Sheet-like and truncated-dodecahedron-like AgI structures via a surfactant-assisted protocol and their morphology-dependent photocatalytic performance†
Dan Xiao
ab,
Guangwei
Geng
ab,
Penglei
Chen
*abc,
Tiesheng
Li
b and
Minghua
Liu
ac
aBeijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, No. 2 Zhongguancun Beiyijie, Beijing 100190, People's Republic of China. E-mail: chenpl@iccas.ac.cn
bCollege of Chemistry and Molecular Engineering, Zhengzhou University, 100 Science Road, Henan, Zhengzhou 450001, People's Republic of China
cUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 28th November 2016
Abstract
Silver halide-based structures have been attracting great interest as efficient visible-light-driven photocatalysts towards the photodegradation of organic pollutants, and those studies focusing on their morphology-dependent catalytic performances have received particular attention. While great advancements in this regard have been witnessed in the past few years with respect to AgCl- and AgBr-based photocatalysts, relevant explorations concerning AgI-based species are relatively rare, even though the excellent durability of AgI-based structures renders them attractive candidates for potential photocatalytic uses. By means of chemical reactions between AgNO3 and tetramethylammonium iodide (TMAI), and AgNO3 and tetrabutylammonium iodide (TBAI), we herein report that AgI structures with a sheet-like and a truncated-dodecahedron-like morphology, respectively, could be controllably synthesized via a surfactant-assisted fabrication protocol. In our synthesis systems, AgNO3 works as the silver source, while the TMAI and TBAI surfactants serve not only as an iodine source but also as a directing reagent for controllable fabrication. It has been demonstrated that our AgI structures could work as visible-light-energized photocatalysts towards the photodegradation of methyl orange. We find that compared to their sheet-like counterparts, the truncated-dodecahedron-like AgI architectures exhibit substantially boosted catalytic performances. Moreover, we disclose that our truncated-dodecahedron-like AgI-based species could display excellent photocatalytic stability, wherein their catalytic reactivity displays only trivial fluctuations under visible-light irradiation even after the photoreactions have been repeated 22 times continuously. Our work might not only introduce a facile protocol for the controllable synthesis of AgI structures but also pave an avenue for facile enhancement of their catalytic performances via morphology alterations.
1 Introduction
Advanced materials of a well-defined and controlled shape have currently gained much attention from scientific communities.1–15 This is because of their fascinating shape-dependent properties and functions, which renders them of both fundamental and technological interest within a broad variety of fields of general concern, including but not limited to optoelectronics, sensors, energy harvesting, conversion and storage, etc.1–15 Amongst these significant areas, particular attention has been devoted to heterogeneous catalysts of a unique morphology, wherein lots of advanced catalysts with a superior catalytic performance have so far been successfully developed through investigation of their morphology-dependent catalytic reactivity.9–15 Practically, the shape-sensitive catalytic performance of a catalyst could, in principle, be elucidated with regard to the distinct catalytic reactivity aroused by a certain surface or crystal plane that is predominately enclosed within an anisotropic structure.9–15 By means of investigation of morphology-dependent catalytic performances, the underlying relationships between the surface atomic arrangement and the catalytic reactivity, which are among the most significant topics in the area of heterogeneous catalysis, could be revealed. This enables them to be ideal scientific test-beds for heterogeneous catalysis investigation, and will in turn provide researchers with important scientific guidance for the design of emerging catalysts with excellent performances.9–15Among various catalysts, the development of highly efficient photocatalysts for the photodegradation of organic pollutants, especially those capable of being driven by visible-light energy, has become an issue of paramount significance.16–20 On the one hand, this is owing to the fact that, as industrialization and globalization proceed, the overwhelming environmental problems aroused by organic pollutants have caused an appeal for more varied and alternative ways of establishing high-performance photocatalysts for efficient elimination of organic pollutants.16–20 On the other hand, compared to photocatalysts driven by UV-light (which only accounts for ∼5% of solar energy), those driven by visible-light (which accounts for ∼45% of solar energy) could make more efficient use of solar energy. Thus, in view of light harvesting and utilization, visible-light drivable photocatalysts are strongly desired.16–20 In the frontier field of visible-light-energized photocatalysis, silver halide-based (AgX, X = Cl, Br, or I) materials have recently attracted ever increasing interest as emerging photocatalytic species, although they are traditionally used as the primary source materials in photographic film and are unstable upon photoirradiation.21–25 Thus far, numerous efforts have been made to investigate the shape-dependent catalytic reactivity of AgCl- and AgBr-based species, wherein advanced catalysts with superior performances have been identified.25–36 Nevertheless, relevant explorations with regard to AgI-based species are relatively rare. This might be owing to the fact that the synthesis of AgI architectures with a tuned morphology is generally tedious work.37,38 As a matter of fact, one of the most distinguished advantages of AgI species over their AgCl and AgBr counterparts is their relatively high photo-stability, which is one of the most significant requirements for high-quality photocatalysts of potential use.39 Considering this issue, it is of significance to fabricate AgI-based structures of well-defined morphology and investigate their facet-dependent photocatalytic performances.
Motivated by the above-mentioned background, we herein demonstrate that by simply conducting a chemical reaction between AgNO3 and surfactants with an iodide counteranion, AgI architectures of controlled morphology can be facilely fabricated under ambient conditions. It was found that when tetramethylammonium iodide (TMAI) is employed, sheet-like AgI structures could be produced, while truncated-dodecahedron-like AgI structures can be manufactured when tetrabutylammonium iodide (TBAI) is used. In our surfactant-assisted fabrication protocol, AgNO3 serves as a silver source, while TMAI and TBAI surfactants not only play the role of an iodine source but also that of a directing reagent for controllable fabrication. We show that the as-fabricated AgI-based architectures could work as photocatalysts for the degradation of methyl orange (MO) pollutants under visible light irradiation, and the truncated-dodecahedron-like AgI structures display substantially enhanced photocatalytic performances compared to their sheet-like counterparts. In addition, it has been shown that the catalytic performance of our truncated-dodecahedron-like AgI species exhibits merely slight fluctuations even after the photoreaction was conducted 22 times consecutively. Such an excellent photo-stability implies a bright future and potential uses.
