The structure and volume phase transition behavior of poly(N-vinylcaprolactam)-based hybrid microgels containing carbon nanodots†
Wenhui
Sun
and
Peiyi
Wu
*
The State Key Laboratory of Molecular Engineering of Polymers, Collaborative Innovation Center of Polymers and Polymer Composite Materials, Department of Macromolecular Science and Laboratory for Advanced Materials, Fudan University, Shanghai 200433, China. E-mail: peiyiwu@fudan.edu.cn
First published on 22nd November 2016
Abstract
In this paper, we investigated the internal structure and the volume phase transition (VPT) behavior of poly(N-vinylcaprolactam-co-vinylimidazole)/polymerizable carbon nanodot (P(VCL-co-VIM)/PCND) microgels with different amounts of PCNDs. Our study shows that compared to the pure P(VCL-co-VIM) microgel, the hybrid microgels undergo a two-step VPT as the temperature increases and a core–shell(–corona) structure of the hybrid microgels is formed by copolymerization with PCNDs. A change in the amount of PCNDs has effects on both of the volume phase transition temperature and internal structure of microgels. Moreover, based on FT-IR in combination with perturbation correlation moving window (PCMW) and two-dimensional correlation spectral (2Dcos) analyses, the difference in VPT behavior between the shell and the core (corona) structure of the hybrid microgels at the molecular level is elucidated. The core/shell of the hybrid microgels fixed with hydrophilic PCNDs has a higher transition temperature during heating and a more compact structure due to the additional crosslinkers PCNDs.
1. Introduction
Microgels or nanogels are cross-linked polymer networks that are colloidally dispersed in a solvent. Their unique abilities to swell or shrink in response to external stimuli like temperature, pH, and ionic strength have been applied in a multitude of fields, such as drug delivery,1,2 sensing,3,4 nanoreactors,5,6 and catalysis.7 Among them, the most studied thermosensitive microgel is based on poly(N-isopropylacrylamide) (PNIPAM) with a volume phase transition temperature (VPTT) around body temperature (32 °C).8–11 Compared to PNIPAM, poly(N-vinylcaprolactam) (PVCL) microgel is also attractive due to its outstanding biocompatibility,12–14 and the lower critical solution temperature (LCST) of PVCL can be easily tuned by changing the molecular weight and concentration.15 Upon increasing the temperature, PVCL chains transform gradually from hydrophilic to hydrophobic in aqueous solution, and the process is totally reversible upon cooling.16,17 There are a few reports on the internal structure of the PVCL-based microgels. For instance, Gerald J. Schneider et al. revealed that PVCL microgels have a heterogeneous internal structure with a high density core and a looser shell because of the reactivity ratio mismatch between cross-linker and monomer.18 A similar core–shell structure is also found in poly(N-vinylcaprolactam-co-glycidyl methacrylate) (PVCL/PGMA) microgel.19 Due to the large reactivity difference between VCL and GMA,20 microgels consisting of a reactive PGMA-rich core and a thermoresponsive PVCL-rich shell were finally formed. On the other hand, in view of the good biocompatibility of such microgels, introducing highly fluorescent materials can be very useful in biomedical applications such as biolabeling, diagnosis of infections and genetic diseases.19 Winnik et al.21 prepared PVCL-based core–shell structured microgels containing CdSe quantum dots (QDs) that are well known for their good photostability and high quantum yield. However, the non-covalent linkage between polymers and QDs often results in instability,22 and the intrinsic environmental risks from semiconductor QDs make their hybrid polymers unsuitable for biological and environmental applications.23 In addition, QDs may easily agglomerate in biological systems due to their highly dynamic surface architectures.24,25As an alternative, carbon nanodots (CNDs) are another important class of fluorescent nanomaterials which are very suitable for biomedical applications due to their low toxicity, good water dispersibility and photostability.26 Herein, we develop a facile route to prepare core–shell and core–shell–corona structured PVCL-based microgels by copolymerization with vinyl-functionalized CNDs (PCNDs). A series of PVCL/PCND hybrid microgels with different amounts of CNDs were synthesized. Due to the difference in reactivity between VCL and PCNDs, we obtained microgels with a heterogeneous structure, i.e. a dense PVCL/PCND hybrid core and a loose PVCL-rich shell.
Recently, transverse relaxation nuclear magnetic resonance measurements have been utilized to investigate the structure and composition of microgels.27–29 However, it is difficult for 1H NMR to trace the changes in other non-proton chemical groups, such as the ester group. In this case, we need to take advantage of FTIR measurements. In addition, hydrogen bonding is proved to be of great importance for the delicate balance between hydrophobic and hydrophilic interactions in microgels. The polymer–water hydrogen bonds are dominant below the VPTT, leading to the swelling of microgels. During heating above the VPTT, the polymer–water hydrogen bonds break gradually and hydrophobic interactions dominate resulting in the collapse of microgels. It is commonly recognized that FTIR is a suitable method for studying the behavior of the core and the shell during the volume phase transition as the characteristic peaks of the core and shell can be identified and separately studied in the IR spectra.30,31 Therefore, in this work, we additionally attempted to use spectral methods to distinguish the specific roles of the inner core and outer shell of the PVCL/PCND hybrid microgels during the volume phase transition process. Two advanced spectral analysis techniques based on FTIR, perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2Dcos), were also employed to detect the subtle information on microscopic variations of delicate interactions in the PVCL-based microgels during the VPT process, and with it, the relationship between the structure and the thermosensitive behavior of the core–shell PVCL-based microgels at the molecular level was elucidated.
