ is 0.66 with an ωlog of 1368 K at 400 GPa. By using the Allen–Dynes modified McMillan equation,40 we obtained the superconducting transition temperature (Tc) of 43.9–44.8 K (μ* = 0.1–0.13). Also, we found that coupling of the electrons to hydrogen vibrations in the frequency region of 33–60 THz contributes ∼64.1% of the total λ.
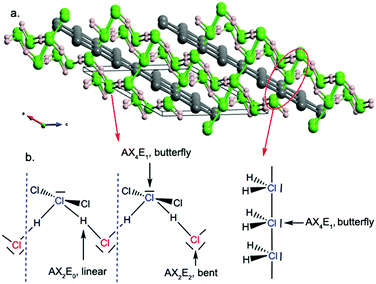
In C2/c-H3Cl, the HCl units are also packed in symmetric zigzag chains, between which non-bonded H2 molecules are located – formally two (H2Cl2)n polymers and four H2 units per unit cell (Fig. 5a). The presence of H2 reduces the pressure required to symmetrize H-bonds in the Cl–H–Cl chain in Cmc21-HCl. The C2/c structure can be viewed as a host–guest inclusion compound: a stacked 1D planar inorganic polymeric HCl chain with encapsulated H2 units in the van der Waals space. Symmetrization of the HCl chain occurs at ∼20 GPa, whereas in HCl it takes place at a much higher pressure of ∼50 GPa. Therefore, H2 dimers act by either adding extra mechanical pressure or through chemical doping in the symmetrization process, lowering the driving force of the Peierls instability.
Obviously, free rotation is allowed around the bonding H–Cl chain, thus, a non-planar form may exist. In the higher-pressure P212121 phase (Fig. 5b), slightly helical zigzag HCl chains run along the b-axis. The dihedral angle H–Cl–Cl–H is 14.1° at 100 GPa. There are four H2 dimers per unit cell located in the cavities between two slightly helical chains. Above 271 GPa, a P2/m structure is stable (Fig. 5c). One can observe graphene-like arrays formed together by Cl and H atoms, some parallel and some perpendicular to the ac plane. The graphene-like nets are composed by the staggered packing of symmetric HCl zigzag chains. H2 units are observed in channels formed by graphene-like nets.
3.3 H5Cl: the existence of H3+ and H5+ units
The crystal structures of H5Cl phases are shown in Fig. 6. The low-pressure Pc structure contains planar chains composed of triangular H3 units connected to two Cl atoms. The structure also contains isolated H2 dimers (H–H = 0.748 Å at 150 GPa). Formally, the H3 unit may be viewed as the well-known H3+ cation interacting with one Cl− anion. The H3+ cation deviates slightly from an equilateral triangle (H–H = 0.864–0.913 Å at 150 GPa), which is the shape experimentally and theoretically observed in the gas phase H3+ for this smallest and simplest aromatic molecule.41 The H–H bond lengths are elongated by 19% relative to the calculated distance in encapsulated H2 dimers or the experimental free gas H2 (0.74 Å). Such elongation is expected for an electron-deficient system (Fig. 6c). The stability of H3+ can be attributed to the occupation of the a1′ bonding molecular orbital (MO) and the large HOMO a1′-LUMO e′ gap. The formal H–H bond order is 1/3 per H–H link (1 in H2), explaining the longer H–H distances for H3+ (H3+ can be described by the well-known three-center two-electron bonding scheme). H3+ is stabilized in this solid-state compound by electrostatic interactions with the anionic chloride network.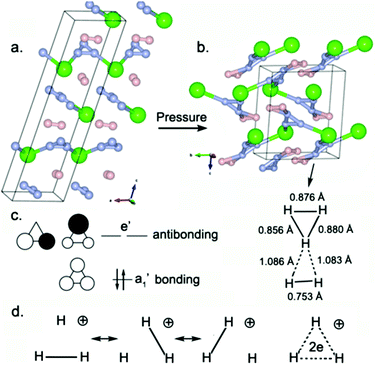 | ||
| Fig. 6 Crystal structures of H5Cl in the (a) Pc phase at 150 GPa and (b) P21/c phase at 350 GPa. (c and d) Molecular orbital diagram and Lewis structures for the H3+ cation. | ||
H2 acts as an electron donor in combination with a proton to yield H3+. By analogy, H2 may also combine with H3+, leading to the higher homolog H5+ (Fig. S7, ESI†). The existence of H3+ and H5+ has been firmly established by mass spectrometry and quantum-mechanical calculations.41–44 In fact, gas phase charged hydrogen clusters are a well-known stable species, provided that the number of hydrogen atoms is odd.45,46 The H5+ ion appears in the high-pressure P21/c phase of H5Cl. The H–H distances reveal the existence of the planar H5+ unit: all H–H distances are in the range of 0.753–1.086 Å at 350 GPa. In the pseudo-equilateral H3 unit, the H–H bond lengths are 0.856–0.880 Å. Two H–H bonds are significantly longer (1.083–1.086 Å) than the H–H bonds reported in H3 units, indicating weaker bonds. The shortest distance is 0.753 Å, similar to the length of the H–H covalent bond in free H2 gas. Therefore, this planar H5 unit may be viewed as H3+ interacting with a slightly activated H2 dimer. A five-center four-electron bonding scheme is assigned: such a bonding mode has been previously observed in protonated hydrogen-based clusters,47 the global-minimum structure of which is, however, non-planar. The calculated electronic properties show that this compound is an insulator up to 400 GPa (Fig. S5, ESI†). Obviously, a hydrogen-rich system is not more easily metallized than a chlorine-rich system.