This might be the first report so far concerning the shape-dependent photocatalytic behaviour of AgI-based species. We suggest that the significance of our work might be two-fold. First, our surfactant-assisted fabrication protocol might pave an avenue for easy synthesis of AgI structures with a controllable shape, wherein significantly boosted catalytic performances could be facilely realized via investigation of the morphology-sensitive catalytic behaviour. Second, but not least, taking into account the rich diversity of surfactants with a halide counteranion, our method might be applicable not only to AgI-based structures but also to AgCl- and AgBr-based species with a controlled shape by means of using surfactants with different structures. Apparently, these benefits might provide researchers with new opportunities to uncover shape-sensitive photocatalytic performances, which is among the core subjects in the field of catalysis.
2 Experimental section
2.1 Chemicals and reagents
Silver nitrate (AgNO3, Alfa Aesar, >99%), tetramethylammonium iodide (TMAI, TCI), tetrabutylammonium iodide (TBAI, TCI, A.R. >99.0%), methyl orange (MO, Alfa Aesar, >98%), Nafion (Aldrich, 10 wt%, dispersion in water) and Na2SO4 (AR, Beijing Chemical Works) were used as received without further purification or treatment. Ultrapure Milli-Q water (18 MΩ cm) was used whenever water is mentioned, in all cases.2.2 Controllable synthesis of AgI structures of tuned morphology
To produce the sheet-like AgI structures, a 500 μL aqueous solution of AgNO3 (5 × 10−2 mol L−1) was added dropwise into a 10 mL aqueous solution of TMAI (0.1 mol L−1) within ca. 5 minutes at room temperature under vigorous magnetic stirring, soon after which a yellowish coloured dispersion was obtained. The stirring was continuously maintained for another 20 minutes under ambient conditions. Subsequently, the as-obtained dispersion was subjected to high speed centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 minutes), and the obtained solids were adequately washed and collected using ultrapure Milli-Q water by repeating the centrifugation and resuspension 3 times. To obtain the truncated-dodecahedron-like AgI structures, similar operations were carried out, except that a TBAI surfactant (5 × 10−3 mol L−1) was employed. The as-synthesized structures were subsequently subjected to various characterization methods and experiments.
000 rpm, 5 minutes), and the obtained solids were adequately washed and collected using ultrapure Milli-Q water by repeating the centrifugation and resuspension 3 times. To obtain the truncated-dodecahedron-like AgI structures, similar operations were carried out, except that a TBAI surfactant (5 × 10−3 mol L−1) was employed. The as-synthesized structures were subsequently subjected to various characterization methods and experiments.
2.3 Photocatalytic performance
To conduct the photocatalytic experiments, 20 mg of the as-prepared AgI structures was dispersed in a 6 mL aqueous solution of MO molecules (15 mg L−1), and a quartz cuvette was used as the photoreactor. A 500 W xenon arc lamp installed in a laboratory lamp housing system (CHF-XM35-500 W, Beijing Trusttech Co. Ltd, China) was used as the light source. The light passed through a 10 cm water filter and a UV cutoff filter (>400 nm) before entering the photoreactor. The reaction dispersion was kept for 30 minutes in a dark room to achieve an equilibrium adsorption state before visible-light illumination. The dark adsorption time was designed to be 30 minutes, since it was found that even when a longer adsorption time of 24 hours was applied, similar results were obtained. During the photocatalytic process, an aliquot of the reaction dispersion (0.3 mL) was taken out from the system for real-time sampling. The photodegradation of MO over the catalysts was monitored by means of measuring the real-time UV-vis absorption of the MO dye at ca. 464 nm. For evaluation of the photocatalytic reactivity, C was the concentration of substrate molecules at a real-time t, and C0 was the concentration of the molecules immediately before the dispersion was kept in dark room. The rate constant of the reaction, in terms of the correlation between ln(C/C0) and the reaction time (t), was deduced through kinetic linear simulation of the photocatalytic performance curves.2.4 Fabrication of the electrodes and electrochemical measurements
For the investigation using electrochemical impedance spectroscopy (EIS), indium tin oxide (ITO) glass electrodes were modified with our AgI species. To achieve this, ca. 5 mg of the AgI structures was dispersed in a Nafion water solution, and the obtained paste was cast onto the ITO electrode surface (1 cm × 1 cm). The electrochemical impedance investigations were carried out with a conventional three-electrode cell using an electrochemical station (CHI660e, CH Instruments, USA) at room temperature. In these measurements, a saturated calomel (saturated KCl) electrode (SCE) was used as the reference electrode, platinum wire was employed as the counter electrode, and our AgI structure-modified ITO electrode was used as the working electrode. An aqueous solution of Na2SO4 (0.1 M) was used as the electrolyte. The impedance spectra were recorded with the help of ZPlot/ZView software under an ac perturbation signal of 5 mV over a frequency range from 10 kHz to 0.1 Hz at a potential of 0.1 V. The photocurrent experiments were also performed using the three-electrode system. In this case, the working electrode was intermittently irradiated with visible-light during the measurements, and the photocurrent–time characteristics were recorded with an electrochemical analyzer.2.5 Apparatus and measurements
