Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogen free 1,2,3- and 1,2,4-triazolide based ionic liquids: synthesis and properties†
Aleksandr
Savateev
 *a,
Clemens
Liedel
*a,
Clemens
Liedel
 a,
Steffen
Tröger-Müller
a,
Alberto S.
de León
b,
Markus
Antonietti
a and
Dariya
Dontsova
a,
Steffen
Tröger-Müller
a,
Alberto S.
de León
b,
Markus
Antonietti
a and
Dariya
Dontsova
 a
a
aMax-Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, 14424 Potsdam, Germany. E-mail: oleksandr.savatieiev@mpikg.mpg.de
bMax-Planck Institute of Colloids and Interfaces, Mechano(bio)chemistry, Research Campus Golm, 14424 Potsdam, Germany
First published on 29th August 2017
Abstract
Triazoles have been successfully used as building blocks to create “fully organic” ILs featuring on both sides organic ions, i.e., 1,2,3- or 1,2,4-triazolide anions and 1,2,4-triazolium or imidazolium cations. Glass transition temperatures, densities and viscosities of these ILs were determined. Their electrochemical and thermal stability, and also conductivity, are higher than those for known ILs.
Ionic liquids (ILs) are in the focus of modern materials chemistry and, for instance, used as a medium to perform chemical transformation as well as for electrochemical and even biological applications.1–3 The vast majority of ILs are represented by an organic cation, typically imidazolium, and an inorganic or halogenated counterion. The pathways of anion decompositions were explored both theoretically and experimentally.4 For example, the reduction of the triflimidate anion, Tf2N⊖, starts at −2.0 V vs. Fc+/Fc and includes cleavage of the S–N bond followed by the formation of CF3⊖.5 In contrast, PF6⊖ is slowly oxidized on the anode producing toxic gaseous PF5 and F˙ radical, which upon hydrogen abstraction is converted to HF.6 On the other hand, B–F bond cleavage in BF4⊖ occurs upon contact with water even under very mild conditions.7 Considering potential risks that fluorinated ILs might carry, a good alternative could be halogen free ILs.
“Fully organic” ILs, having both organic cations and anions, are less common8 and usually comprise simple anions like acetate, bringing however obvious restrictions in use.9 In this context, 1,2,3- and 1,2,4-triazoles are an interesting choice of heterocycles which form both stable cations and anions. This enables to set up a new class of ILs. The advantage of “fully organic” triazole-based ILs is their chemical stability, but they also possess many nitrogen atoms which can participate in H-bonding with the solute. This makes them potentially more compatible with many organic substances since “similia similibus solvuntur”.
Triazolium cations (A, Fig. 1) are stabilized via conjugation with nitrogen lone pairs as well as through the inductive effect of electron-donating functional groups, which additionally may affect the charge distribution within the molecule affording task specific ILs for targeted application.10 The advantage of triazolium ILs compared to widely used imidazolium ones is the reduction in number or even suppression of side-reactions caused by deprotonation of the ring carbon atoms of heterocyclic cations.11 Some 1,2,3-triazolium ILs were successfully used as a medium for the Baylis–Hillman reaction11 and for (±)-centrolobine synthesis12 while bicyclic triazolium ILs were applied as molecular solvents for rutaecarpine synthesis.13 Besides, several 1,2,3-triazolium ILs with tosylate and TfO⊖ anions were synthesized and characterized.14
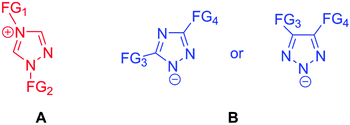 | ||
| Fig. 1 Cationic triazole-1,2,4-based IL (A) and anionic triazole-1,2,4- (B, left) or triazole-1,2,3-based ILs (B, right). FG stands for ‘functional group’. | ||
Conversely, in the triazolide anion (B, Fig. 1) three nitrogen atoms can effectively stabilize negative charges and lower the IL viscosity.15 In addition, due to the smaller size of the organic anion compared, for example, to Tf2N⊖, the ion mobility of the IL is expected to be higher. As a result of the low acidity of 1,2,4- and 1,2,3-triazoles, their corresponding anions are considered to be superbases,16 and several 1,2,3-triazolide ILs were used for CO2 capture17 and CO2 electrochemical reduction.18–20 The triazole-1,2,4-ide anion was employed as a part of highly energetic ILs as well.21 However, their electrochemical properties remain unexplored.
