Switching enzyme specificity from phosphate to resveratrol glucosylation†
Michael
Kraus
a,
Clemens
Grimm
*b and
Jürgen
Seibel
 *a
*a
aDepartment of Organic Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: seibel@chemie.uni-wuerzburg.de
bDepartment of Biochemistry, Theodor Boveri-Institute, University of Würzburg Am Hubland, 97074 Wuerzburg, Germany. E-mail: Clemens.grimm@uni-wuerzburg.de
First published on 13th October 2017
Abstract
Here we present a point mutation-triggered domain shift which switches the acceptor preference of a sucrose phosphorylase from phosphate to a variety of large polyphenolic compounds including resveratrol and quercetin, enabling their efficient glucosylation. The variant possesses a high affinity for aromatic substrates due to newly introduced π–π- and hydrophobic interactions in the altered active site. The domain shift brings about a substantially enlarged and multifunctional active site for polyphenol glucosylation and rare disaccharide production. The crystal structure of the variant with its product resveratrol-3-α-D-glucoside allows the prediction of the substrate scope and regioselectivity of the aromatic compounds’ glucosylation sites.
Polyphenols, in particular the extensively studied resveratrol and quercetin, exhibit antitumor activities1,2 and play a key role in lifespan and health span extension.3–5 Glycosylation is desired in order to increase bioavailability, fine-tune bioactivities and pharmaceutical properties and to improve delivery of polyphenol drugs to target cells.6,7 Thus engineering of carbohydrate processing enzymes towards accepting polyphenols as substrates is the subject of several recent investigations.7,9–14 We chose sucrose phosphorylase (SP, EC 2.4.1.7, GH13) as the target for protein engineering because it is an industrially important enzyme that utilizes the abundant glucosyl donor substrate sucrose to transfer glucose moieties to various acceptors.15–17 However, SPs do not glucosylate resveratrol or other polyphenols efficiently due to the spatial limitations of the active site (Fig. 1).9,10,16 The overall poor solubility of this compounds in aqueous system presents a further challenge and call for a high affinity of the engineered enzymes towards their target substrates.10 In general, the introduction of new activities into enzymes has been achieved by state of the art protein engineering including directed evolution18 and rational de novo approaches where a theoretical active site is constructed in silico and accommodated in an existing protein scaffold.19 To date, optimization of de novo enzymes via directed evolution is required to achieve activities comparable to naturally occurring proteins.20 Therefore structure based, (semi-) rational exchanges remain a common tool and are performed mostly to enlarge the active site, or to fine-tune its polarity and ligand protein interactions.21 We followed an unconventional strategy during the redesign of Bifidobacterium adolescentis sucrose phosphorylase (BaSP)9 and exchanged a glutamine residue (Gln345), located at the acceptor binding site against phenylalanine, which actually introduces a larger sidechain into the active site. By introducing a non-polar aromatic residue into the acceptor binding site, we envisioned to enable π–π stacking with the desired aromatic substrates. Furthermore the exchanged glutamine is involved in phosphate binding and may play a role in utilizing glucose as an acceptor. Reducing this undesired side reactions by eliminating polar interactions to this substrate was an additional goal of the design strategy.
 | ||
| Fig. 1 Enabling of aromatic compounds glucosylation via domain shift in BaSP Q345F; (A) electron density of bound resveratrol-3-α-D-glucoside 1.0σ; (B) active site dimensions and substrate positioning in the wild-type enzyme, dotted line: required position of resveratrol; (C) domain shift responsible for the creation of a novel active site, green: BaSP Q345F, grey: BaSP wild-type (PDB ID 2gdv chain B); (D) engineered active site of BaSP Q345F with bound resveratrol-3-α-D-glucoside. | ||
The Q345F exchange in fact enables the efficient glucosylation of bulky polyphenol species (Scheme 1). This effect is explained by the crystal structure of the engineered BaSP in complex with glucosylated resveratrol (Fig. 1A). The active site volume increases from 272 to 557 Å3 providing the space required for resveratrol and flavonoid accommodation and glucosylation (Fig. 1B and C) due to an initially unexpected shift of one domain (Fig. 1D).