Scanning electron microscopy (SEM) measurements were conducted with a Hitachi S-4800 system, for which an accelerating voltage of 10 kV was employed. Energy dispersive X-ray spectroscopy (EDX) was conducted with a Horiba EMAX X-act energy dispersive spectroscopy system that was attached to a Hitachi S-4800, and an accelerating voltage of 15 kV was applied. The X-ray diffraction (XRD) measurements were performed using a PANalytical X'Pert PRO instrument with Cu Kα radiation. The X-ray photoelectron spectroscopy (XPS) was carried out with a VG Scientific Model ESCALab220i-XL electron spectrometer using 300 W Al Kα radiation. The binding energies were referenced to the C1s line at 284.8 eV from adventitious carbon. The UV-visible diffuse reflectance spectroscopy (DRS) spectra of our AgI architectures were obtained using an UV-vis spectrophotometer (SHIMADZU UV-2600), wherein BaSO4 was used as the reference. The performance in the photocatalytic reaction was estimated by measuring the real-time UV-vis absorptions of the MO molecules at a desired time, which were measured using a JASCO UV-3900 spectrophotometer. The specific surface areas of our AgI-based structures were measured by means of nitrogen gas adsorption at −196 °C using a TriStar II 3020 (Micromeritics, USA) after the samples were degassed under vacuum at 120 °C overnight, and the specific surface areas were estimated using a Brunauer–Emmett–Teller (BET) method. The total organic carbon (TOC) of our photoreaction systems was measured using an Analytik Jena Multi N/C 2100 TOC analyzer.3 Results and discussion
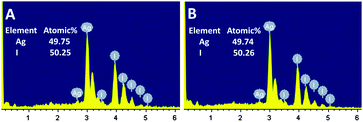
Experimentally, our AgI nanostructures were formulated by means of a surfactant-assisted fabrication protocol. To achieve this, an aqueous solution of AgNO3 was added dropwise into an aqueous solution of TMAI or TBAI surfactant at room temperature under vigorous magnetic stirring. Typically, it was found that an opaque dispersion with a light yellow colour was produced soon after the addition of AgNO3. After the stirring had proceeded for 20 minutes, the dispersion was subjected to centrifugation, and the precipitates were washed with ultrapure Milli-Q water 3 times by means of repeating the centrifugation and resuspension. As illustrated in Fig. 1A, when an aqueous solution of TMAI was used as the host solution, sheet-like nanostructures with a lateral dimension of ca. 100–300 nm and a thickness of ca. 50–80 nm were obtained. In contrast, when TBAI was employed instead, truncated-dodecahedron-like (Fig. 1B) architectures with a size of ca. 400–550 nm were produced. These results indicate that the morphology of our products could be handily tuned using TMAI and TBAI surfactants.The components in our products were examined by means of energy-dispersive X-ray spectroscopy (EDX) analysis. As shown in Fig. 2A, in the case of the sheet-like structures, silver (Ag) and iodide (I) elements could be detected distinctly. A semiquantitative analysis indicated that the atomic ratio between the Ag and I elements was approximately 49.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.25. This value is nearly the same as the theoretical stoichiometric atomic ratio between the Ag and I elements of AgI, which should be 50
50.25. This value is nearly the same as the theoretical stoichiometric atomic ratio between the Ag and I elements of AgI, which should be 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. In the case of the truncated-dodecahedron-like structures, similar results were obtained, as shown in Fig. 2B. These results basically suggest that the as-prepared nanostructures are AgI species. To confirm this, we carried out X-ray photoelectron spectroscopy (XPS) investigations. As presented in Fig. 3A, it can be seen that two bands at ca. 374.2 and 368.2 eV, which were attributed to Ag 3d3/2 and Ag 3d5/2 binding energies of the Ag+ species of AgI, respectively,39–42 could be observed in the XPS spectra of the sheet-like structures. In addition, two bands at ca. 630.8 and 619.4 eV, which were attributed to I 3d3/2 and I 3d5/2 binding energies of the I− species of AgI, respectively, could also be observed,39–41 as shown in Fig. 3B. On the basis of these XPS results, the molar ratio between the Ag+ and I− species could be semiquantitatively evaluated to be ca. 1
50. In the case of the truncated-dodecahedron-like structures, similar results were obtained, as shown in Fig. 2B. These results basically suggest that the as-prepared nanostructures are AgI species. To confirm this, we carried out X-ray photoelectron spectroscopy (XPS) investigations. As presented in Fig. 3A, it can be seen that two bands at ca. 374.2 and 368.2 eV, which were attributed to Ag 3d3/2 and Ag 3d5/2 binding energies of the Ag+ species of AgI, respectively,39–42 could be observed in the XPS spectra of the sheet-like structures. In addition, two bands at ca. 630.8 and 619.4 eV, which were attributed to I 3d3/2 and I 3d5/2 binding energies of the I− species of AgI, respectively, could also be observed,39–41 as shown in Fig. 3B. On the basis of these XPS results, the molar ratio between the Ag+ and I− species could be semiquantitatively evaluated to be ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As shown in Fig. 3C and D, similar results were obtained from the XPS spectra of the truncated-dodecahedron-like structures. Together with the results of the EDX investigations (Fig. 2), these results further validate that the synthesized structures are AgI species.
1. As shown in Fig. 3C and D, similar results were obtained from the XPS spectra of the truncated-dodecahedron-like structures. Together with the results of the EDX investigations (Fig. 2), these results further validate that the synthesized structures are AgI species.