Triazoles could be an excellent platform to synthesize “fully organic” ILs, comprising triazolium cations and triazolide anions. However, throughout the challenging design a number of criteria should be kept in mind. These include the acidity of the target triazole, which functional groups to use to adjust the triazole properties, and the synthetic approach to employ.
Herein we report on the synthesis of a set of “fully organic” triazole based ILs and their properties.
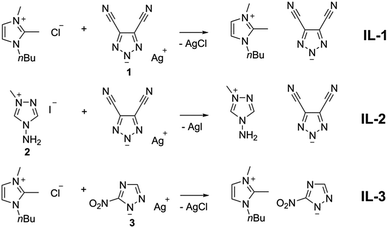
Triazoles, bearing NO2 and CN functional groups, were selected as anion precursors due to the ability of these groups to stabilize efficiently a negative charge via a (–M) mesomeric effect. IL precursors 2H-1,2,3-triazole-4,5-dicarbonitrile,22 3-nitro-1H-1,2,4-triazole,23 1-methyl-4-amino-4H-1,2,4-triazol-1-ium iodide242 were synthesized by us according to the procedures given in the literature.
The ILs studied in the current work were obtained in high yields via ion metathesis reactions as depicted in Fig. 2. The method provides ILs with high purity, as shown below, and without laborious purification procedures. The chemical composition of the ILs is very close to the calculated one (Table S1, ESI†). IL-1, IL-2 and IL-3 are free of any foreign elements, e.g. Ag, Cl, and I, which was unambiguously proved by EDX analysis (Fig. S2 and Table S1, ESI†).25,26 The water mass fraction (ww) in the ILs after drying under vacuum (0.1 mbar, 65 °C, 4 h) determined using Karl–Fischer titration in IL-1 was ww = 0.00832 ± 0.0002, IL-2 ww = 0.0144 ± 0.0008 and IL-3 ww = 0.0102 ± 0.0004 (Table S1, ESI†).
The structures of ILs are consistent with 1H and 13C NMR spectra including two-dimensional techniques (Fig. S3–S23, ESI†). The full assignment of peaks in the NMR spectra to specific nuclei was performed (Fig. S24, ESI†). In HR-MS spectra the masses of the cation and anion were observed independently in the positive and negative ion modes proving that all these compounds can give stable charged species (Fig. S25–S27, ESI†). Combining 1H NMR data with elemental analysis and water content the purity of the ILs (in mass fraction) was determined to be 0.968–0.982 (Table S1, ESI†).
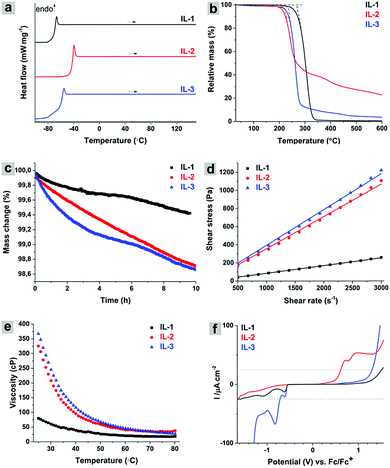
At room temperature IL-1, IL-2, and IL-3 are viscous liquids. The glass transition temperatures (Tg) of the ILs were determined from differential scanning calorimetry (DSC) curves (Fig. 3a) and are summarized in Table 1. The ILs are stable in the temperature range of −100… + 150 °C as proved by the identity of Tg values during cyclic heating/cooling (Fig. S28, ESI†). IL-1 has Tg = −66.6 °C, which is higher than that for 1-butyl-3-methyl-1H-imidazol-3-ium 4,5-dicyano-1,2,3-triazol-2-ide, −87 °C (heating rate 2 °C min−1), evidently due to the presence of a methyl group in the second position of the imidazole ring.15
| IL | T g , °C | T dec , °C | μ , cP | ρ , g cm−3 | κ , mS cm−1 | Cathodic limitf, V vs. Fc/Fc+ | Anodic limitf, V vs. Fc/Fc+ | ECWg, V |
|---|---|---|---|---|---|---|---|---|
| a Glass transition temperatures determined by DSC. b Onset of decomposition. c Dynamic (shear) viscosity measured at 25 °C. d Measured at 25 °C in triplicate. e Measured at 25 °C. f Cathodic and anodic limits were determined from LSV (scan rate 1 mV s−1) using a glassy carbon working electrode, a Pt counter electrode and an Ag/AgNO3/Bu4N+H2PO4− reference electrode (Fig. 3f) applying a current density cutoff at j = 20 μA cm−2. g Electrochemical window (ECW) = anodic limit − cathodic limit. | ||||||||