2. Results and discussion
2.1 Synthesis of PVCL/PCND hybrid microgels
A series of thermoresponsive PVCL/PCND hybrid microgels were prepared via the emulsion polymerization of VCL and the co-monomer 1-vinylimidazole (VIM) using N,N′-methylenebis (acrylamide) (BIS) as the cross-linker, N-cetyl-N,N,N-trimethylammonium bromide (CTAB) as the surfactant and 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AIBA) as the initiator with different amounts of fluorescent PCNDs introduced into the reaction solution. PCNDs were synthesized by microwave assisted pyrolysis and subsequent surface vinylation with glycidyl methacrylate (TEM and 1H NMR characterizations of PCNDs in Fig. S1 and S2, ESI†).32 More details of the synthesis and characterizations of the PCNDs and hybrid microgels can be found in the ESI.† The strategy for the synthesis of PVCL-based microgels is depicted in Scheme 1, and samples PVCL-0, PVCL-10%, and PVCL-50% represent the amounts of PCNDs of 0, 10% and 50%, respectively. The FTIR spectra of the dried microgels indicate that the microgels contain a certain amount of VIM and various amounts of PCNDs (Fig. S3, ESI†). Note that the electrostatic repulsion between protonated 1-vinylimidazole and PCNDs with protonated amino groups can effectively diminish the possibility of the fixation of PCNDs in the hybrid microgels from entrapping them within the microgel network via the electrostatic interaction or physical adsorption. The C–H stretching vibration at 3107 cm−1 can be ascribed to the![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 end in the vinyl segments of the PVIM portion.33 Compared to the absorption band at 3110 cm−1 for free VIM, the observed shift to a lower wavenumber suggests the formation of a polymeric structure.33 In addition, the IR peak at 1715 cm−1 (–COOR) and the band at 1558 cm−1 (amide II) of PCNDs can be seen in the IR spectra of PVCL/PCND hybrid microgels indicating the successful fixation of the PCNDs into the microgels.32
CH2 end in the vinyl segments of the PVIM portion.33 Compared to the absorption band at 3110 cm−1 for free VIM, the observed shift to a lower wavenumber suggests the formation of a polymeric structure.33 In addition, the IR peak at 1715 cm−1 (–COOR) and the band at 1558 cm−1 (amide II) of PCNDs can be seen in the IR spectra of PVCL/PCND hybrid microgels indicating the successful fixation of the PCNDs into the microgels.32
 | ||
| Scheme 1 Schematic illustration of the preparation of P(VCL-co-VIM)/PCND microgels containing different amounts of PCNDs. | ||
Fig. 1 shows the TEM images of PVCL-based microgels with different amounts of PCNDs. The average diameters of microgels determined by TEM are ca. 168, 85, and 338 nm for PVCL-0, PVCL-10% and PVCL-50%, respectively. In the TEM images, PCNDs appear as dark black spots. The images show that PCNDs are distributed uniformly inside the core of the PVCL-10% microgel, while for PVCL-50% some aggregation exists. In addition, the PVCL-50% microgel consists of a PVCL-based shell, in which the PCNDs are distributed uniformly, and a PVCL-based gel corona with much less PCNDs. It suggests that the hybrid microgel has a core–shell(–corona) structure, due to the higher reactivity of PCNDs compared to VCL during polymerization (Fig. S4, ESI†), leading to the faster consumption of PCNDs than VCL. Note that the cross-linker BIS and PCND reacted faster than VCL, suggesting that BIS and PCND located mainly in the core of PVCL-10%.
2.2 Structure and thermoresponsivity of PVCL/PCND hybrid microgels
To investigate the thermoresponsivity of the PVCL-based microgels, dynamic light scattering (DLS) was employed to measure the temperature-dependent variation of the hydrodynamic diameter of microgels (1 mg mL−1). As shown in Fig. S5 (ESI†), all the three microgels have narrow size distributions. When the amounts of PCNDs added were less than 10 wt%, increasing contents of PCNDs in the microgels induced a decrease in the size of microgel particles due to the incorporation of PCNDs which act as additional cross-linkers while restricting the swelling of the microgel networks (Fig. S6, ESI†). For the PVCL-50% microgel, a larger size compared to the PVCL-10% microgel may be caused by the formation of an inner core from PCND self-polymerization. The tough PCND cores in PVCL-50% serve as the structural support for the microgels, surrounded by a PVCL-rich shell. When PCNDs are added in large quantities, at first they will take part in a self-polymerization reaction resulting from both the high concentration of PCNDs and higher reactivity of PCNDs compared to VCL. After self-polymerization of the PCNDs, the free radical copolymerization of the residual PCNDs and VCL forms a uniform shell structure, followed by the copolymerization of the rest of VCL with VIM to form a corona. The formation of a corona for the PVCL-50% microgel is speculated from the previous findings of Winnik et al.,21 who found that the VIM units mainly accumulate in a loosely crosslinked VCL-rich shell for the one-step synthesis of PVCL/acetoacetoxyethyl methacrylate (AAEM)/VIM microgels with an AAEM-rich core. Note that compared with the sizes determined by TEM, the hydrodynamic diameters measured by DLS are much larger, owing to a swollen state for the DLS measurement and a collapsed and dried state for the TEM observation.Fig. 2 shows the change in hydrodynamic diameters of microgels as a function of temperature. The VPTT of PVCL-0 is found to be 34 °C. A two-step VPT occurs at around 32 and 36 °C for PVCL-10% corresponding to the shell and core, respectively, as well as 32 and 38 °C for PVCL-50% corresponding to the corona and shell, respectively. Two different transition temperatures are observed for both the hybrid microgels suggesting the distinct structures between the inner core and shell from their outer layer. The higher VPTT of the hybrid microgels should come from the incorporation of hydrophilic PCNDs in the core of PVCL-10% or the shell of PVCL-50%.34,35 As shown in Fig. S7 (ESI†), the hydrodynamic diameters of PVCL-10% and PVCL-50% show good reversibility after 5 cycles of the heating–cooling process between 20 and 54 °C, suggesting their prospects for preparing thermosensitive sensors.