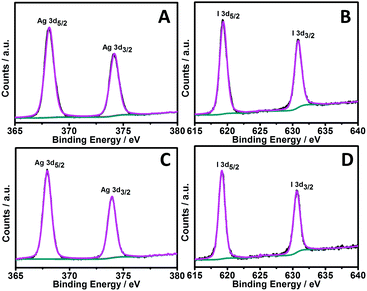 | ||
| Fig. 3 Typical XPS spectra for Ag 3d (A and C) and I 3d (B and D) of the as-fabricated sheet-like (A and B) and truncated-dodecahedron-like (C and D) AgI structures. | ||
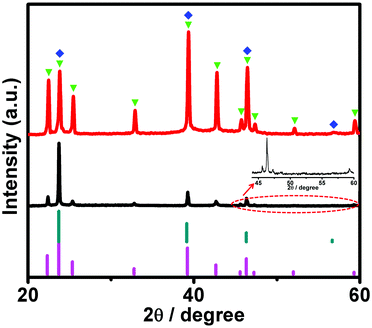
The powder X-ray diffraction (PXRD) patterns of our products were also examined to further support the above proposals. In Fig. 4 (black curve), it can be seen that the PXRD spectra of the sheet-like architectures exhibit evident diffractions peaks (2θ) at 22.4°, 25.4°, 32.8°, 42.7°, 45.7°, 47.3°, 52.1°, and 59.4°, which could be well indexed to the characteristic {100}, {101}, {102}, {103}, {200}, {201}, {202}, and {203} planes of a typical hexagonal phase (β-AgI, JCPDS File Card No. 09-0374), respectively.40,41 At the same time, three diffraction peaks at 23.8°, 39.3°, and 46.4°, which could either be ascribed to the {002}, {110}, and {112} facets of β-AgI, or the {111}, {220}, and {311} facets of γ-AgI (JCPDS File Card No. 09-0399),39,43 respectively, could also be observed. We note that it is hard to assign this set of peaks unambiguously to γ-AgI or β-AgI species. This is owing to the fact that they have very close diffraction positions.39,44 Regardless of this issue, a peak at 56.8°, which could be well indexed to the {400} facet of γ-AgI, could be also observed. In the case of the PXRD spectra of the truncated-dodecahedron-like structures, similar results were observed, as illustrated in Fig. 4 (red curve). These results suggest the coexistence of γ-AgI and β-AgI phases in our AgI architectures. Similar results have been reported previously, wherein it has been suggested that AgI species are commonly a mixture of γ-AgI and β-AgI phases.44,45
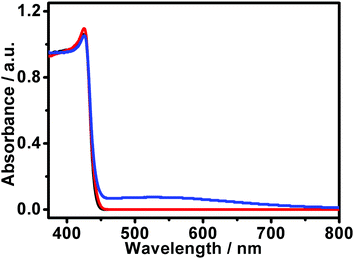
For visible-light-energized photocatalysts, it is essential that they can exhibit satisfactory absorptions in the visible region so as to attain efficient light harvesting and a high catalytic performance. Fig. 5 shows typical UV-visible diffuse reflectance spectra of the AgI structures. It can be seen that both the samples display evident absorptions in the UV region with their absorption edge extended to ca. 454 nm, which is in the visible region. These results imply that the AgI structures might be catalytically active under visible-light illumination.
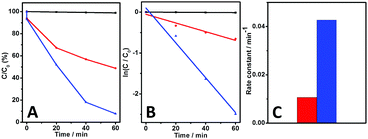
The photocatalytic performances of our AgI structures were evaluated by investigating the photodegradation of MO pollutants under visible-light irradiation. The photoreaction process was tracked by measuring the real-time UV-vis absorption of the MO molecules at 464 nm. Practically, prior to the visible-light irradiation, an adsorption process in the dark was conducted to reach an equilibrium adsorption state. As shown in Fig. 6A (black curve) and Fig. S1 (ESI†), merely a negligible photodegradation of the MO molecules could be observed under visible-light irradiation when there was no catalyst in the photoreaction system. This result indicates that self-photo-sensitized decomposition of the MO molecules could be basically neglected under our experimental conditions. On the other hand, when the sheet-like AgI nanostructures were used as the photocatalyst, ca. 48.8% of the MO molecules were eliminated within 60 minutes under the similar experimental conditions, as presented in Fig. 6A (red curve) and Fig. S2 (ESI†). This indicates that our AgI structures could serve as visible-light-driven photocatalysts for the photodegradation of MO pollutants. Interestingly, it was found that nearly 100% of the MO molecules were photodecomposed when the truncated-dodecahedron-like AgI nanostructures were employed as the photocatalyst (Fig. 6A, blue curve, Fig. S3, ESI†).
As plotted in Fig. 6B, a good linear correlation between ln(C/C0) and the reaction time (t) could be observed. This indicates that the decomposition of MO molecules photocatalyzed by our AgI-based structures follows first-order kinetics, −dC/dt = kC.46 Here, C represents the real-time concentration of the MO molecules, t stands for the reaction time, and k is the rate constant for the photoreaction. The results show that the rate constant of the catalytic reaction using the sheet-like AgI structures was estimated to be 0.011 min−1 (Fig. 6B, red curve), while with the truncated-dodecahedron-like structures it increased to ca. 0.043 min−1 (Fig. 6B, blue curve). These facts suggest that compared to the sheet-like AgI architectures, our truncated-dodecahedron-like structures could display an enhanced catalytic performance towards the photoelimination of MO molecules (Fig. 6C). In addition to the above investigations, the TOC values of our photoreaction systems were also measured. The results show that the initial TOC value of the original MO solution was ca. 8.71 mg L−1. After the photoreactions, the TOC value decreased to 4.59 and 1.93 mg L−1 when our sheet-like and truncated-dodecahedron-like AgI nanostructures were employed as the photocatalyst, respectively. These results indicate mineralization of the MO molecules. On the other hand, it can be seen that the TOC value of the photoreaction system containing our sheet-like AgI structures decreases by ca. 47.3% upon being irradiated with visible light for 60 minutes, while that containing our truncated-dodecahedron-like AgI architectures decreases by ca. 77.8% under similar experimental conditions. This trend is in agreement with that estimated from the UV-vis spectra of the photoreactions systems shown in Fig. 6, Fig. S2 and S3 (ESI†), further validating that the truncated-dodecahedron-like structures are superior photocatalysts compared to their sheet-like counterparts.