| IL-1 | −66.6 ± 0.1 | 268 | 85 ± 2 | 1.107 ± 0.001 | 2.122 ± 0.185 | −1.60 | 1.51 | 3.02 |
| IL-2 | −39.0 ± 0.3 | 219 | 376 ± 20 | 1.325 ± 0.001 | 1.180 ± 0.008 | −1.64 | 0.61 | 2.25 |
| IL-3 | −55.4 ± 0.2 | 234 | 414 ± 20 | 1.181 ± 0.001 | 0.731 ± 0.110 | −1.3 | 1.42 | 2.72 |
Interestingly, due to the compact size of the 1,2,4-triazolium cation, one could expect a high melting point of IL-2. However, this is not the case, as IL-2 still possesses a relatively low Tg = −39.0 °C, probably due to the influence of the anion. IL-3 demonstrates quite low Tg = −55.4 °C that could be explained, at least partially, by the lower symmetry of the anion compared, for example, to 1,2,3-triazolide.
The thermal stability and thermal decomposition pathways of the synthesized ILs were explored using thermogravimetric analysis mass spectroscopy (TGA-MS). The onset of decomposition (Tdec) values extracted from the TGA curves (Fig. 3b) are summarized in Table 1. Tdec values could be used as a descriptor of IL thermal stability upon short exposure to high temperatures. We have monitored charged species with m/z in the range of 1–200 that are formed during the IL heating up to 600 °C. The ion current curves where the change of signal was detected are shown in Fig. S29, S31 and S33 (ESI†).
IL-1 starts decomposing at 268 °C and complete decomposition occurs at 400 °C suggesting that only gaseous products are formed. Importantly, the Tdec value of IL-1 is higher than those for some of the known imidazolium ILs with organic anions.27,28 The decomposition of the 4,5-dicyano-1,2,3-triazol-2-ide anion involves the formation of C2N2˙+ (m/z 52), N2˙+ (m/z 28), CN+ (m/z 26) and CH+ (m/z 13) species (Fig. S29, ESI†). The decomposition of the 3-butyl-1,2-dimethyl-1H-imidazol-3-ium cation probably involves the elimination of the n-butyl radical and its subsequent fragmentation along with dehydration as supported by a series of charged species with m/z 54, 53, 52, 51, 50 (C4 fragments: C4H6˙+, C4H5+, C4H4˙+, C4H3+, C4H2˙+), 44, 43, 42, 41, 40, 39 (C3 fragments: C3H8˙+, C3H7+, C3H6˙+, C3H5+, C3H4˙+, C3H3+) and 30, 29, 28, 27, 26 (C2 fragments C2H6˙+, C2H5+, C2H4˙+, C2H3+, C2H2˙+). The charged species with m/z 68 and 66 that could be assigned to the products of imidazole ring decomposition were also detected. The proposed mechanism of IL-1 thermal decomposition is schematically depicted in Fig. S30 (ESI†).
IL-2 has the onset of decomposition at 219 °C. It loses 60% of initial mass at 350 °C. The decomposition of IL-2 in the first step could involve the transfer of a methyl cation from the cation to the anion leading to the formation of 4H-1,2,4-triazol-4-amine and 2-methyl-2H-1,2,3-triazole-4,5-dicarbonitrile (Fig. S32, ESI†). The latter is decomposed to C2N2˙+ (m/z 52), C2N+ (m/z 38) and methanediazonium (CH3N2+, m/z 43) as initial fragments. CH3N2+, upon subsequent fragmentation, gives rise to other species, such as CH2N2˙+ (m/z 42), CH2N2˙+ (m/z 40), diazene (N2H2˙+, m/z 30), CH3N+ (m/z 29), etc. (Fig. S31, ESI†). The decomposition of 4H-1,2,4-triazol-4-amine, in turn, involves the evolution of nitrogen (N2˙+, m/z 28), ammonia (NH3˙+, m/z 17) and C2HN˙+ (m/z 39) (Fig. S31, ESI†). The latter could give HCN˙+ (m/z 27) and CN+ (m/z 26) charged species, while the liberation of ammonia is supported by the presence of N+ (m/z 14), NH˙+ (m/z 15), NH2+ (m/z 16) and NH4+ (m/z 18).