As the observed domain shift might be of further interest to manipulate the active site of TIM-barrel enzymes in particular the vast GH13 family, we moved on to a closer inspection of its underlying mechanics. The central domain of SP and the GH13 family with its 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 members is a TIM-barrel which harbours the enzyme's active site near the C-terminal ends of its eight parallel β-strands. The loops at the C-terminal end of the strands are often replaced by versatile domains and thus structurally and functionally diverse active sites are created. In sucrose phosphorylases the acceptor binding site is defined by two of these domains, domain B (residues 86–166) and domain B′ (residues 292–355, Fig. 1D and Fig. S1, ESI†).22 In the BaSP variant Q345F the domain shift responsible for the active site remodelling consists of domain B moving 3.3 Å away from B′ (Fig. 1D). This rearrangement is independent of the presence of a ligand in the active site (Fig. S3, ESI†). Therefore an induced fit effect as the reason behind the domain shift can be excluded. Ultimately, the Q345F exchange causes an opening of BaSP and enables resveratrol and flavonoid accommodation via a provoked fit effect. A potentially adverse effect connected to the observed domain shift could be the displacement of the highly conserved residues His88 and Gln160 involved in substrate binding (Fig. 2). These residues coordinate the 4-OH and 6-OH of the donor glucose moiety. An additional defined water molecule is found in the BaSP Q345F crystal structure coordinated by residues His88 and Gln160. The water molecule bridges the increased distance and recovers the lost hydrogen bond to the 6-OH of the glucose moiety, thus healing the distortions in the active site (Fig. 2). The engineered variant BaSP Q345F glucosylates various polyphenolic acceptors efficiently, achieving a yield of 97% in the synthesis of resveratrol-3-α-D-glucoside from resveratrol (Fig. 3). More importantly, our engineered variant displays a high affinity for the desired aromatic acceptors (KM 0.08–1.55 mM), while the affinity for phosphate is simultaneously reduced (KM from 4.8 to 26 mM, Table 1 and ESI†).
000 members is a TIM-barrel which harbours the enzyme's active site near the C-terminal ends of its eight parallel β-strands. The loops at the C-terminal end of the strands are often replaced by versatile domains and thus structurally and functionally diverse active sites are created. In sucrose phosphorylases the acceptor binding site is defined by two of these domains, domain B (residues 86–166) and domain B′ (residues 292–355, Fig. 1D and Fig. S1, ESI†).22 In the BaSP variant Q345F the domain shift responsible for the active site remodelling consists of domain B moving 3.3 Å away from B′ (Fig. 1D). This rearrangement is independent of the presence of a ligand in the active site (Fig. S3, ESI†). Therefore an induced fit effect as the reason behind the domain shift can be excluded. Ultimately, the Q345F exchange causes an opening of BaSP and enables resveratrol and flavonoid accommodation via a provoked fit effect. A potentially adverse effect connected to the observed domain shift could be the displacement of the highly conserved residues His88 and Gln160 involved in substrate binding (Fig. 2). These residues coordinate the 4-OH and 6-OH of the donor glucose moiety. An additional defined water molecule is found in the BaSP Q345F crystal structure coordinated by residues His88 and Gln160. The water molecule bridges the increased distance and recovers the lost hydrogen bond to the 6-OH of the glucose moiety, thus healing the distortions in the active site (Fig. 2). The engineered variant BaSP Q345F glucosylates various polyphenolic acceptors efficiently, achieving a yield of 97% in the synthesis of resveratrol-3-α-D-glucoside from resveratrol (Fig. 3). More importantly, our engineered variant displays a high affinity for the desired aromatic acceptors (KM 0.08–1.55 mM), while the affinity for phosphate is simultaneously reduced (KM from 4.8 to 26 mM, Table 1 and ESI†).