It is well known that morphology-sensitive catalytic performances can generally be understood in terms of the different catalytic reactivities induced by certain crystal facets, preferentially bound by anisotropic structures.9–15 As validated in the above-sections, using the PXRD patterns (Fig. 4), both of our AgI architectures are a mixture of β-AgI and γ-AgI. The diffraction peaks at ca. 39.3° and 23.8° could be indexed to the {110} and {002} facets of β-AgI, respectively. At the same time, these two peaks could also be assigned to the {220} and {111} planes of γ-AgI, respectively. On the one hand, when these peaks are indexed to β-AgI (JCPDS File Card No. 09-0374), the intensity ratio between the {110} and {002} facets, estimated using the {002} facet as the reference (namely, 100), is ca. 85. On the other hand, when they are ascribed to γ-AgI (JCPDS File Card No. 09-0399), the intensity ratio between the {220} and {111} planes, evaluated using the {111} plane as the reference, is ca. 60. From the PXRD pattern of the truncated-dodecahedron-like structures (Fig. 4, red curve), it can be seen that the corresponding ratio between these two peaks is ca. 161. This value is substantially larger than 85 and 60, which were estimated based on the JCPDS files for β-AgI and γ-AgI, respectively. These results indicate that our truncated-dodecahedron-like AgI structures are relatively enriched with {110} facets of either β-AgI or γ-AgI. On the basis of a similar comparison method, it can be clearly seen from Fig. 4 (black curve) that our sheet-like AgI structures are enriched with either {001} facets of β-AgI or {111} facets of γ-AgI.
Generally, compared to low-index facets, high-index crystal planes have a higher density of catalytically active sites, such as steps, ledges, kinks, corners, and edges, which endow crystal facets of a high-index with a much higher catalytic activity.9,46,47 In the case of photocatalysts, these active sites are suggested to promote a more effective separation of photoinduced electron–hole pairs, which is one of the most critical requirements for high-performance photocatalysts.48 On the basis of these basic explanations, it could be suggested that the superior catalytic performance of our truncated-dodecahedron-like structures compared to their sheet-like counterparts (Fig. 6) might be due to the fact that the truncated-dodecahedron-like AgI structures are relatively enriched with {110} facets of either β-AgI or γ-AgI, which are relatively high-index crystal facets and thus could promote a more efficient photoinduced electron–hole separation.39 On the other hand, the inferior catalytic reactivity of the sheet-like architecture suggests that they might be relatively enriched with low-index {001} facets of β-AgI, while the content of high-index {111} facets of γ-AgI might be relatively low, however we could not evaluate the respective content of β-AgI and γ-AgI at this stage.
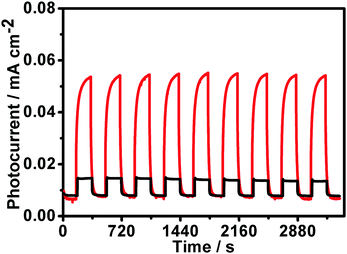
To verify that compared to the their sheet-like counterparts, the truncated-dodecahedron-like structures have a more efficient photoinduced electron–hole separation capability, the transient photocurrent responses of our AgI structures during intermittent visible-light irradiation were examined in terms of photocurrent–time response (I–t) curves. As shown in Fig. 7, the ITO electrodes modified with both of our structures exhibited distinct increases in the photocurrent when the light was switched on, while the photocurrents decreased to their initial values when the light irradiation was switched off. The as-observed reversible photocurrent responses could be obtained reproducibly over a couple of light switched-on/off cycles. These observations imply separation and transportation of the photoinduced electron–hole pairs in our AgI architectures upon light irradiation. Specifically, for the ITO electrode modified with the sheet-like AgI structures, relatively small photocurrent responses with a weak current intensity could be detected (Fig. 7, black curve). In contrast, in the case of the ITO electrode modified with the truncated-dodecahedron-like AgI species, much more distinct photocurrent responses with an evidently boosted photocurrent intensity could be observed (Fig. 7, red curve). These results suggest that compared with the case of the sheet-like structures, there exists more efficient charge separation during the photocatalytic process with our truncated-dodecahedron-like AgI structures. This is in good accordance with the photocatalytic results that our truncated-dodecahedron-like AgI architecture could exhibit a substantially enhanced catalytic performance compared to the sheet-like structures (Fig. 6).
In order to further confirm these results, the electrochemical impedance spectroscopy (EIS) spectra of the AgI architectures were examined in terms of their Nyquist plots. As illustrated in Fig. 8, it can be seen that the size of the arc radius for the electrode modified with the truncated-dodecahedron-like AgI species is evidently smaller than that of the electrode modified with the sheet-like species. This result indicates a decrease in the solid state interface layer resistance and the charge transfer resistance on the surface of the truncated-dodecahedron-like structures.31,49–51 Together with the above-mentioned results of PXRD (Fig. 4), the photocatalytic performance (Fig. 6), and the transient photocurrent responses (Fig. 7), this result further verifies that compared to the sheet-like AgI structures, more efficient photogenerated electron–hole separation occurs in the truncated-dodecahedron-like structures, which is facilitated by their enriched {110} facets, which confer them with superior catalytic reactivity.