3.4 H4Cl7: chlorine-based Kagomé layers intercalated with zigzag HCl chains
Besides H-rich HxCly compounds, we also uncovered a Cl-rich compound H4Cl7, which has never been reported before. H4Cl7 has two structures in the pressure range 0–400 GPa, i.e. C2/m (87–280 GPa) and C2 (280–400 GPa). In the low-pressure C2/m phase (Fig. 7a), two different networks can be found, namely two (HCl)2 zigzag chains intercalated between planar Kagomé (Cl3)2 covalent layers (4H2Cl2 + 2Cl3 = H8Cl14). The 1D zigzag chain has the topology encountered in the Cmcm-HCl phase with two-coordinate bent chlorine atoms and two-coordinate (almost) linear hydrogens (Cl–H = 1.46–1.47 Å; H–Cl–H = 98.7°; Cl–H–Cl = 173.5°). Besides, the C2/m structure contains a 2D hexagonal network of chlorine atoms comprising triangles and hexagons, shown in Fig. 7b. The planar Kagomé (Cl3)2 covalent layers possess four-coordinate (with rectangular planar coordination) chlorine atoms with Cl–Cl bond distances of 2.236–2.273 Å and bond angles of 60.5° and 119.5° at 100 GPa. Seven electrons may be formally assigned to each chlorine atom, thus according to the VSEPR model, the chlorine atom is the AX4E3/2 type, a rectangular planar AX4 unit (one electron less than that of XeF448).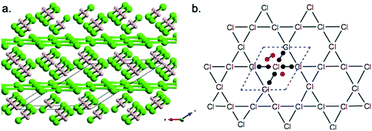 | ||
| Fig. 7 (a) Crystal structure of C2/m-H4Cl7 at 100 GPa. (b) VSEPR analysis of the planar Kagomé (Cl3)2 covalent layers. | ||
The high-pressure C2 phase has even more complex substructures (Fig. 8a). First, the 2D Kagomé chlorine (3636) layers found in the low-pressure C2/m phase still exist (Cl–Cl = 2.146–2.162 Å at 350 GPa). Second, between these planar Kagomé layers, there are two zigzag (HCl)2 chains with two types of coordinated chlorine atoms: one is coordinated to two bridging hydrogen atoms (H–Cl = 1.367–1.387 Å), the other is four-coordinated to two bridging hydrogen atoms (H–Cl = 1.375–1.379 Å) and two chlorine atoms through short and long bonds (Cl–Cl = 2.051–2.223 Å). Finally, one may see 1D linear chlorine chains running through the center of Cl6 hexagons with alternating distances (2.006 Å and 2.183 Å). Even though this phase displays delocalized bonding (e.g. the Cl–Cl long distance of 2.223 Å, signature of an electron-deficient covalent bond), its local structures are rationalized by using VSEPR rules from single Lewis resonant structures (Fig. 8b and Fig. S8, ESI†). For the 1D zigzag (HCl)2 chains, the two-coordinate chlorine atoms are AX2E2 type with a bent geometry (H–Cl–H = 99.2°). The four-coordinate chlorine atoms are AX4E1 type with a local butterfly-like structure (H–Cl–H = 92.3°, Cl–Cl–Cl = 172.4°). The two-coordinate hydrogen atom is AX2E0 type with a (almost) linear geometry. DFT calculations show that both C2/m- and C2-H4Cl7 phases are metallic, mainly due to the Cl-p states.