Interestingly, the introduction of a nitro group into the triazole ring did not destabilize the respective anion significantly. IL-3 starts decomposing at 234 °C and loses 90% of the initial mass at 300 °C. The N-butylimidazolium cation follows the decomposition pattern described for IL-1 above and includes the elimination of butyl radical that upon further decay gives a set of characteristic C4, C3 and C2 fragments (Fig. S34, ESI†). On the other hand, anion decomposition could involve elimination of NO+ (m/z 30) accompanied by OH+ ion (m/z 17), N2˙+ (m/z 28) and presumably HC2N˙+ (m/z 39) (Fig. S33, ESI†). The latter, however, overlaps with the signal of C3H3+.
In order to estimate long term thermal stability of ILs, we performed isothermal TGA at 120 °C for 10 h. This temperature exceeds the typical operational temperature for the most common processes and could serve on a first approximation as a parameter describing the longstanding stability of an IL.29 Under the applied conditions, IL-1 has lost 0.5% of the initial mass, while IL-2 and IL-3 lost about 1.3% (Fig. 3c). The densities of the synthesized ILs are in the range of 1.107–1.325 g cm−3 (Table 1). Typically the presence of water affects the densities of ILs within the range of 1–2%.30
The novel ILs behave as Newtonian fluids in the range of shear rates 500–3000 s−1 (Fig. 3d). IL-1 has the lowest dynamic viscosity of 85 cP at 25 °C, among the ILs presented in the current work (Table 1). IL-2 and IL-3 have viscosities of 376 and 414 cP, respectively. The viscosities of the ILs gradually decrease with temperature and reach a plateau at 70–80 °C (Fig. 3e). The viscosity of the ILs also depends on the water content and for [C4mim][Tf2N], for example, it is 33% lower when the water content increases from 10 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 ppm.31 Nevertheless, IL-1 possesses a lower viscosity compared, for example, to [C4mim][PF6] (397 cP, ww = 0.0117, 25 °C) and [C6mim][PF6] (452 cP, ww = 0.008837, 25 °C) having nearly the same water content.32
480 ppm.31 Nevertheless, IL-1 possesses a lower viscosity compared, for example, to [C4mim][PF6] (397 cP, ww = 0.0117, 25 °C) and [C6mim][PF6] (452 cP, ww = 0.008837, 25 °C) having nearly the same water content.32
The conductivities of ILs were determined using electrochemical impedance spectroscopy (EIS) (Table 1). Widegren and Magee have shown that the conductivity of ILs depends on the water content and for dry [C6mim][Tf2N] (ww = 10−5), for example, it is 2.18 mS cm−1, which is 30% lower compared to that of the same IL, but measured under wet conditions (3.06 mS cm−1, ww = 0.00898, 25 °C).33 The conductivities of the newly synthesized ILs are comparable, for example, to those of aforementioned [C6mim][Tf2N] and [C4mim][Tf2N] (5.42 mS cm−1, ww = 0.00885, 25 °C).34 In addition, the conductivities of the ILs reported here are higher than those of [aP4443][Ala] (0.244 mS cm−1, ww = 0.0169), [aP4443][Val] (0.116 mS cm−1, ww = 0.0157) and [aP4443][Leu] (0.114 mS cm−1, ww = 0.0151) measured at 25 °C and having higher water contents.35
