Facile synthesis of CoNix nanoparticles embedded in nitrogen–carbon frameworks for highly efficient electrocatalytic oxygen evolution†
Jiawei
Wan
,
Wenxing
Chen
,
Chen
Chen
 ,
Qing
Peng
,
Dingsheng
Wang
,
Qing
Peng
,
Dingsheng
Wang
 * and
Yadong
Li
* and
Yadong
Li
Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: wangdingsheng@mail.tsinghua.edu.cn
First published on 17th October 2017
Abstract
We report a pyrolysis reduction method to prepare highly uniform dispersed CoNix nanoparticles embedded in nitrogen–carbon frameworks. With an appropriate value of Ni/Co, the obtained CoNi0.37–CN catalyst exhibited excellent properties and stability in electrocatalytic oxygen evolution, which are superior to those of the commercial IrO2.
Electrocatalytic oxygen evolution reaction (OER) is an important half reaction in water splitting,1–3 which is believed to be one of the most important sources of green energy. Commercial catalysts for the OER reaction are usually based on noble metals or noble metal oxides,4–6 which are effective, but expensive. Hence, it is important and necessary to design catalysts with high catalytic activity and low cost for the OER reaction. Most of these catalysts are metal oxides and hydroxides, which usually contain the elements Fe, Co, and Ni as the active sites.7–15 Notably, some of these metals have been constructed with others to design a highly efficient catalyst for water oxidations. Cobalt–nickel bimetallic catalysts have exhibited higher catalytic activity than cobalt only catalysts in the OER reaction due to the incorporation of the nickel metal.12,16,17 However, most of them are metal oxides and hydroxides which have relatively low electronic conductivity and suffer from low stability during the long term electrochemical reaction, extremely limiting their practical applications in electrochemical devices.
Porous carbon materials have exhibited good properties in many fields for energy applications,18–22 such as energy storage and conversion devices, gas storages and catalytic reactions, owing to their large surface areas, suitable size distribution of pores, and good stability. Porous carbon materials can be easily designed with plenty of methods, including chemical activation, physical carbonization and so on. What's more, they can also be used as good supports to fabricate porous nanomaterials with excellent properties and good stability in energy applications.
Here, we report a facile method to prepare highly uniform dispersed CoNix nanoparticles embedded in nitrogen–carbon frameworks (CoNix–CN) with a highly porous graphitic shell, which could not only support a conductive porous carbon layer, but also maintain the stability of CoNix nanoparticles during the long term electrochemical reaction. Furthermore, we investigate the impact of compositions of binary catalysts on their electrocatalytic OER performance.
The synthetic process of CoNix–CN includes two steps. Firstly, a mixture solution was prepared by full mixing of Co(NO3)2, Ni(NO3)2, 2-methylimidazole (2MI) and sodium dodecyl sulfate (SDS) (please see details in the ESI†). After an hour reaction, the product was collected with centrifugation and washed with deionized water and ethanol. Then, the obtained centrifugal product was coated by a thin shell of phenol-formaldehyde resin (PF), which is the source of the graphitic shell. After centrifugation, washing, and vacuum drying, the desired product was pyrolysis treated at 800 °C in a nitrogen atmosphere for 3 h and then the catalyst of CoNix–CN was successfully prepared.
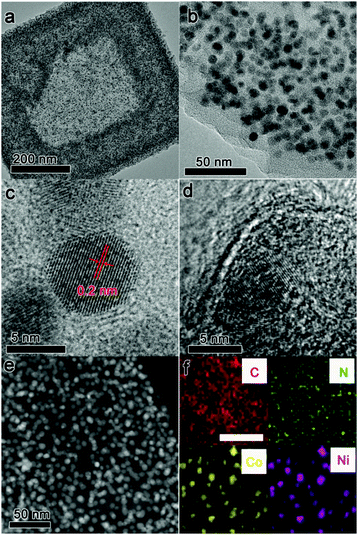
A transmission electron microscopy (TEM) image of CoNix–CN is displayed in Fig. 1a, which demonstrates the highly uniform dispersed nanoparticles embedded in a framework. With the large magnification of CoNix–CN in Fig. 1b, the uniform diameters of the nanoparticles can be clearly observed. From the high-resolution transmission electron microscopy (HRTEM) image of CoNix–CN (Fig. 1c), the lattice spacing of 0.2 nm can be observed. The porous graphitic shell can be noticed in the HRTEM image in Fig. 1d, which is derived from the PF shell. The dark field scanning transmission electron microscopy (DF-STEM) image (Fig. 1e) displays the uniform dispersed bright spots embedded in the framework, which represent the uniform dispersion of nanoparticles embedded in the framework. The overall SEM of CoNix–CN is displayed in Fig. S1 (ESI†), and the CN frameworks can be obviously recognized. The elemental mapping images in Fig. 1f suggest the uniform distributions of C and N over the entire framework, which can indicate the CN framework in this work. And signals of Co and Ni are only present in the bright spots of the structure, suggesting that CoNix nanoparticles are embedded in the CN framework.