4 Conclusion
In summary, we have extensively explored the stable stoichiometries and structures in the H–Cl system up to 400 GPa. Besides HCl, we also discovered three hydrogen-rich compounds, H2Cl, H3Cl and H5Cl, and a chlorine-rich compound H4Cl7. HCl and H3Cl show reentrant behavior. With increasing pressure, they will first decompose into adjacent stoichiometries, then become stable at a higher pressure again. Our results show that the ZPE contribution to the total energy will significantly affect the stability of HxCly compounds. After considering ZPE corrections, P4/nmm-HCl has lower enthalpy than the previously proposed C2/m structure, and C2/c-H3Cl has a lower enthalpy than the Cc structure proposed by Duan et al. Besides, we found a Pc structure for H5Cl at low pressures, which is more energetically favorable than the Cc structure. And H7Cl is metastable with or without ZPE correction in the whole studied pressure range.The newly discovered HxCly compounds possess peculiar structural characteristics and rich chemical bonding types. Here, we used VSEPR and molecular orbital theory to rationalize their formation. The zigzag shape of H–Cl⋯H–Cl chains can be explained by the Lewis acid model based on electrostatic potential calculations. Under high pressure, asymmetric zigzag chains become symmetric. In H5Cl, triangular H3+ cations are stabilized by electrostatic interactions with the anionic chloride network. Further increase of pressure makes H2 dimers combine with H3+ cations to form H5+ units. Additionally, we found chlorine-based Kagomé layers in H4Cl7, intercalated with zigzag HCl chains. These findings of unexpected complexity in the simple H–Cl system will be helpful in understanding the effect of pressure on chemical bonding.
Acknowledgements
We thank the National Natural Science Foundation of China (Grants No. 51372203, 51332004 and 51571166), the Foreign Talents Introduction and Academic Exchange Program (Grant No. B08040), Poitiers University (mésocentre de calculs Thor) and CNRS, the Government of the Russian Federation (Grant No. 14.A12.31.0003), and the Fundamental Research Funds for the Central Universities of China (Grant No. 3102016JKBJJGZ02) for financial support. We also acknowledge the high performance computing center of NWPU (China), and TGCC/Curie GENCI (France) under project no. 2016087539 for allocation of computing time on their machines.References
- E. Wigner and H. B. Huntington, J. Chem. Phys., 1935, 3, 764–770 CrossRef CAS.
- W. Grochala, R. Hoffmann, J. Feng and N. W. Ashcroft, Angew. Chem., Int. Ed., 2007, 46, 3620 CrossRef CAS PubMed.
- E. Zurek and W. Grochala, Phys. Chem. Chem. Phys., 2015, 17, 2917–2934 RSC.
- W. Zhang, A. R. Oganov, A. F. Goncharov, Q. Zhu, S. E. Boulfelfel, A. O. Lyakhov, E. Stavrou, M. Somayazulu, V. B. Prakapenka and Z. Konôpková, Science, 2012, 342, 1502–1505 CrossRef PubMed.
- G. Saleh and A. R. Oganov, Phys. Chem. Chem. Phys., 2016, 18, 2840–2849 RSC.
- N. W. Ashcroft, Phys. Rev. Lett., 1968, 21, 1748–1749 CrossRef CAS.
- D. Duan, Y. Liu, F. Tian, D. Li, X. Huang, Z. Zhao, H. Yu, B. Liu, W. Tian and T. Cui, Sci. Rep., 2014, 4, 6968 CrossRef CAS PubMed.
- A. P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov and S. I. Shylin, Nature, 2015, 525, 73–76 CrossRef CAS PubMed.
- N. W. Ashcroft, Phys. Rev. Lett., 2004, 92, 187002 CrossRef CAS PubMed.
- J. Obriot, F. Fondère, P. Marteau and M. Allavena, J. Chem. Phys., 1983, 79, 33–42 CrossRef CAS.
- L. Zhang, Y. Wang, X. Zhang and Y. Ma, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 1558–1564 Search PubMed.
