Reorganized, weak C–H⋯O interactions directly modify the mechanical properties and compaction performance of a series of nitrobenzoic acids†
Abstract
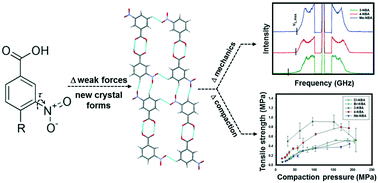
Strong hydrogen-bonding interactions are predictably leveraged for local molecular docking; however, weak intermolecular interactions are being increasingly recognized as pivotal governors of extended, supramolecular assembly. In this report, a series of nitrobenzoic acids (NBA) – m-nitrobenzoic acid (3-NBA), p-nitrobenzoic acid (4-NBA), 4-methyl-3-NBA (Me-NBA), 4-chloro-3-NBA (Cl-NBA) and 4-bromo-3-NBA (Br-NBA) – are utilized to systematically adjust weak C–H⋯O (nitro) and C–H⋯O (carboxyl) bonding interactions and discriminate their influence on the crystal structure, aggregate elasticity and compaction performance. The expected carboxylic acid dimer persists across the entire NBA series; however, alternative organization of C–H⋯O interactions yields distinct 1-d tapes. The acoustic frequency distributions obtained from powder Brillouin light scattering (BLS) spectra provide supportive interpretation of the strength and anisotropy of the reorganized intermolecular interactions. Aggregate Young's moduli displayed a narrow range from 13.5 GPa for Br-NBA to 9.8 GPa for 3-NBA. However, the maximum longitudinal moduli (Mmax), calculated from the high-frequency cutoff, revealed significant differences in the strength of intermolecular interactions along the 1-d tapes. For 3-NBA, the extended organization of several co-linear C–H⋯O (nitro) interactions yielded an Mmax of 42.0 GPa, while the remaining NBA materials displayed an Mmax near 25 GPa. From powder compaction studies, the tabletability rank order was observed as: 3-NBA > 4-NBA > Me-NBA ≈ Br-NBA ≈ Cl-NBA; however, none of the materials achieved a tensile strength greater than 1 MPa. Overall, even minor redistribution of weak intermolecular interactions significantly modifies supramolecular assembly in NBA and detailing the specific contribution of these weak forces is required for substantive structure–performance correlation.


 Please wait while we load your content...
Please wait while we load your content...