 | ||
| Fig. 2 Coordination of the glucose moiety of resveratrol-3-α-D-glucoside in the -1 site of BaSP D192N/Q345F: outlines indicate corresponding positions in the structure of the wild-type enzyme (PDB ID code 2GDV chain B). | ||
 | ||
| Fig. 3 Glucosylation pattern of BaSP Q345F. Red arrow main glucosylation site, dashed arrow, secondary glucosylation site. | ||
| Substrate | K M [mM] | K cat [s−1] | K cat/KM [M−1 s−1] |
|---|---|---|---|
| Values based on Michaelis–Menten fittings. A detailed analysis including Lineweaver–Burk plot, Hanes–Woolf plot and direct linear plot8 included in ESI. | |||
| Phosphate | 25.7 ± 1.86 | 0.179 ± 0.007 | 6.96 |
| Resveratrol | 0.92 ± 0.09 | 0.131 ± 0.002 | 142 |
| Quercetin | 0.52 ± 0.06 | 0.094 ± 0.003 | 181 |
| Fisetin | 0.32 ± 0.12 | 0.067 ± 0.004 | 209 |
| (−)-Epicatechin | 1.55 ± 0.43 | 0.104 ± 0.007 | 67.1 |
| (+)-Catechin | 0.95 ± 0.41 | 0.074 ± 0.0101 | 77.9 |
| Naringenin | 0.08 ± 0.01 | 0.002 ± 0.0001 | 250 |
K M values for the wild-type enzyme with polyphenolic substrates could not be determined due to it's virtually non-existing affinity towards this compounds. The variants improved transfer to polyphenolic substrates is due the significant stabilizing interactions between the enzyme and the novel acceptor substrates and to the creation of space in the active site. Investigation of both the product profiles and the crystal structure of BaSP Q345F in complex with resveratrol grant further insight into the variants binding mechanism. BaSP Q345F prefers 1,2- and 1,3-aromatic diols and utilizes the 3-OH of resveratrol and the 3′-OH of flavonoids, while ignoring the 4′-OH of both acceptors (Fig. 3). This fact is explained by the chemical environment of the resveratrol moiety of resveratrol-3-α-D-glucoside in the engineered acceptor binding site of the enzyme. The second non-glucosylated OH group of resveratrol is coordinated by Glu232 and the peptide nitrogen of Ala193 (Fig. 4), and a comparable arrangement is possible for the 1,2- and 1,3-diol motifs present in flavonoids (Fig. 3). If a carbonyl functionality is present in 4-position of a flavonoid the variant glucosylates the acceptor at position 7, whereas the catechins which lack the carbonyl functionality are glucosylated at the 5-OH moiety (Fig. 3). Glucosylation at the 4′-position of flavonoids would however require an almost linear orientation of the glucose moiety and all three flavonoid rings, which would cause a steric clash with the residues outlining the active site, in particular Tyr132, Tyr196 and Phe205. We therefore conclude that this region constitutes a newly identified hotspot for the modification of SP regioselectivity towards complex aromatic acceptors. Detailed analysis of product distributions revealed that (−)-epicatechin is glucosylated in equal measures in positions 3′ and 5 while (+)-catechin, is almost exclusively glucosylated in 3′-position. Superimposition of both flavanols with the resveratrol moiety bound to the active site (Fig. S4, ESI†) point to a hydrogen bond between the 3-OH moiety of (−)-epicatechin and Arg135 as the cause of this variance in regioselectivity.
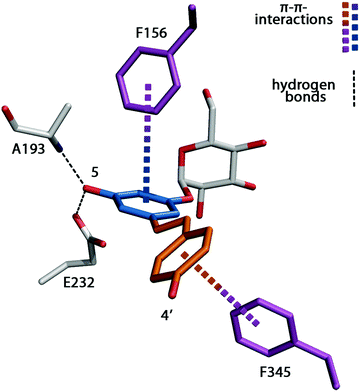
Our initial strategy to introduce π–π-interactions between the polyphenols and the enzyme to force the acceptor substrate in a productive position for glucosylation is reflected in Fig. 4. The binding of nonpolar substrates is further facilitated through the increased hydrophobic and aromatic character of the engineered active site. The interaction interface between the resveratrol moiety and the enzyme has an area of 308 Å2. In addition, T-type π–π stacking interactions between Phe156 and the A-ring of resveratrol stabilize the substrate (Fig. 4). A second T-shape π–π interaction exists between the π-system formed by the central double bond and the B-ring of resveratrol and the engineered residue Phe345 (Fig. 4).