- C. Chen, Y. Xu, X. Sun and S. Wang, J. Phys. Chem. C, 2015, 119, 17039–17043 CAS.
- H. Shimizu, K. Kamabuchi, T. Kume and S. Sasaki, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 11727–11732 CrossRef CAS.
- K. Aoki, E. Katoh, H. Yamawaki, M. Sakashita and H. Fujihisa, Phys. B, 1999, 265, 83–86 CrossRef CAS.
- D. Duan, F. Tian, Z. He, X. Meng, L. Wang, C. Chen, X. Zhao, B. Liu and T. Cui, J. Chem. Phys., 2010, 133, 074507 CrossRef PubMed.
- Z. Wang, H. Wang, J. S. Tse, T. Iitaka and Y. Ma, Chem. Sci., 2014, 6, 522–526 RSC.
- D. Duan, X. Huang, F. Tian, Y. Liu, D. Li, H. Yu, B. Liu, W. Tian and T. Cui, J. Phys. Chem. A, 2015, 119, 11059–11065 CrossRef CAS PubMed.
- A. R. Oganov and C. W. Glass, J. Chem. Phys., 2006, 124, 244704 CrossRef PubMed.
- A. O. Lyakhov, A. R. Oganov, H. T. Stokes and Q. Zhu, Comput. Phys. Commun., 2013, 184, 1172–1182 CrossRef CAS.
- A. R. Oganov, A. O. Lyakhov and M. Valle, Acc. Chem. Res., 2011, 44, 227–237 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- E. R. Davidson, Chem. Phys. Lett., 1996, 260, 514–518 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria and M. A. Robb, et al., Gaussian, 2001 Search PubMed.
- F. A. Bulat, A. Toro-Labbé, T. Brinck, J. S. Murray and P. Politzer, J. Mol. Model., 2010, 16, 1679–1691 CrossRef CAS PubMed.
- C. J. Pickard and R. J. Needs, Nat. Phys., 2007, 3, 473–476 CrossRef CAS.
- P. Li, G. Gao and Y. Ma, J. Chem. Phys., 2012, 137, 2840–2847 Search PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- G. Bao, D. Duan, D. Zhou, X. Jin, B. Liu and T. Cui, J. Phys. Chem. B, 2010, 114, 13933–13939 CrossRef CAS PubMed.
- G. Bao, D. Duan, F. Tian, L. Wang, B. Liu and T. Cui, J. Chem. Phys., 2011, 134, 123–127 CrossRef PubMed.
- T. Clark, M. Hennemann, J. S. Murray and P. Politzer, J. Mol. Model., 2007, 13, 291–296 CrossRef CAS PubMed.
- T. Clark, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2013, 3, 13–20 CrossRef CAS.
- Y. Duan and D. C. Sorescu, J. Chem. Phys., 2010, 133, 304–311 Search PubMed.
- P. Li, G. Gao and Y. Ma, J. Chem. Phys., 2012, 137, 2840–2847 Search PubMed.
- A. Simon, Angew. Chem., Int. Ed. Engl., 1997, 36, 1788–1806 CrossRef.
- P. B. Allen and R. C. Dynes, Phys. Rev. B: Solid State, 1975, 12, 905–922 CrossRef CAS.
- R. D. Poshusta and F. A. Matsen, J. Chem. Phys., 1967, 47, 4795–4799 CrossRef CAS.
- J. J. Thomson, J. Rönt. Soc., 1922, 18, 150–151 CrossRef.
- R. E. Christoffersen, J. Chem. Phys., 1964, 41, 960–971 CrossRef CAS.
- P. H. Dawson and A. W. Tickner, J. Chem. Phys., 1962, 37, 672–673 CrossRef CAS.
- H. Chermette and I. Ymmud, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 165427 CrossRef.
- M. Farizon, B. Farizon-Mazuy, N. de Castro Faria and H. Chermette, Chem. Phys. Lett., 1991, 177, 451–457 CrossRef CAS.
- I. Štich, D. Marx, M. Parrinello and K. Terakura, J. Chem. Phys., 1997, 107, 9482–9492 CrossRef.
- Q. Zhu, D. Y. Jung, A. R. Oganov, C. W. Glass, C. Gatti and A. O. Lyakhov, Nat. Chem., 2013, 5, 61–65 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp08708f |
| This journal is © the Owner Societies 2017 |