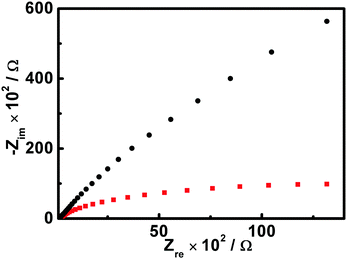 | ||
| Fig. 8 Typical EIS spectra of the ITO electrodes modified with the sheet-like AgI species (black curve) and the truncated-dodecahedron-like AgI (red curve) structures. | ||
In addition, the specific surface areas of our AgI structures were also measured using a BET method. The results show that the BET surface area for the sheet-like AgI structures was ca. 4.03 m2 g−1, while that of the truncated-dodecahedron-like AgI structures was ca. 2.71 m2 g−1, which is evidently smaller than the former case. As demonstrated in the above sections, compared to the sheet-like AgI structures, the truncated-dodecahedron-like counterparts display a substantially enhanced photocatalytic performance (Fig. 6). Together with the results from XRD (Fig. 4), the transient photocurrent responses (Fig. 7), and the EIS (Fig. 8), these values indicate that the enhanced catalytic activity of the truncated-dodecahedron-like structures does not result from their relatively lower specific surface area but is due to the fact that they are comparatively enriched with {110} facets of either β-AgI or γ-AgI, which could promote a more efficient separation of photogenerated electrons and holes.39
Practically, in addition to a superior catalytic reactivity, a good recyclability of the catalyst is another significant criterion strongly required for catalytic species of potential use. The durability of our truncated-dodecahedron-like AgI structures was evaluated by conducting the MO photodecomposition reaction repeatedly dozens of times under visible-light irradiation. As shown in Fig. 9, it can be seen that the photocatalytic performance displays only slight fluctuations under visible-light irradiation, even after the reaction had been repeated 22 times continuously and consecutively. This indicates that the truncated-dodecahedron-like AgI structures could work as highly stable catalysts for the photobleaching of organic pollutants under visible light irradiation. As is well-known, AgX species are traditionally photo-susceptible materials, which could generally be decomposed into Ag0 upon light irradiation. Recently, it has been disclosed that the photo-generation of Ag0 on the AgX species before or during the photocatalytic process could lead to the formation of Ag/AgX hybrids.21–25 The existence of metallic Ag0 species could in turn endow the composite with so-called “self-stability”, rendering the Ag/AgX materials photo-stable materials to some extent.39,52 In such materials, the artificially or naturally generated plasmonic metallic Ag0 species plays a crucial role, in which the photogenerated electrons that originally would have been captured by Ag+ cations would now be caught by O2via the plasmonic metallic Ag0 formed at the initial stage of the light irradiation, resulting in the generation of photostable Ag/AgX composites.39,52
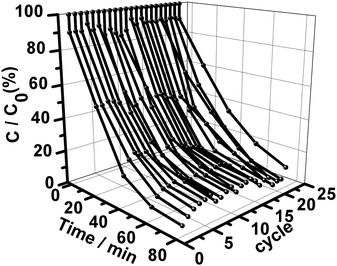 | ||
| Fig. 9 The photocatalytic behavior on consecutive cycling of the truncated-dodecahedron-like AgI structures for the photodegradation of MO molecules under visible-light irradiation. | ||
For our present case, the in situ generation of plasmonic metallic Ag0 species in the truncated-dodecahedron-like structures could be verified using UV-visible diffuse reflectance spectra obtained after the photocatalytic reactions. As shown in Fig. 5 (blue curve), it can be seen that compared to the corresponding curve obtained before the catalytic process, there appears to be a broad and strong absorption around 450–750 nm. This result indicates in situ generation of a metallic Ag0 species during the photocatalytic reaction, which could result in plasmonic absorptions in the visible region. At the same time, this suggests the formation of a Ag/AgI composite. To provide more evidence for this proposal, the samples obtained after the photocatalytic reactions were also investigated using EDX, as shown in Fig. S4 (ESI†). Herein, the semiquantitative analysis results show that the atomic ratio between the silver and iodide species was about 50.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.15. This value is higher than that of the sample evaluated before the photocatalytic process, which was very close to 50
49.15. This value is higher than that of the sample evaluated before the photocatalytic process, which was very close to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (Fig. 2). This experimental result is in agreement with the conclusion derived from the UV-visible diffuse reflectance spectral analysis (Fig. 5), further indicating that a metallic Ag0 species was generated in situ during the photocatalytic reaction.
50 (Fig. 2). This experimental result is in agreement with the conclusion derived from the UV-visible diffuse reflectance spectral analysis (Fig. 5), further indicating that a metallic Ag0 species was generated in situ during the photocatalytic reaction.
On the basis of EDX analysis, the molar ratio between the Ag0 and Ag+ species could be estimated to be approximately 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This could be further confirmed using XPS spectra, as shown in Fig. S5 (ESI†). It was found that compared to those obtained before the photocatalytic reactions, the XPS spectra of the truncated-dodecahedron-like structures obtained after the catalytic reactions display an almost similar pattern, except that two additional bands around 368.4 and 374.7 eV, which could be ascribed to Ag 3d5/2 and Ag 3d3/2 binding energies of the metallic Ag0 species,39,42,53 respectively, could also be observed following deconvolution of the silver-related bands. On the basis of the XPS results, the molar ratio between Ag0 and Ag+ was semiquantitatively estimated to be ∼0.03
1. This could be further confirmed using XPS spectra, as shown in Fig. S5 (ESI†). It was found that compared to those obtained before the photocatalytic reactions, the XPS spectra of the truncated-dodecahedron-like structures obtained after the catalytic reactions display an almost similar pattern, except that two additional bands around 368.4 and 374.7 eV, which could be ascribed to Ag 3d5/2 and Ag 3d3/2 binding energies of the metallic Ag0 species,39,42,53 respectively, could also be observed following deconvolution of the silver-related bands. On the basis of the XPS results, the molar ratio between Ag0 and Ag+ was semiquantitatively estimated to be ∼0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This value is similar to that obtained from the EDX analysis (Fig. S4, ESI†). Actually, we have also attempted to provide further evidence for the photoinduced generation of a metallic Ag0 species in our samples by means of obtaining PXRD spectra of the samples after the photocatalytic reactions. Unfortunately, we found that no distinct diffraction peaks attributed to metallic Ag0 species could be observed (figures not shown). This outcome is similar to results reported previously, wherein the reason might be ascribed to the limited amount of Ag0, the small particle size, and a high dispersion in the composite.39,52,54 Nevertheless, our experimental results from the UV-visible diffuse reflectance spectra (Fig. 5, blue curve), EDX analysis (Fig. S4, ESI†), and XPS spectra (Fig. S5, ESI†) strongly indicate in situ photoinduced generation of a metallic Ag0 species during the photocatalytic reactions, leading to the formation of a Ag/AgI species, and thus conferring our truncated-dodecahedron-like structures with an excellent recyclability. Additionally, a SEM image of the truncated-dodecahedron-like structures after the photocatalytic process was also obtained, as shown in Fig. S6 (ESI†). It can be seen that the morphology of the sample displays only slight changes after the catalytic reaction. This further indicates the good stability of our AgI-based photocatalysts.