While the domain shift-created novel active site of BaSP Q345F is particularly well suited for large polyphenols, it displays a certain degree of substrate promiscuity. In the absence of suitable acceptors BaSP slowly hydrolyses sucrose and utilizes the resulting glucose as an acceptor to form maltose (4-O-α-D-glucopyranosyl-D-glucose) and nigerose (3-O-α-D-glucopyranosyl-D-glucose) in case of the Q345F variant.23 In contrast the wild-type enzyme synthesizes maltose and kojibiose (2-O-α-D-glucopyranosyl-D-glucose).24 A crystal structure (Fig. S2, ESI†) of the variant in complex with the non-natural product nigerose illustrated that nigerose production is enabled by the same, engineered acceptor binding site that allows polyphenol glucosylation.
In summary, we present the creation of a new multifunctional acceptor binding site via a domain shift and the introduction of favourable π–π-interactions. To the best of our knowledge this is the first example of an active site remodelling by a domain shift which is visualized by protein structures with and without the substrates. The domain shift is triggered by a single amino acid exchange and is responsible for remodelling the acceptor-binding site of BaSP into a polyphenol binding site. The engineered variant is capable of the glucosylation of a wide variety of bulky flavonoids, including quercetin and resveratrol as well as of the synthesis of rare disaccharides. The crystal structures of our engineered sucrose phosphorylase, in complex with its respective products resveratrol-3-α-D-glucoside and nigerose explain the mode of substrate binding and may reveal hotspots for future modification of this and possibly other SP variants.
We thank the team of beamline ID30B of ESRF Grenoble, France for their excellent support during data collection. The corresponding coordinates and structure factors are available from the PDB under accession code 5M9X (BaSP D192N/Q345F in complex with resveratrol-3-α-D-glucoside), 5MAN (BaSP D192N/Q345F in complex with nigerose) and 5MB2 (BaSP Q345F apo form).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Jang, L. Cai, G. O. Udeani, K. V. Slowing, C. F. Thomas, C. W. Beecher, H. H. Fong, N. R. Farnsworth, A. D. Kinghorn, R. G. Mehta, R. C. Moon and J. M. Pezzuto, Science, 1997, 275, 218–220 CrossRef CAS PubMed.
- S. Srivastava, R. R. Somasagara, M. Hegde, M. Nishana, S. K. Tadi, M. Srivastava, B. Choudhary and S. C. Raghavan, Sci. Rep., 2016, 6, 24049 CrossRef CAS PubMed.
- M. Sajish and P. Schimmel, Nature, 2015, 519, 370–373 CrossRef CAS PubMed.
- J. A. Baur, K. J. Pearson, N. L. Price, H. A. Jamieson, C. Lerin, A. Kalra, V. V. Prabhu, J. S. Allard, G. Lopez-Lluch, K. Lewis, P. J. Pistell, S. Poosala, K. G. Becker, O. Boss, D. Gwinn, M. Wang, S. Ramaswamy, K. W. Fishbein, R. G. Spencer, E. G. Lakatta, D. Le Couteur, R. J. Shaw, P. Navas, P. Puigserver, D. K. Ingram, R. de Cabo and D. A. Sinclair, Nature, 2006, 444, 337–342 CrossRef CAS PubMed.
- K. T. Howitz, K. J. Bitterman, H. Y. Cohen, D. W. Lamming, S. Lavu, J. G. Wood, R. E. Zipkin, P. Chung, A. Kisielewski, L.-L. Zhang, B. Scherer and D. A. Sinclair, Nature, 2003, 425, 191–196 CrossRef CAS PubMed.