In order to overcome the influence of viscosity, especially for IL-3, and to achieve equilibrium conditions, linear sweep voltammetry (LSV) measurements were performed at a low scan rate of 1 mV s−1 (Fig. 3f).36 The electrochemical window (ECW) of the IL naturally depends on the current density cutoff used for calculations.37 We applied a very low current density cutoff, j = 20 μA cm−2, which is considerably lower than that generally used in the literature, in order to avoid overestimation of the ECW (Table 1). Thus, IL-1 has the widest electrochemical window (ECW) among the studied compounds, 3.02 V. On the other hand, IL-2 has the highest stability on the cathodic side, −1.64 V. The ECW of IL-3 is 2.72 V, and the anodic limit, 1.42 V, is higher than those in the cases of IL-1 and IL-2. This implies that 5-nitro-1,2,4-triazol-1-ide is more stable against oxidation than 4,5-dicyanotriazole-1,2,3-2-ide. In comparison with other ILs, the ECWs of the electrolytes presented here are wider despite using much lower current density cutoff values. To support this the following ILs having similar water contents could be mentioned: [C4mpyrr][Tf2N] (2.0 V, ww = 0.011407, j = 1 mA cm−2), [C4mim][I] (2.0 V, ww = 0.011349, j = 1 mA cm−2) and [N6,2,2,2][Tf2N] (2.2 V, ww = 0.0082, j = 1 mA cm−2), all measured at +25 °C using a Pt microdisc as the working electrode and a Pt wire as the quasi-reference electrode.37
Three new “fully organic” ILs, which are built only from C, N, O, and H elements and comprise heterocycles as cation and anion building blocks, have been synthesized. Their physical properties were measured and compared to known ILs taking into account the water contents. The obtained results suggest that the electrolytes reported here have higher conductivity, wider ECW and lower viscosities than those of some of the known ILs. And these are in addition to good thermal stability. All these features make the ILs reported here suitable for energy storage applications. Moreover, due to the basic properties of the anions and the presence of a large number of nitrogen atoms, these ILs could be used as a reaction medium to accomplish desired chemical transformations, for example, CO2 reduction.
The work was supported by Deutsche Forschungsgemeinschaft (grant number DFG-An 156 13-1). We thank Antje Völkel, Ursula Lubahn and Sylvia Pirok for TGA-MS, DSC and elemental analysis, respectively. Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.References
- S. V. Malhotra, Ionic Liquids in Organic Synthesis, American Chemical Society, Washington, DC, 2007 Search PubMed.
- A. A. J. Torriero, Electrochemistry in Ionic Liquids, Springer International Publishing, 2015 Search PubMed.
- Y. Zhang, X. Chen, J. Lan, J. You and L. Chen, Chem. Biol. Drug Des., 2009, 74, 282–288 CAS.
- N. D. Vos, C. Maton and C. V. Stevens, ChemElectroChem, 2014, 1, 1258–1270 CrossRef.
- P. C. Howlett, E. I. Izgorodina, M. Forsyth and D. R. MacFarlane, Z. Phys. Chem., 2006, 220, 1483–1498 CrossRef CAS.
- V. R. Koch, A. Dominey, C. Nanlundiah and M. J. Ondrechen, J. Electrochem. Soc., 1996, 143, 798–803 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, I. M. Marrucho, J. A. P. Coutinho and A. M. Fernandes, J. Phys. Chem. A, 2010, 114, 3744–3749 CrossRef CAS PubMed.
- J. M. Aizpurua, R. M. Fratila, Z. Monasterio, N. Pérez-Esnaola, E. Andreieff, A. Irastorza and M. Sagartzazu-Aizpurua, New J. Chem., 2014, 38, 474–480 RSC.
- H. F. D. Almeida, H. Passos, J. A. Lopes-da-Silva, A. M. Fernandes, M. G. Freire and J. A. P. Coutinho, J. Chem. Eng. Data, 2012, 57, 3005–3013 CrossRef CAS.
- C. Yue, D. Fang, L. Liu and T.-F. Yi, J. Mol. Liq., 2011, 163, 99–121 CrossRef CAS.
- Y. Jeong and J.-S. Ryu, J. Org. Chem., 2010, 75, 4183–4191 CrossRef CAS PubMed.
- Y. Jeong, D.-Y. Kim, Y. Choi and J.-S. Ryu, Org. Biomol. Chem., 2011, 9, 374–378 CAS.
- M.-C. Tseng, H.-T. Cheng, M.-J. Shen and Y.-H. Chu, Org. Lett., 2011, 13, 4434–4437 CrossRef CAS PubMed.
- S. Sanghi, E. Willett, C. Versek, M. Tuominen and E. B. Coughlin, RSC Adv., 2012, 2, 848–853 RSC.
- S. Kitaoka, K. Nobuoka, N. Yoshiiwa, T. Harran and Y. Ishikawa, Chem. Lett., 2010, 39, 1142–1143 CrossRef CAS.