 | ||
| Fig. 2 Variation of the hydrodynamic diameters (Dh) of (a) PVCL-0, (b) PVCL-10%, and (c) PVCL-50% microgels. | ||
In addition, to explore the structure of the hybrid microgels, the fluorescence emission intensity (1 mg mL−1) was evaluated (Fig. S8, ESI†). The lower photoluminescence (PL) intensity of PVCL-50% compared to PVCL-10% may arise from the co-effect of two factors: first, the thicker corona of the PVCL-50% microgel would scatter more photons that come from the PCNDs inside the microgels; second, PCND-rich cores would lead to self-quenching. Moreover, the observed lower intensity of PVCL-50% was accompanied by a CND concentration-dependent PL red-shift due to the self-absorbing/excitation effect as the CND concentration increased.
These findings are in line with our observations that the PVCL-50% microgel consists of a PCND-rich core, a PVCL/PCND hybrid shell and a PVCL-rich corona, and the PVCL-10% microgel has a relatively large PVCL/PCND hybrid core surrounded by a thinner PVCL-rich shell.
2.3 Conventional IR analysis
For further study of the influence of the fixed PCNDs on the structures and VPTs of the hybrid microgels, the temperature-dependent FTIR spectra of PVCL-10% and PVCL-50% were recorded. It should be mentioned that we choose D2O instead of H2O as the solvent here for eliminating the overlap of the δ(OH) band of H2O around 1640 cm−1 with the amide I region of PVCL and the broad ν(OH) band of H2O around 3300 cm−1 with the C–H stretching region of PVCL. The temperature-dependent FTIR spectra of PVCL-0 were also collected for comparison, as presented in Fig. 3. In the following discussion, we will mainly focus on two regions: C–H stretching region (2990–2835 cm−1) and amide I region (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, 1645–1570 cm−1) which can be used to trace nearly all thermoresponsive group motions of the PVCL-based microgels. We can find that all the C–H stretching bands shift to lower wavenumbers as the temperature increases, while C
O stretching, 1645–1570 cm−1) which can be used to trace nearly all thermoresponsive group motions of the PVCL-based microgels. We can find that all the C–H stretching bands shift to lower wavenumbers as the temperature increases, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O exhibits a binary spectral intensity change in all the three microgels. The variations in C–H stretching bands can be interpreted as the hydrophobic hydration of C–H groups with neighboring water molecules. As reported, a higher number of water molecules surrounding C–H groups would lead to a higher vibrational frequency.36 In this case, we can conclude that all the C–H groups of the three microgels undergo dehydration upon heating. On the other hand, the amide I region consists of two parts: hydrogen bonded and free C
O exhibits a binary spectral intensity change in all the three microgels. The variations in C–H stretching bands can be interpreted as the hydrophobic hydration of C–H groups with neighboring water molecules. As reported, a higher number of water molecules surrounding C–H groups would lead to a higher vibrational frequency.36 In this case, we can conclude that all the C–H groups of the three microgels undergo dehydration upon heating. On the other hand, the amide I region consists of two parts: hydrogen bonded and free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, corresponding to the lower frequency and the higher one, respectively. Therefore, the variations of amide I groups upon heating can be explained by the transformation of hydrogen bonded carbonyls with water to free carbonyls. Based on this point, the three microgel systems have similar spectral change tendencies in the VPTs, and during heating water molecules are expelled outside leading to the collapse of microgel networks.
O, corresponding to the lower frequency and the higher one, respectively. Therefore, the variations of amide I groups upon heating can be explained by the transformation of hydrogen bonded carbonyls with water to free carbonyls. Based on this point, the three microgel systems have similar spectral change tendencies in the VPTs, and during heating water molecules are expelled outside leading to the collapse of microgel networks.
