Polymorphism, pseudo-polymorphism, and conformerism in the crystal structure of piperazine-N,N′-bis(N,O-diphenyl phosphoramidate)†
Abstract
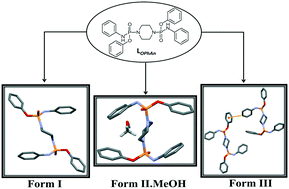
Herein, we report on the synthesis of a novel bisphosphoramidate compound (LOPhAn), (C6H5O)(C6H5NH)P(O)NC4H8N(O)P(OC6H5)(NHC6H5), with two polymorphic (I and III) and one methanol solvated pseudo-polymorphic (II.MeOH) structures in the solid state obtained by crystal growth from different solvents. Single crystal X-ray analysis revealed that Form (I) has one extended conformer of LOPhAn in the solid state, while Form (II) demonstrated one twisted conformer as well as the coexistence of solvent molecules (methanol) in the lattice. Form (III) shows conformerism in the solid state, and two different molecular conformers are found in this structure: one extended and one slightly twisted that has a different conformation from the others. The crystal structures of these polymorphs were investigated using geometrical analysis, the non-covalent interaction (NCI) approach, hydrogen bonding topological graphs and Hirshfeld surface analysis. The geometrical orientations and intramolecular and intermolecular interactions of these three forms were compared with structurally related crystal structures within CSD. The stability of isolated conformers in the gas phase and the lattice energy of structures were studied theoretically. Furthermore, the thermodynamic relationships of the polymorphs are examined through calorimetric analysis.


 Please wait while we load your content...
Please wait while we load your content...