- C. Zhang, B. R. Griffith, Q. Fu, C. Albermann, X. Fu, I. K. Lee, L. Li and J. S. Thorson, Science, 2006, 313, 1291–1294 CrossRef CAS PubMed.
- R. W. Gantt, P. Peltier-Pain and J. S. Thorson, Nat. Prod. Rep., 2011, 28, 1811–1853 RSC.
- R. Eisenthal and A. Cornish-Bowden, Biochem. J., 1974, 139, 715–720 CrossRef CAS PubMed.
- M. Kraus, C. Grimm and J. Seibel, ChemBioChem, 2016, 17, 33–36 CrossRef CAS PubMed.
- M. E. Dirks-Hofmeister, T. Verhaeghe, K. De Winter and T. Desmet, Angew. Chem., Int. Ed., 2015, 54, 9289–9292 CrossRef CAS PubMed.
- C. Liang, Y. Zhang, Y. Jia, W. Wenzhao, Y. Li, S. Lu, J.-M. Jin and S.-Y. Tang, Sci. Rep., 2016, 6, 21051 CrossRef CAS PubMed.
- Y. Malbert, S. Pizzut-Serin, S. Massou, E. Cambon, S. Laguerre, P. Monsan, F. Lefoulon, S. Morel, I. André and M. Remaud-Simeon, ChemCatChem, 2014, 6, 2282–2291 CrossRef CAS.
- T. Desmet, W. Soetaert, P. Bojarová, V. Křen, L. Dijkhuizen, V. Eastwick-Field and A. Schiller, Chem. – Eur. J., 2012, 18, 10786–10801 CrossRef CAS PubMed.
- M. Zhou, A. Hamza, C.-G. Zhan and J. S. Thorson, J. Nat. Prod., 2013, 76, 279–286 CrossRef CAS PubMed.
- C. Goedl, T. Sawangwan, M. Mueller, A. Schwarz and B. Nidetzky, Angew. Chem., Int. Ed., 2008, 47, 10086–10089 CrossRef CAS PubMed.
- D. Aerts, T. F. Verhaeghe, B. I. Roman, C. V. Stevens, T. Desmet and W. Soetaert, Carbohydr. Res., 2011, 346, 1860–1867 CrossRef CAS PubMed.
- V. Lombard, H. Golaconda Ramulu, E. Drula, P. M. Coutinho and B. Henrissat, Nucleic Acids Res., 2014, 42, D490–D495 CrossRef CAS PubMed.
- P. S. Coelho, E. M. Brustad, A. Kannan and F. H. Arnold, Science, 2013, 339, 307–310 CrossRef CAS PubMed.
- J. B. Siegel, A. Zanghellini, H. M. Lovick, G. Kiss, A. R. Lambert, J. L. S. Clair, J. L. Gallaher, D. Hilvert, M. H. Gelb, B. L. Stoddard, K. N. Houk, F. E. Michael and D. Baker, Science, 2010, 329, 309–313 CrossRef CAS PubMed.
- R. Obexer, A. Godina, X. Garrabou, P. R. E. Mittl, D. Baker, A. D. Griffiths and D. Hilvert, Nat. Chem., 2017, 9, 50–56 CAS.
- C. K. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Huisman and G. J. Hughes, Science, 2010, 329, 305–309 CrossRef CAS PubMed.
- O. Mirza, L. K. Skov, D. Sprogoe, L. A. van den Broek, G. Beldman, J. S. Kastrup and M. Gajhede, J. Biol. Chem., 2006, 281, 35576–35584 CrossRef CAS PubMed.
- M. Kraus, J. Gorl, M. Timm and J. Seibel, Chem. Commun., 2016, 52, 4625–4627 RSC.
- T. Verhaeghe, K. De Winter, M. Berland, R. De Vreese, M. D'Hooghe, B. Offmann and T. Desmet, Chem. Commun., 2016, 52, 3687–3689 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc05993k |
| This journal is © The Royal Society of Chemistry 2017 |