 | ||
| Fig. 3 Temperature-dependent FTIR spectra of the three P(VCL-co-VIM)/PCND microgels in D2O (10 wt%) during heating from 23 to 54 °C with an increment of 1 °C, respectively. | ||
To quantitatively evaluate the impact of temperature on the hydration status of different chemical groups during the VPTs of the three kinds of microgels, the temperature-dependent frequency shifts of νas(CH2) and the integral area changes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were plotted, as presented in Fig. 4. The wavenumbers of the C–H groups of the three microgels show a sharp shift around VPTTs and a gradual shift above VPTTs, a typical asymmetric sigmoid curve. The changes in amide I during the heating process result from the transformation of hydrogen bonded C
O were plotted, as presented in Fig. 4. The wavenumbers of the C–H groups of the three microgels show a sharp shift around VPTTs and a gradual shift above VPTTs, a typical asymmetric sigmoid curve. The changes in amide I during the heating process result from the transformation of hydrogen bonded C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with water to free C
O with water to free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, reflected by the emergence of characteristic bands for free C
O, reflected by the emergence of characteristic bands for free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in PVCL-based microgels after volume phase transition. For the hybrid microgels, the initial shrinkage causes a continuous increase in the number of free carbonyls before turning points at ∼33 °C, which is approximate equal to the VPTT of PVCL-0. This thermal transition behavior should correspond to the gradual dehydration of the C
O in PVCL-based microgels after volume phase transition. For the hybrid microgels, the initial shrinkage causes a continuous increase in the number of free carbonyls before turning points at ∼33 °C, which is approximate equal to the VPTT of PVCL-0. This thermal transition behavior should correspond to the gradual dehydration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the shell of the PVCL-10% microgel and the corona of the PVCL-50% microgel, since the outer layer of both the hybrid microgels has a structure similar to that of the PVCL-0 microgel. With further increase in the temperature, sharp changes occur at 35 and 38 °C for PVCL-10% and PVCL-50%, respectively, when more water molecules are expelled outside the microgels. The second transition points reveal that the hybrid microgels contain another form of the PVCL-based network architecture that should be distinct from their outer layer. It also fits our previous speculation that the core of the PVCL-10% microgels and the shell of the PVCL-50% microgels are fixed with hydrophilic PCNDs resulting in a higher transition temperature. It is noted that the transitions of the hydrated C–H groups are more continuous than those of the relative hydrophilic C
O groups in the shell of the PVCL-10% microgel and the corona of the PVCL-50% microgel, since the outer layer of both the hybrid microgels has a structure similar to that of the PVCL-0 microgel. With further increase in the temperature, sharp changes occur at 35 and 38 °C for PVCL-10% and PVCL-50%, respectively, when more water molecules are expelled outside the microgels. The second transition points reveal that the hybrid microgels contain another form of the PVCL-based network architecture that should be distinct from their outer layer. It also fits our previous speculation that the core of the PVCL-10% microgels and the shell of the PVCL-50% microgels are fixed with hydrophilic PCNDs resulting in a higher transition temperature. It is noted that the transitions of the hydrated C–H groups are more continuous than those of the relative hydrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in both the hybrid microgels. In other words, in the temperature range with the relatively flat curves of C
O groups in both the hybrid microgels. In other words, in the temperature range with the relatively flat curves of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, the C–H groups responds prior to C
O groups, the C–H groups responds prior to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups upon heating, indicating that the core in the PVCL-10% microgel and the shell in the PVCL-50% microgel would firstly undergo conformational changes and then start their dehydration process in solution. Note that the C–H groups of PVCL-10% undergo the highest degree of dehydration among the three microgels, while for the C
O groups upon heating, indicating that the core in the PVCL-10% microgel and the shell in the PVCL-50% microgel would firstly undergo conformational changes and then start their dehydration process in solution. Note that the C–H groups of PVCL-10% undergo the highest degree of dehydration among the three microgels, while for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, the degree of dehydration increases with the reduced concentration of PCNDs (Fig. S9, ESI†). The different effect of PCND concentration on the dehydration process of C
O groups, the degree of dehydration increases with the reduced concentration of PCNDs (Fig. S9, ESI†). The different effect of PCND concentration on the dehydration process of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–H groups may be attributed to the fact that C
O and C–H groups may be attributed to the fact that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups are more hydrophilic than C–H groups. The hybrid microgels with a higher concentration of PCNDs are more hydrophilic, leading to less dehydration of hydrophilic C
O groups are more hydrophilic than C–H groups. The hybrid microgels with a higher concentration of PCNDs are more hydrophilic, leading to less dehydration of hydrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The change in C–H groups will be further discussed by following the 2Dcos analysis.
O. The change in C–H groups will be further discussed by following the 2Dcos analysis.
2.4 Perturbation correlation moving window (PCMW)
As for the curves discussed above, it is hard to determine the transition temperatures accurately during the heating process. Therefore, a PCMW method was employed to explicitly show the transition temperatures and ranges in these three microgels. A detailed description of PCMW is presented in the ESI.†
Fig. 5 shows the PCMW synchronous and asynchronous spectra of PVCL-0 and PVCL-10% to investigate the VPTs upon heating from 23 to 54 °C, respectively. For PVCL-50%, the PCMW spectra are presented in Fig. S10 (ESI†). For clarity, we plotted all the transition points and regions read from PCMW maps in Fig. S11 (ESI†). As mentioned above, the PVCL-10% microgel possesses a relatively dehydrated core and a loosely cross-linked shell and PVCL-50% has a relatively dehydrated shell and a corona with a lower cross-linking density. Thus, the dehydrated C–H groups of these two microgels have an obviously earlier response than the hydrated ones upon heating indicating that C–H groups located in the relatively dehydrated core of PVCL-10% or the shell of PVCL-50% are more sensitive to the temperature rise. Besides, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the hybrid microgels have two VPTTs, and the higher one correspond to the core of PVCL-10% or the shell of PVCL-50% fixed with PCNDs. To further investigate the core structure of the PVCL-50% microgel, we also drew the PCMW synchronous map of the C
O groups of the hybrid microgels have two VPTTs, and the higher one correspond to the core of PVCL-10% or the shell of PVCL-50% fixed with PCNDs. To further investigate the core structure of the PVCL-50% microgel, we also drew the PCMW synchronous map of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O region of PCNDs fixed in it (Fig. S12, ESI†). Note that the PCNDs themselves have no thermal sensitivity, while they will undergo a dehydration process in response to the temperature rise under the influence of adjacent PVCL segments. It can be seen that the transition points with regard to the hydrated C
O region of PCNDs fixed in it (Fig. S12, ESI†). Note that the PCNDs themselves have no thermal sensitivity, while they will undergo a dehydration process in response to the temperature rise under the influence of adjacent PVCL segments. It can be seen that the transition points with regard to the hydrated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the PCNDs locate at 35 and 46 °C, which are in the transition regions of the hydrated and dehydrated states of the shell of PVCL-50%, respectively, indicating that the PCNDs undergo two dehydration processes during heating. In the first stage, the PCNDs that are fixed in the shell of PVCL-50% undergo dehydration (35 °C) prior to the shrinkage of the shell in the PVCL-50% microgel (38 °C). Such a behavior is reasonable if we consider that a reduction of the effective charge density of hydrolyzed amino groups in the PCNDs is a prerequisite for further shrinkage of the interior of the microgels.37 After the dehydration process of the shell finishes at about 47 °C upon heating, the network of the shell becomes compact and the innermost PCND-rich core begins to dehydrate (46 °C), driven by the squeezing effect to remove more water molecules. The present mechanism can be proved in the following 2Dcos analysis.
