Connectivity pattern modifies excited state relaxation dynamics of fluorophore–photoswitch molecular dyads†
Abstract
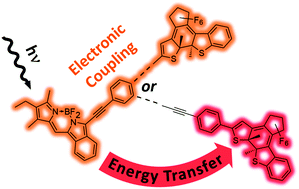
In order to modulate the emission of BODIPY fluorophores, they were connected to a diarylethene (DAE) photoswitch via phenylene–ethynylene linkers of different lengths and orientations. The latter allowed for modulation of the electronic coupling in the prepared four BODIPY–DAE dyads, which were compared also to appropriate BODIPY and DAE model compounds by steady state as well as time-resolved spectroscopies. In their open isomers, all dyads show comparable luminescence behavior indicative of an unperturbed BODIPY fluorophore. In strong contrast, in the closed isomers the BODIPY emission is efficiently quenched but the deactivation mechanism depends on the nature of the linker. The most promising dyad was rendered water-soluble by means of micellar encapsulation and aqueous suspensions were investigated by fluorescence spectroscopy and microscopy. Our results (i) illustrate that the electronic communication between the BODIPY and DAE units can indeed be fine-tuned by the nature of the linker to achieve fluorescence modulation while maintaining photoswitchability and (ii) highlight potential applications to image and control biological processes with high spatio-temporal resolution.


 Please wait while we load your content...
Please wait while we load your content...