1. This value is similar to that obtained from the EDX analysis (Fig. S4, ESI†). Actually, we have also attempted to provide further evidence for the photoinduced generation of a metallic Ag0 species in our samples by means of obtaining PXRD spectra of the samples after the photocatalytic reactions. Unfortunately, we found that no distinct diffraction peaks attributed to metallic Ag0 species could be observed (figures not shown). This outcome is similar to results reported previously, wherein the reason might be ascribed to the limited amount of Ag0, the small particle size, and a high dispersion in the composite.39,52,54 Nevertheless, our experimental results from the UV-visible diffuse reflectance spectra (Fig. 5, blue curve), EDX analysis (Fig. S4, ESI†), and XPS spectra (Fig. S5, ESI†) strongly indicate in situ photoinduced generation of a metallic Ag0 species during the photocatalytic reactions, leading to the formation of a Ag/AgI species, and thus conferring our truncated-dodecahedron-like structures with an excellent recyclability. Additionally, a SEM image of the truncated-dodecahedron-like structures after the photocatalytic process was also obtained, as shown in Fig. S6 (ESI†). It can be seen that the morphology of the sample displays only slight changes after the catalytic reaction. This further indicates the good stability of our AgI-based photocatalysts.
4 Conclusions
In summary, we herein report that sheet-like and truncated-dodecahedron-like AgI architectures can be facilely synthesized in a controllable manner via a surfactant-assisted fabrication protocol. In this method, surfactants with an iodide counteranion not only play the role of an iodine source but also serve as a directing reagent for controllable synthesis. It was found that the as-fabricated AgI structures could act as visible-light-driven photocatalysts towards the photobleaching of MO molecules, wherein compared to the sheet-like AgI architectures, the truncated-dodecahedron-like structures displayed an evidently boosted catalytic performance. Our experimental results suggest that these structures are enriched with different crystal facets. This endows them with distinct photoinduced electron–hole separation capabilities, and thus leads to their evidently different photocatalytic reactivities. Importantly, we show that besides the superior catalytic reactivity, our truncated-dodecahedron-like structures can also display an extremely high photocatalytic durability. This establishes an important foundation for their future use. Considering the abundance of diverse surfactants with a halide counteranion,55,56 our new method might be applicable not only to the controllable fabrication of AgI-based structures but also to AgCl- and AgBr-based architectures, from which suitable visible-light-energized photocatalysts might be developed via investigation of their shape-dependent catalytic reactivity.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (grants 21673253, 21372225, 21321063, and 91027042), the National Key Basic Research Project of China (grant 2013CB834504), and the Chinese Academy of Sciences (grants XDA09030200, XDB12020200 and 1731300500015).Notes and references
- J. Xiao and L. Qi, Nanoscale, 2011, 3, 1383–1396 RSC.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389–458 CrossRef CAS PubMed.
- S. Liu and Z. Tang, J. Mater. Chem., 2010, 20, 24–35 RSC.
- N. E. Motl, A. F. Smith, C. J. DeSantisa and S. E. Skrabalak, Chem. Soc. Rev., 2014, 43, 3823–3834 RSC.
- X. Liu and M. T. Swihart, Chem. Soc. Rev., 2014, 43, 3908–3920 RSC.
- P. C. Ray, Chem. Rev., 2010, 110, 5332–5365 CrossRef CAS PubMed.
- H. Liu, J. Xu, Y. Li and Y. Li, Acc. Chem. Res., 2010, 43, 1496–1508 CrossRef CAS PubMed.
- Q. H. Cui, Y. S. Zhao and J. Yao, Adv. Mater., 2014, 26, 6852–6870 CrossRef CAS PubMed.
- K. Zhou and Y. Li, Angew. Chem., Int. Ed., 2012, 51, 602–613 CrossRef CAS PubMed.
- G. Li and Z. Tang, Nanoscale, 2014, 6, 3995–4011 RSC.
- G. Liu, J. C. Yu, G. Q. Lu and H.-M. Cheng, Chem. Commun., 2011, 47, 6763–6783 RSC.
- Z.-Y. Zhou, N. Tian, J.-T. Li, I. Broadwell and S.-G. Sun, Chem. Soc. Rev., 2011, 40, 4167–4185 RSC.
- F. Zaera, ChemSusChem, 2013, 6, 1797–1820 CrossRef CAS PubMed.
- Y. Li and W. Shen, Chem. Soc. Rev., 2014, 43, 1543–1574 RSC.
- Q. Chen, Y. Jia, S. Xie and Z. Xie, Chem. Soc. Rev., 2016, 45, 3207–3220 RSC.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan and X. Yang, Chem. Soc. Rev., 2016, 45, 3701–3730 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- W. Wang, M. O. Tadé and Z. Shao, Chem. Soc. Rev., 2015, 44, 5371–5408 RSC.