O groups of the PCNDs locate at 35 and 46 °C, which are in the transition regions of the hydrated and dehydrated states of the shell of PVCL-50%, respectively, indicating that the PCNDs undergo two dehydration processes during heating. In the first stage, the PCNDs that are fixed in the shell of PVCL-50% undergo dehydration (35 °C) prior to the shrinkage of the shell in the PVCL-50% microgel (38 °C). Such a behavior is reasonable if we consider that a reduction of the effective charge density of hydrolyzed amino groups in the PCNDs is a prerequisite for further shrinkage of the interior of the microgels.37 After the dehydration process of the shell finishes at about 47 °C upon heating, the network of the shell becomes compact and the innermost PCND-rich core begins to dehydrate (46 °C), driven by the squeezing effect to remove more water molecules. The present mechanism can be proved in the following 2Dcos analysis.
2.5 Two-dimensional correlation analysis (2Dcos)
Based on the phase transition evolving ranges determined by PCMW, 2Dcos analysis was carried out from 23 to 54 °C for PVCL-0 and the two hybrid microgels during heating, respectively. 2Dcos consists of the synchronous spectra which exhibit simultaneous changes between two given wavenumbers and the asynchronous spectra which can achieve an enhanced spectral resolution. The judging rule can be extracted from Noda's rule.38 In brief, when the cross-peaks (ν1, ν2, and assume ν1 > ν2) in synchronous and asynchronous spectra have the same symbol, it indicates the variation at ν1 prior to that of ν2, and vice versa. A detailed description of 2Dcos is presented in the ESI.† In order to discuss more conveniently, all the bands detected from the asynchronous spectra of the PVCL-0 microgel and the relevant assignments are shown in Table S1 (ESI†). Derived from the 2Dcos spectra, the sequence order for the different groups of PVCL-0 can be deduced upon heating as follows (→means prior to): 1622 → 2877 → 2968 → 2870 → 2952 → 1608 → 1589 → 1632 → 2852 → 2924 cm−1 (Fig. S13, ESI†). Since the hydrophobic C–H groups respond earlier than the relative hydrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, it reveals that the conformational change of the C–H groups occurs before the dehydration of the C
O groups, it reveals that the conformational change of the C–H groups occurs before the dehydration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups during heating. For the vibrational modes of C–H groups, the symmetric stretching vibration changes prior to the asymmetric stretching vibration upon heating. The direction of the asymmetric stretching vibration is parallel to the chain axis, while that of the symmetric stretching vibration is vertical with respect to the chain axis as previously reported.39 Therefore, it is deduced that the side chains collapse prior to main chains during the conformational changing process. Such sequence order that is opposite to that of the PVCL-4VP microgels as we reported previously14 may be attributed to the effect of the conformational change of the PVIM segments in the side chains during the VPT. As discussed above, the VIM units tend to accumulate in the loosely crosslinked VCL-rich outer layer of the PVCL-based microgels. Therefore, we can conclude that with increasing temperature above the LCST, the VIM units in the shell turn toward the outside of the shrinking microgels due to its hydrophilic nature, and the adjacent PVCL segments are forced to adjust their hydrophilic and hydrophobic groups in the perpendicular direction by the rearrangement of the VIM units.
O groups during heating. For the vibrational modes of C–H groups, the symmetric stretching vibration changes prior to the asymmetric stretching vibration upon heating. The direction of the asymmetric stretching vibration is parallel to the chain axis, while that of the symmetric stretching vibration is vertical with respect to the chain axis as previously reported.39 Therefore, it is deduced that the side chains collapse prior to main chains during the conformational changing process. Such sequence order that is opposite to that of the PVCL-4VP microgels as we reported previously14 may be attributed to the effect of the conformational change of the PVIM segments in the side chains during the VPT. As discussed above, the VIM units tend to accumulate in the loosely crosslinked VCL-rich outer layer of the PVCL-based microgels. Therefore, we can conclude that with increasing temperature above the LCST, the VIM units in the shell turn toward the outside of the shrinking microgels due to its hydrophilic nature, and the adjacent PVCL segments are forced to adjust their hydrophilic and hydrophobic groups in the perpendicular direction by the rearrangement of the VIM units.