To synthesize different compositions of CoNix–CN, various values of Ni/Co were adjusted to prepare CoNix–CN nanomaterials. Four different Ni/Co ratios were adjusted and the corresponding CoNix–CN nanomaterials are investigated in Fig. 2. From the statistical results in Fig. 2a–d, the diameters of the nanoparticles for the four samples are 5.07 ± 0.32, 5.13 ± 0.31, 5.09 ± 0.25, and 5.10 ± 0.23 nm, respectively. All samples have uniformly dispersed CoNix nanoparticles with almost the same size. The nitrogen-doped carbon frameworks could be formed from PF during the high temperature thermal treatment under a N2 atmosphere and these could reduce metal oxides to metals at high temperature, therefore maintaining the structure of CoNix–CN. On the other hand, the frameworks at the outside of the overall structure could limit the expansion of the metals and prevent CoNix nanoparticles from diffusing and aggregating. In contrast, the sample without the PF shell was produced using the same method as CoNix–CN, except for step two. The corresponding product is displayed in Fig. S2 (ESI†); the diameter distribution of the nanoparticles reaches a value of 43.23 nm, which is much larger than those of the CoNix–CN nanomaterials. And the CN frameworks become broken after pyrolysis treatment. In a word, the PF shell can not only maintain the structure of CoNix–CN, but also prevent CoNix nanoparticles from aggregating seriously during the high temperature pyrolysis treatment. Next, line scan measurement was performed to discuss the values of Ni/Co for the four samples and the ratio values change from 0.37, 0.81, and 1.42 to 1.86, indicating the samples of CoNi0.37–CN, CoNi0.81–CN, CoNi1.42–CN and CoNi1.86–CN, respectively. Moreover, the line scan results illustrate the uniform distributions of Co and Ni over the entire nanoparticles (Fig. 2e–h) without the partial nucleation of Co or Ni on the surface of CoNix.
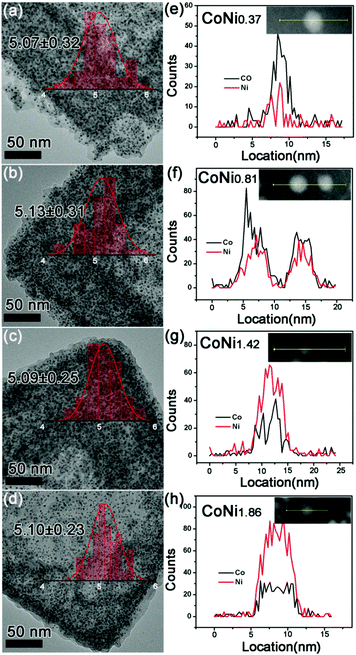 | ||
| Fig. 2 Diameter size distribution of (a) CoNi0.37–CN, (b) CoNi0.81–CN, (c) CoNi1.42–CN, and (d) CoNi1.86–CN; line scan of (e) CoNi0.37–CN, (f) CoNi0.81–CN, (g) CoNi1.42–CN, and (h) CoNi1.86–CN. | ||
Furthermore, the crystalline structure and surface chemical constitution of the CoNix–CN nanomaterials are investigated in Fig. 3. A seen from Fig. 3a, the derived catalysts present similar XRD patterns, which contain two parts of CoNix and graphite (JCPDS# 41-1487),23 indicating similar crystalline structures of the CoNix–CN nanomaterials. In addition to the broader peak of graphite at 2 theta of 26°, the diffraction peaks of the four samples are located between those of pure Co (JCPDS# 15-0806) and pure Ni (JCPDS# 04-0850), which belong to the face-centered cubic (fcc) phase. And there are no additional diffraction peaks corresponding to pure Co or Ni to be discerned, suggesting that the obtained products are Co–Ni alloys rather than a mixture of Co and Ni, which can also be supported by the line scan results in Fig. 2. In addition, the lattice spacing of 0.2 nm (Fig. 1c) closely matches the lattice spacing in the (111) plane of pure Co (0.2047 nm) and pure Ni (0.2041 nm) judging from standard pure Co (JCPDS# 15-0806) and pure Ni (JCPDS# 04-0850), which can support the view of the fcc phase for the CoNix nanoparticles. X-ray photon spectroscopy (XPS) spectra were acquired to investigate the chemical surface characteristics of the four samples. From the Co 2p and Ni 2p core level XPS spectra exhibited in Fig. 3b and c, respectively, it can be concluded that Co and Ni are almost in zero valences,24 indicating the alloy structure of the CoNix nanoparticles.
