A nonmonotonic dependence of the contact angles on the surface polarity for a model solid surface
Abstract
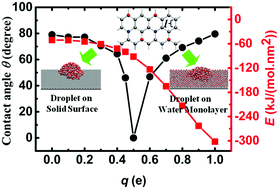
Based on molecular dynamics simulations, we found a nonmonotonic relationship between the contact angle of water droplets and the surface polarity on a solid surface with specific hexagonal charge patterns at room temperature. The contact angle firstly decreases and then increases as polarity (denoted as charge q) increases from 0 e to 1.0 e with a vertex value of q = 0.5 e. We observed a different wetting behavior for a water droplet on a conventional nonwetted solid surface when q ≤ 0.5 e, and a water droplet on an ordered water monolayer adsorbed on a highly polar solid surface when q > 0.5 e. The solid–water interaction, density of water, hydrogen bonds, and water structures were analyzed. Remarkably, there was up to six times difference in the solid–water interactions despite the same value of the apparent contact angle values.


 Please wait while we load your content...
Please wait while we load your content...