As for the hybrid microgels, the 2Dcos spectra of the PVCL-10% and PVCL-50% microgels during heating from 23 to 54 °C are shown in Fig. 6 and Fig. S14 (ESI†), respectively. In the C–H region of PVCL-10%, two distinct kinds of dehydrated C–H bands appear at 2926 and 2916 cm−1, respectively. The one at 2926 cm−1 is in accordance with PVCL-0, and the appearance of the lower one indicates that there exists another structure in PVCL-10% which is less hydrated than that in PVCL-0. As mentioned before, the C–H groups in the core of PVCL-10% are less dehydrated than the shell, thus we can attribute the new band at 2916 cm−1 to the C–H groups that are close to PCNDs in the core. Similarly, the C–H bands at 2960, 2943 and 2914 cm−1 detected in the asynchronous spectra of the PVCL-50% microgel can be assigned to less hydrated C–H groups neighboring PCNDs in the shell. For clarity, Table 1 and Table S2 (ESI†) list all the bands detected in the 2Dcos spectra and their tentative assignments, respectively. We can deduce the specific order for the heating process of PVCL-10% in D2O: 2916 → 1608 → 1618 → 1587 → 2950 → 2868 → 1632 → 2926 → 2858 cm−1. Regardless of the differences in stretching modes, we have C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → C–H, indicating the phase transition is driven by the hydrogen bonding transformation of amide groups. It is interesting to note that the band at 2916 cm−1 which is attributed to the dehydrated C–H groups that are close to PCNDs shows an earlier response than all the bands during the VPT. This is because the fixed PCNDs with hydrophilicity will “attract” most water molecules in the core, and endow the inner core of PVCL-10% with a more compact network. As a result, the hydrophobic C–H groups in the core of PVCL-10% locate in a relatively hydrophobic environment and undergo a more efficient dehydration than those in the core of PVCL-0 during the VPTT, in line with the above conventional IR analysis. In addition, the order of the vibration modes for C–H groups in the PVCL-10% microgel is the reverse of that in the PVCL-0 microgel, and the backbones of the PVCL-10% microgel change prior to the side chains during heating. This may be attributed to the fact that the PCND-crosslinkers with an earlier dehydration process induce the changes in neighboring groups in the main chains prior to the ones in the side chains. In all, the VPT process of the PVCL-10% microgel begins from the relatively dehydrated core, and then the C
O → C–H, indicating the phase transition is driven by the hydrogen bonding transformation of amide groups. It is interesting to note that the band at 2916 cm−1 which is attributed to the dehydrated C–H groups that are close to PCNDs shows an earlier response than all the bands during the VPT. This is because the fixed PCNDs with hydrophilicity will “attract” most water molecules in the core, and endow the inner core of PVCL-10% with a more compact network. As a result, the hydrophobic C–H groups in the core of PVCL-10% locate in a relatively hydrophobic environment and undergo a more efficient dehydration than those in the core of PVCL-0 during the VPTT, in line with the above conventional IR analysis. In addition, the order of the vibration modes for C–H groups in the PVCL-10% microgel is the reverse of that in the PVCL-0 microgel, and the backbones of the PVCL-10% microgel change prior to the side chains during heating. This may be attributed to the fact that the PCND-crosslinkers with an earlier dehydration process induce the changes in neighboring groups in the main chains prior to the ones in the side chains. In all, the VPT process of the PVCL-10% microgel begins from the relatively dehydrated core, and then the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–H groups associated with water molecules in the shell start to dehydrate. When the temperature increases above the VPTT of the core, which is higher than normal owing to the hydrophilic property of PCNDs, more water molecules are gradually squeezed out from the compact core.
O and C–H groups associated with water molecules in the shell start to dehydrate. When the temperature increases above the VPTT of the core, which is higher than normal owing to the hydrophilic property of PCNDs, more water molecules are gradually squeezed out from the compact core.
| Wavenumber (cm−1) | Tentative assignments |
|---|---|
| 2950 | ν as(hydrated CH2) |
| 2926 | ν as(dehydrated CH2) |
| 2916 | ν as(dehydrated CH2, close to PCNDs) |
| 2868 | ν s(hydrated CH2) |
| 2858 | ν s(dehydrated CH2) |
| 1632 |
ν(free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| 1618 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–O–D⋯O O⋯D–O–D⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 1608 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–O–D) O⋯D–O–D) |
| 1587 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯(D–O–D)2) O⋯(D–O–D)2) |
For the PVCL-50% microgel, the final sequence for the groups during heating is: 1606 → 1589 → 2968 → 2870 → 2960 → 2914 → 2950 → 2935 → 2852 → 2943 → 1618 → 1635 cm−1. One can see that the C–H groups close to PCNDs show a later response than the other C–H groups during the VPT. It could be explained by the higher VPTT of the shell where the C–H groups close to PCNDs are mainly distributed. From the overall sequence order, we can find that when the temperature reaches the phase transition temperature of the corona with a structure similar to that of PVCL-0, the hydrogen bonds formed between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of PVCL and water will break, and then the main chains of PVCL start to collapse. Subsequently, with further increase in the temperature the less hydrated shell with a higher transition temperature begins to shrink and expels out water molecules. Combined with the PCMW analysis of PCNDs, water is forced to squeeze out of the hydrophilic PCND-core following the VPT process of the corona and shell finishing with more compact networks. For clarity, the VPTs of PVCL-0 and the two hybrid microgels during heating are illustrated in Scheme 2. PVCL-0 exhibits a typically thermal-sensitive behavior, which has a relatively sharp transition around the VPTT, while for the hybrid microgels, a two-step VPT upon heating can be observed, and the core of PVCL-10% and the shell of PVCL-50% with higher VPTTs dehydrate following the VPT process of their layer. Moreover, the core of PVCL-50% is forced to dehydrate after the dehydration of the shell.
O groups of PVCL and water will break, and then the main chains of PVCL start to collapse. Subsequently, with further increase in the temperature the less hydrated shell with a higher transition temperature begins to shrink and expels out water molecules. Combined with the PCMW analysis of PCNDs, water is forced to squeeze out of the hydrophilic PCND-core following the VPT process of the corona and shell finishing with more compact networks. For clarity, the VPTs of PVCL-0 and the two hybrid microgels during heating are illustrated in Scheme 2. PVCL-0 exhibits a typically thermal-sensitive behavior, which has a relatively sharp transition around the VPTT, while for the hybrid microgels, a two-step VPT upon heating can be observed, and the core of PVCL-10% and the shell of PVCL-50% with higher VPTTs dehydrate following the VPT process of their layer. Moreover, the core of PVCL-50% is forced to dehydrate after the dehydration of the shell.