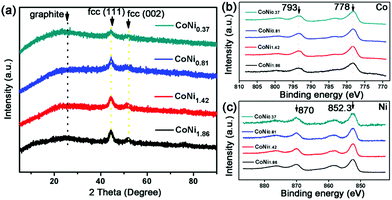 | ||
| Fig. 3 (a) XRD patterns of CoNix–CN, (b) Co 2p XPS spectra of CoNix–CN, and (c) Ni 2p XPS spectra of CoNix–CN. | ||
The porous structure of the CoNix–CN nanomaterials was investigated with Brunauer–Emmett–Teller (BET) measurement. The BET specific surface areas and pore sizes of the four CoNix–CN nanomaterials are displayed in Fig. 4. The adsorption–desorption isotherms of the four samples exhibit type IV curves and the surface areas are 425 m2 g−1 (CONi0.37), 413 m2 g−1 (CoNi0.81), 431 m2 g−1 (CoNi1.42) and 444 m2 g−1 (CoNi1.86), respectively, indicating the porous structure with large surface area. And the pore diameter sizes are 3.55 (CoNi0.37), 3.84 (CoNi0.81), 3.93 (CoNi1.42), and 3.69 nm (CoNi1.86), respectively, suggesting a mesoporous structure of the CN frameworks. Considering the larger diameter sizes of the CoNix alloys than that of the pore sizes of the CN frameworks, the alloys cannot go through the channels of the CN frameworks and will be well retained. In addition, the pore sizes of the framework are large enough to allow the ionic liquid to pass smoothly. Due to the pore structure of the CN framework, it can support good electrolyte transport channels and good protection of the CoNix nanoparticles in a long term electrochemical reaction.
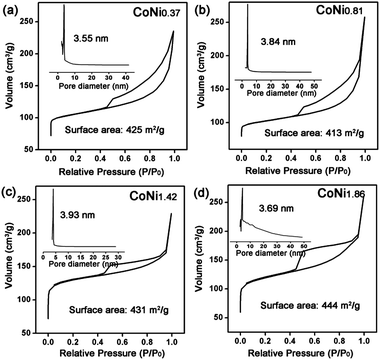 | ||
| Fig. 4 N2 adsorption/desorption isotherms, and pore size distributions of (a) CoNi0.37–CN, (b) CoNi0.81–CN, (c) CoNi1.42–CN and (d) CoNi1.86–CN. | ||
To discuss the impact of the Ni/Co value on the electrochemical performance of the CoNix–CN catalysts, electrocatalytic OER tests of the CoNix–CN nanomaterials and other contrast samples were carried out using a linear sweep voltammetry (LSV) method in 1.0 M KOH solution in a standard three-electrode system (please see details in the ESI†). From the LSV results in Fig. 5a, the anodic current density of CoNi0.37–CN exhibits a sharp onset of OER current at 1.48 V versus the reversible hydrogen electrode (RHE). When the thermodynamic OER potential (E0H2O/O2 = 1.229 V) is used as the reference, CoNi0.37–CN demonstrates the smallest overpotential of 0.25 V among all CoNix–CN samples, e.g., 0.3 V for CoNi0.81–CN, 0.34 V for CoNi1.42–CN, and 0.38 V for CoNi1.86–CN, indicating the best electrocatalytic performance for OER among them. Besides the onset potential, the operational potential at 10 mA cm−2 is another key parameter for OER performance evaluation. The CoNi0.37–CN possesses an overpotential of 0.32 V at 10 mA cm−2, smaller than that of CoNi0.81–CN (0.38 V), CoNi1.42–CN (0.41 V), and CoNi1.86–CN (0.46 V) (Fig. 5a), suggesting the best catalytic properties of CoNi0.37–CN of the four. Therefore, it is beneficial to improve the OER performance with an appropriate value of Ni/Co and the CoNi0.37–CN exhibits the best properties