- Z. Lou, Z. Wang, B. Huang and Y. Dai, ChemCatChem, 2014, 6, 2456–2476 CrossRef CAS.
- M. Zhu, P. Chen and M. Liu, Prog. Chem., 2013, 25, 209–220 CAS.
- S. Sarina, E. R. Waclawik and H. Zhu, Green Chem., 2013, 15, 1814–1833 RSC.
- P. Wang, B. Huang, Y. Dai and M.-H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813–9825 RSC.
- B. Tian and J. Zhang, Catal. Surv. Asia, 2012, 16, 210–230 CrossRef CAS.
- Y. Shen, P. Chen, D. Xiao, C. Chen, M. Zhu, T. Li, W. Ma and M. Liu, Langmuir, 2015, 31, 602–610 CrossRef CAS PubMed.
- Z. Zhou, X. Peng, L. Zhong, L. Wu, X. Cao and R. C. Sun, Carbohydr. Polym., 2016, 136, 322–328 CrossRef CAS PubMed.
- H. Wang, X. Lang, R. Hao, L. Guo, J. Li, L. Wang and X. Han, Nano Energy, 2016, 19, 8–16 CrossRef CAS.
- Z. Y. Lin, J. Xiao, J. H. Yan, P. Liu, L. H. Li and G. W. Yang, J. Mater. Chem. A, 2015, 3, 7649–7658 CAS.
- H. Zhang, Y. Lu, H. Liu and J. Fang, Nanoscale, 2015, 7, 11591–11601 RSC.
- Y. Wang, P. Chen, Y. Shen, C. Chen, C. Yang and M. Liu, Phys. Chem. Chem. Phys., 2015, 17, 25182–25190 RSC.
- H. Wang, X. Lang, J. Gao, W. Liu, D. Wu, Y. Wu, L. Guo and J. Li, Chem. – Eur. J., 2012, 18, 4620–4626 CrossRef CAS PubMed.
- H. Wang, Y. Liu, P. Hu, L. He, J. Li and L. Guo, ChemCatChem, 2013, 5, 1426–1430 CrossRef CAS.
- Q. Liang, Y. Shi, W. Ma, Z. Li and X. Yang, Appl. Catal., A, 2013, 455, 199–205 CrossRef CAS.
- H. Wang, J. Gao, T. Guo, R. Wang, L. Guo, Y. Liu and J. Li, Chem. Commun., 2012, 48, 275–277 RSC.
- H. Wang, J. Yang, X. Li, H. Zhang, J. Li and L. Guo, Small, 2012, 8, 2802–2806 CrossRef CAS PubMed.
- Q. Kuang, X. Zheng and S. Yang, Chem. – Eur. J., 2014, 20, 2637–2645 CrossRef CAS PubMed.
- W. Jiang, C. An, J. Liu, S. Wang, L. Zhao, W. Guo and J. Liu, Dalton Trans., 2014, 43, 300–305 RSC.
- B. Lei, M. Zhu, P. Chen, C. Chen, W. Ma, T. Li and M. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 4160–4169 CAS.
- S. Feng, H. Xu, L. Liu, Y. Song, H. Li, Y. Xu, J. Xia, S. Yin and J. Yan, Colloids Surf., A, 2012, 410, 23–30 CrossRef CAS.
- H. Xu, J. Yan, Y. Xu, Y. Song, H. Li, J. Xia, C. Huang and H. Wan, Appl. Catal., B, 2013, 129, 182–193 CrossRef CAS.
- M. Zhu, P. Chen and M. Liu, Langmuir, 2012, 28, 3385–3390 CrossRef CAS PubMed.
- J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Appl. Surf. Sci., 2011, 257, 7083–7089 CrossRef CAS.
- S. Ghosh, A. Saraswathi, S. S. Indi, S. L. Hoti and H. N. Vasan, Langmuir, 2012, 28, 8550–8561 CrossRef CAS PubMed.
- A. R. Abbasi and A. Morsali, Ultrason. Sonochem., 2010, 17, 572–578 CrossRef CAS PubMed.
- M. Zhu, P. Chen, W. Ma, B. Lei and M. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 6386–6392 CAS.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS PubMed.
- H. Kato, K. Asakura and A. Kudo, J. Am. Chem. Soc., 2003, 125, 3082–3089 CrossRef CAS PubMed.
- D. Xiao, T. Li, Y. Wang, P. Chen, G. Geng and M. Liu, RSC Adv., 2016, 6, 47062–47071 RSC.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2009, 4, 380–386 CrossRef PubMed.
- M. Adachi, M. Sakamoto, J. Jiu, Y. Ogata and S. Isoda, J. Phys. Chem. B, 2006, 110, 13872–13880 CrossRef CAS PubMed.
- H. Yu, L. Liu, X. Wang, P. Wang, J. Yu and Y. Wang, Dalton Trans., 2012, 41, 10405–10411 RSC.
- M. Zhu, P. Chen and M. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS PubMed.
- C. Hu, T. Peng, X. Hu, Y. Nie, X. Zhou, J. Qu and H. He, J. Am. Chem. Soc., 2010, 132, 857–862 CrossRef CAS PubMed.
- M. S. Kamal, J. Surfactants Deterg., 2016, 19, 223–236 CrossRef CAS.
- L. Zhu, Y. Tang and Y. Wang, J. Surfactants Deterg., 2016, 19, 237–247 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Typical real-time absorption spectra of MO molecules during the photodegradation process, and EDX analysis and XPS spectra of the truncated-dodecahedron-like AgI architectures after the photocatalytic process. See DOI: 10.1039/c6cp06948g |
| This journal is © the Owner Societies 2017 |