Vibrational intensities in the mobile block Hessian approximation†
Abstract
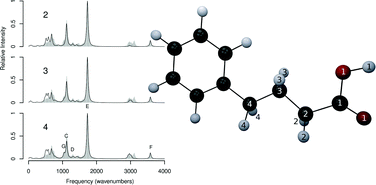
Herein we present a proof of concept for the recovery of vibrational intensities from density functional theory vibrational calculations performed in the Mobile Block Hessian (MBH) approximation, which constrains the internal degrees of freedom of designated subsets of a molecule. We compare and contrast the behaviour of this methodology with respect to conventional vibrational calculations, and characterise the performance and accuracy of our method with respect to the size of MBH constrained regions within a variety of species. We demonstrate the viability of this method as a means by which to obtain vibrational intensities for regions of interest within a molecule whilst potentially dramatically reducing computational expense with respect to conventional all-atom vibrational calculations, and discuss caveats for application.


 Please wait while we load your content...
Please wait while we load your content...