in the OER. In contrast, a pure sample of Co–CN without Ni metal was prepared and an OER test was performed. From the LSV results in Fig. 5a, the overpotential (V vs. RHE) of 0.36 V for Co–CN is smaller than 0.38 V for CoNi1.86–CN. However, it is larger than that of CoNi0.37–CN (0.25 V), CoNi0.81–CN (0.3 V), and CoNi1.42–CN (0.34 V). To discuss the facile charge transfer states of CoNix–CN samples, an electrochemical impedance spectroscopy (EIS) method was performed. Judged from Fig. S3 (ESI†), the charge transfer resistance of CoNi0.37–CN is the smallest of the four, leading to the best catalytic activity. In order to prove that the determinant of performance is from CoNix, and not the CN framework, pure CN frameworks were prepared. And the C1s and N1s XPS spectra of CoNix–CNs and CN were discussed to confirm the same CN structure of all samples in Fig. S4 (ESI†). The performance of pure CN can hardly be observed in the OER test, indicating the little influence of the CN framework on OER performance in our work. Finally, the commercial IrO2 was measured as the contrast sample using the same method as discussed above. An overpotential (V vs. RHE) of 0.28 V of IrO2 was obtained, which is larger than that of CoNi0.37–CN (0.25 V), suggesting the better properties of CoNi0.37–CN compared to commercial IrO2.
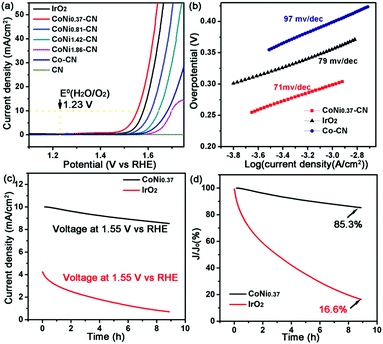 | ||
| Fig. 5 (a) LSV plots, (b) Tafel plots of CoNi0.37–CN, Co–CN, and IrO2, and (c and d) CA plots of CoNi0.37–CN and IrO2. | ||
The catalytic kinetics of CoNi0.37–CN is assessed by Tafel plots, which were recorded with the linear regions, and then subsequently fitted via the Tafel equation25(η = b × log j + a, where η is the overpotential, j is the current density, and b is the Tafel slope) (Fig. 5b). Obviously, the CoNi0.37–CN exhibits enhanced kinetics for the OER, which is approved by its lower Tafel slope of 71 mV dec−1, compared with commercial IrO2 (79 mV dec−1) and Co–CN (97 mV dec−1), as revealed in Fig. 5b.
Except for the activity, the high stability of catalysts toward the OER is also critical for energy conversion systems. As displayed in chronoamperometry (CA) plots in Fig. 5c and d, the current density of CoNi0.37–CN remains 85.3% of 10 mA cm−2 at 1.55 V (V vs. RHE) for 8 h, while the commercial IrO2 only remains 16.6% of 4.23 mA cm−2 at 1.55 V (V vs. RHE) for 8 h, demonstrating the excellent durability of CoNi0.37–CN in alkaline solution. And the surface chemical states of CoNi0.37–CN before and after the CA test are discussed in Fig. S5 (ESI†). An electrochemical CV stability test was also performed to support this conclusion (Fig. S6, ESI†).
In summary, a facile synthesis route is designed to fabricate amorphous CoNix–CN nanomaterials with a tunable Ni/Co value. CoNi0.37–CN not only possesses good properties but also exhibits good stability in OER tests.