- A. D. McNaugh, A. Wilkinson, M. Nic, J. Jirat, B. Kosata and A. Jenkins, IUPAC. Compendium of Chemical Terminology XML on-line corrected version: http://goldbook.iupac.org (2006-), Blackwell Scientific Publications, Oxford, 2nd (the “Gold Book”) edn, 1997.
- R. L. Thompson, W. Shi, E. Albenze, V. A. Kusuma, D. Hopkinson, K. Damodaran, A. S. Lee, J. R. Kitchin, D. R. Luebke and H. Nulwala, RSC Adv., 2014, 4, 12748–12755 RSC.
- N. Hollingsworth, S. F. R. Taylor, M. T. Galante, J. Jacquemin, C. Longo, K. B. Holt, N. H. d. Leeuwad and C. Hardacre, Faraday Discuss., 2015, 183, 389–400 RSC.
- S. F. R. Taylor, C. McCrellis, C. McStay, J. Jacquemin, C. Hardacre, M. Mercy, R. G. Bell and N. H. d. Leeuw, J. Solution Chem., 2015, 44, 511–527 CrossRef CAS.
- J. J. Fillion, H. Xia, M. A. Desilva, M. Quiroz-Guzman and J. F. Brennecke, J. Chem. Eng. Data, 2016, 61, 2897–2914 CrossRef CAS.
- H. Xue, Y. Gao, B. Twamley and J. n. M. Shreeve, Inorg. Chem., 2005, 44, 5068–5072 CrossRef CAS PubMed.
- M.-J. Crawford, K. Karaghiosoff, T. M. Klapötke and F. A. Martin, Inorg. Chem., 2009, 48, 1731–1743 CrossRef CAS PubMed.
- R. Petersen, J. F. Jensen and T. E. Nielsen, Org. Prep. Proced. Int., 2014, 46, 267–271 CrossRef CAS.
- G. Drake, T. Hawkins, K. Tollison, L. Hall, A. Vij and S. Sobaski, in Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities, ed. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington, DC, 2005, vol. 902, ch. 20, pp. 259–302 Search PubMed.
- M. Holzweber, W. E. S. Unger and V.-D. Hodoroaba, Anal. Chem., 2016, 88, 6967–6970 CrossRef CAS PubMed.
- A. R. Santos, R. K. Blundell and P. Licence, Phys. Chem. Chem. Phys., 2015, 17, 11839–11847 RSC.
- M. T. Clough, K. Geyer, P. A. Hunt, J. Mertes and T. Welton, Phys. Chem. Chem. Phys., 2013, 15, 20480–20495 RSC.
- M. Babucci, A. Akçay, V. Balci and A. Uzun, Langmuir, 2015, 31, 9163–9176 CrossRef CAS PubMed.
- A. I. Siriwardana, in Electrochemistry in Ionic Liquids, ed. A. A. J. Torriero, Springer International Publishing, 2015, vol. 2, Applications, ch. 20, pp. 563–603 Search PubMed.
- J. Jacquemin, P. Husson, A. A. H. Padua and V. Majer, Green Chem., 2006, 8, 172–180 RSC.
- J. A. Widegren, A. Laesecke and J. W. Magee, Chem. Commun., 2005, 1610–1612 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- J. A. Widegren and J. W. Magee, J. Chem. Eng. Data, 2007, 52, 2331–2338 CrossRef CAS.
- J. A. Widegren, E. M. Saurer, K. N. Marsh and J. W. Magee, J. Chem. Thermodyn., 2005, 37, 569–575 CrossRef CAS.
- Z. Wang, L. Fu, H. Xu, Y. Shang, L. Zhang and J. Zhang, J. Chem. Eng. Data, 2012, 57, 1057–1063 CrossRef CAS.
- X. Lu, G. Burrell, F. Separovic and C. Zhao, J. Phys. Chem. B, 2012, 116, 9160–9170 CrossRef CAS PubMed.
- A. M. O’Mahony, D. S. Silvester, L. Aldous, C. Hardacre and R. G. Compton, J. Chem. Eng. Data, 2008, 53, 2884–2891 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR and HR-MS spectra, TGA-MS, DSC. See DOI: 10.1039/c7cc05770a |
| This journal is © The Royal Society of Chemistry 2017 |