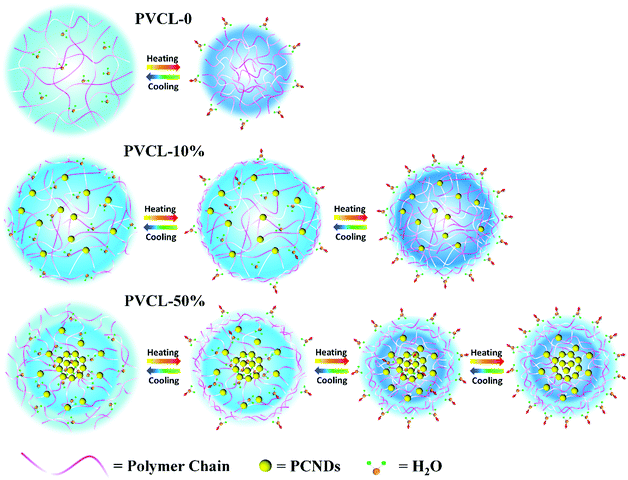 | ||
| Scheme 2 Schematic illustration and comparison of the VPTs of the PVCL-0, PVCL-10% and PVCL-50% hybrid microgels in water. The light blue color represents the distribution density of water molecules. | ||
3. Conclusions
In this work, we studied the internal structures and thermally induced volume phase transition behaviors of three PVCL-based hybrid microgels with different amounts of PCNDs (named PVCL-0, PVCL-10% and PVCL-50%, respectively). Our results indicate that core–shell(–corona) structures of the hybrid microgels with distinct VPTs can be prepared by varying the concentration of PCNDs. The core–shell(–corona) architecture arises from the difference in reactivity between VCL/VIM and PCNDs. The DLS curves of PVCL-0 shows one transition point, while the hybrid microgels undergo a two-step thermal transition during heating. This phenomenon can also be observed by the PCMW spectra and the temperature dependent integral area of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in conventional IR analysis, respectively. From this we can infer that the inner core or shell of the hybrid microgels has distinct structures and components from their outer layer and the higher transition points of the hybrid microgels come from the incorporation of the hydrophilic PCNDs in the core of PVCL-10% or the shell of PVCL-50%. The FLS measurements support the formation of the core of the PVCL-50% microgel by self-polymerization of the PCNDs. Moreover, in combination with the 2Dcos analysis, the C–H groups of PVCL-10% undergo the highest degree of dehydration among the three microgels, which can be attributed to the fact that the fixed PCNDs with hydrophilicity will “attract” most water molecules in the core and endow the inner core of PVCL-10% with a more compact network leading to a relatively hydrophobic environment for the hydrophobic C–H groups, thereby enabling them undergo more efficient dehydration than those of PVCL-0.
O groups in conventional IR analysis, respectively. From this we can infer that the inner core or shell of the hybrid microgels has distinct structures and components from their outer layer and the higher transition points of the hybrid microgels come from the incorporation of the hydrophilic PCNDs in the core of PVCL-10% or the shell of PVCL-50%. The FLS measurements support the formation of the core of the PVCL-50% microgel by self-polymerization of the PCNDs. Moreover, in combination with the 2Dcos analysis, the C–H groups of PVCL-10% undergo the highest degree of dehydration among the three microgels, which can be attributed to the fact that the fixed PCNDs with hydrophilicity will “attract” most water molecules in the core and endow the inner core of PVCL-10% with a more compact network leading to a relatively hydrophobic environment for the hydrophobic C–H groups, thereby enabling them undergo more efficient dehydration than those of PVCL-0.
Acknowledgements
We gratefully acknowledge the financial support from the National Science Foundation of China (NSFC) (No. 21274030 and 51473038).Notes and references
- C. M. Jewell, J.-M. Jung, P. U. Atukorale, R. P. Carney, F. Stellacci and D. J. Irvine, Angew. Chem., Int. Ed., 2011, 50, 12312–12315 CrossRef CAS PubMed.
- J. K. Oh, R. Drumright, D. J. Siegwart and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 448–477 CrossRef CAS.
- M. R. Islam, A. Ahiabu, X. Li and M. J. Serpe, Sensors, 2014, 14, 8984–8995 CrossRef CAS PubMed.
- D.-M. Han, Q. M. Zhang and M. J. Serpe, Nanoscale, 2015, 7, 2784–2789 RSC.
- Y. Lu, S. Proch, M. Schrinner, M. Drechsler, R. Kempe and M. Ballauff, J. Mater. Chem., 2009, 19, 3955–3961 RSC.
- H. Jia, D. Schmitz, A. Ott, A. Pich and Y. Lu, J. Mater. Chem. A, 2015, 3, 6187–6195 CAS.
- Y. Tang, T. Wu, B. Hu, Q. Yang, L. Liu, B. Yu, Y. Ding and S. Ye, Mater. Chem. Phys., 2015, 149, 460–466 CrossRef.
- S. Maccarrone, A. Ghavami, O. Holderer, C. Scherzinger, P. Lindner, W. Richtering, D. Richter and R. G. Winkler, Macromolecules, 2016, 49, 3608–3618 CrossRef CAS.