This work was supported by the China Ministry of Science and Technology under Contract of 2016YFA (0202801), the Foundation for the Author of the National Excellent Doctoral Dissertation of P. R. China (201321) and the National Natural Science Foundation of China (21521091, 21390393, 21471089, 21671117, U1463202).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Zhang, G. Wang, Y. Liu, H. Liu, J. Qu and J. Li, J. Am. Chem. Soc., 2016, 138, 14686–14693 CrossRef CAS PubMed.
- C. C. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- B. M. Hunter, H. B. Gray and A. M. Müller, Chem. Rev., 2016, 116, 14120–14136 CrossRef CAS PubMed.
- J. Zhang, M. Chen, J. Chen, H. Li, S. Wang, Q. Kuang, Z. Cao and Z. Xie, Sci. China Mater., 2017, 60, 685–696 CrossRef.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang and J. K. Norskov, Science, 2016, 353, 1011–1014 CrossRef CAS PubMed.
- Y. Pi, N. Zhang, S. Guo, J. Guo and X. Huang, Nano Lett., 2016, 16, 4424–4430 CrossRef CAS PubMed.
- W. Liao and G. Zhou, Sci. China Mater., 2017, 60, 664–673 CrossRef.
- L. Du, L. Luo, Z. Feng, M. Engelhard, X. Xie, B. Han, J. Sun, J. Zhang, G. Yin, C. Wang, Y. Wang and Y. Shao, Nano Energy, 2017, 39, 245–252 CrossRef CAS.
- Q. Dong, Y. Zhang, Z. Dai, P. Wang, M. Zhao, J. Shao, W. Huang and X. Dong, Nano Res., 2017 DOI:10.1007/s12274-017-1754-5.
- M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS PubMed.
- S. Kundu, B. Malik, A. Prabhakaran, D. K. Pattanayak and V. K. Pillai, Chem. Commun., 2017, 53, 9809–9812 RSC.
- J. Nai, H. Yin, T. You, L. Zheng, J. Zhang, P. Wang, Z. Jin, Y. Tian, J. Liu, Z. Tang and L. Guo, Adv. Energy Mater., 2015, 5, 1401880 CrossRef.
- L. Kong, Z. Ren, S. Du, J. Wu and H. Fu, Chem. Commun., 2014, 50, 4921–4923 RSC.
- S. Du, Z. Ren, J. Zhang, J. Wu, W. Xi, J. Zhu and H. Fu, Chem. Commun., 2015, 51, 8066–8069 RSC.
- Y. P. Zhu, T. Y. Ma, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2017, 56, 1324–1328 CrossRef CAS PubMed.
- J. Wu, Z. Ren, S. Du, L. Kong, B. Liu, W. Xi, J. Zhu and H. Fu, Nano Res., 2016, 9, 713–725 CrossRef CAS.
- X. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen and S. Yang, Angew. Chem., Int. Ed., 2014, 126, 7714–7718 CrossRef.
- A. Stein, Z. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265–293 CrossRef CAS.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS.
- L. Du, Y. Shao, J. Sun, G. Yin, J. Liu and Y. Wang, Nano Energy, 2016, 29, 314–322 CrossRef CAS.
- T. Cao, D. Wang, J. Zhang, C. Cao and Y. Li, Chem. – Eur. J., 2015, 21, 14022–14029 CrossRef CAS PubMed.
- S. Guo, P. Yuan, J. Zhang, P. Jin, H. Sun, K. Lei, X. Pang, Q. Xu and F. Cheng, Chem. Commun., 2017, 53, 9862–9865 RSC.
- Y. Hou, S. Cui, Z. Wen, X. Guo, X. Feng and J. Chen, Small, 2015, 11, 5940–5948 CrossRef CAS PubMed.
- H. Li, J. Liao, Y. Du, T. You, W. Liao and L. Wen, Chem. Commun., 2013, 49, 1768–1770 RSC.
- M. B. Stevens, L. J. Enman, A. S. Batchellor, M. R. Cosby, A. E. Vise, C. D. Trang and S. W. Boettcher, Chem. Mater., 2017, 29, 120–140 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and data (Fig. S1–S6). See DOI: 10.1039/c7cc07115a |
| This journal is © The Royal Society of Chemistry 2017 |