- K. C. Clarke, S. N. Dunham and L. A. Lyon, Chem. Mater., 2015, 27, 1391–1396 CrossRef CAS.
- G. Liu, D. Wang, F. Zhou and W. Liu, Small, 2015, 11, 2807–2816 CrossRef CAS PubMed.
- E. Daly and B. R. Saunders, Phys. Chem. Chem. Phys., 2000, 2, 3187–3193 RSC.
- A. C. W. Lau and C. Wu, Macromolecules, 1999, 32, 581–584 CrossRef CAS.
- P. C. Naha, A. Casey, T. Tenuta, I. Lynch, K. A. Dawson, H. J. Byrne and M. Davoren, Aquat. Toxicol., 2009, 92, 146–154 CrossRef CAS PubMed.
- L. Hou and P. Wu, RSC Adv., 2014, 4, 39231–39241 RSC.
- F. Meeussen, E. Nies, H. Berghmans, S. Verbrugghe, E. Goethals and F. Du Prez, Polymer, 2000, 41, 8597–8602 CrossRef CAS.
- H. Vihola, A. Laukkanen, L. Valtola, H. Tenhu and J. Hirvonen, Biomaterials, 2005, 26, 3055–3064 CrossRef CAS PubMed.
- Y. B. Gao, S. C. F. Au-Yeung and C. Wu, Macromolecules, 1999, 32, 3674–3677 CrossRef CAS.
- F. Schneider, A. Balaceanu, A. Feoktystov, V. Pipich, Y. Wu, J. Allgaier, W. Pyckhout-Hintzen, A. Pich and G. J. Schneider, Langmuir, 2014, 30, 15317–15326 CrossRef CAS PubMed.
- N. Haentzschel, F. Zhang, F. Eckert, A. Pich and M. A. Winnik, Langmuir, 2007, 23, 10793–10800 CrossRef CAS PubMed.
- X. P. Qiu and S. A. Sukhishvili, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 183–191 CrossRef CAS.
- L. Shen, A. Pich, D. Fava, M. F. Wang, S. Kumar, C. Wu, G. D. Scholes and M. A. Winnik, J. Mater. Chem., 2008, 18, 763–770 RSC.
- N. Tomczak, D. Janczewski, M. Han and G. J. Vancso, Prog. Polym. Sci., 2009, 34, 393–430 CrossRef CAS.
- D. Janczewski, N. Tomczak, M.-Y. Han and G. J. Vancso, Nat. Protoc., 2011, 6, 1546–1553 CrossRef CAS PubMed.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- J. P. Ryman-Rasmussen, J. E. Riviere and N. A. Monteiro-Riviere, Nano Lett., 2007, 7, 1344–1348 CrossRef CAS PubMed.
- A. D. Zhao, Z. W. Chen, C. Q. Zhao, N. Gao, J. S. Ren and X. G. Qu, Carbon, 2015, 85, 309–327 CrossRef CAS.
- A. Balaceanu, D. E. Demco, M. Moller and A. Pich, Macromol. Chem. Phys., 2011, 212, 2467–2477 CrossRef CAS.
- S. Schachschal, A. Balaceanu, C. Melian, D. E. Demco, T. Eckert, W. Richtering and A. Pich, Macromolecules, 2010, 43, 4331–4339 CrossRef CAS.
- A. Balaceanu, D. E. Demco, M. Moller and A. Pich, Macromolecules, 2011, 44, 2161–2169 CrossRef CAS.
- L. Hou, K. Ma, Z. An and P. Wu, Macromolecules, 2014, 47, 1144–1154 CrossRef CAS.
- M. Keerl, V. Smirnovas, R. Winter and W. Richtering, Angew. Chem., Int. Ed., 2008, 47, 338–341 CrossRef CAS PubMed.
- P. Zhang, W. C. Li, X. Y. Zhai, C. J. Liu, L. M. Dai and W. G. Liu, Chem. Commun., 2012, 48, 10431–10433 RSC.
- M. Talu, E. U. Derniroglu, S. Yurdakul and S. Badoglu, Spectrochim. Acta, Part A, 2015, 134, 267–275 CrossRef CAS PubMed.
- X. Zhou, Y. Zhou, J. Nie, Z. Ji, J. Xu, X. Zhang and B. Du, ACS Appl. Mater. Interfaces, 2014, 6, 4498–4513 CAS.
- Z. Cao, B. Du, T. Chen, J. Nie, J. Xu and Z. Fan, Langmuir, 2008, 24, 12771–12778 CrossRef CAS PubMed.
- H. Cheng, L. Shen and C. Wu, Macromolecules, 2006, 39, 2325–2329 CrossRef CAS.
- K. Kratz, T. Hellweg and W. Eimer, Colloids Surf., A, 2000, 170, 137–149 CrossRef CAS.
- S. Morita, H. Shinzawa, I. Noda and Y. Ozaki, Appl. Spectrosc., 2006, 60, 398–406 CrossRef CAS PubMed.
- S. Sun and P. Wu, Macromolecules, 2013, 46, 236–246 CrossRef CAS.
- S. T. Sun and P. Y. Wu, J. Phys. Chem. B, 2011, 115, 11609–11618 CrossRef CAS PubMed.
- Y. Maeda, T. Nakamura and I. Ikeda, Macromolecules, 2002, 35, 217–222 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images and 1H NMR spectra of PCNDs, fluorescence emission spectra of hybrid microgels, DLS curves of the hybrid microgels, IR spectra of PCNDs and hybrid microgels, experimental section and detailed description of PCMW and 2Dcos. See DOI: 10.1039/c6cp06862f |
| This journal is © the Owner Societies 2017 |




