Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultraclean pure shift NMR†
Pinelopi
Moutzouri
 a,
Yingxian
Chen
a,
Mohammadali
Foroozandeh
a,
Yingxian
Chen
a,
Mohammadali
Foroozandeh
 a,
Peter
Kiraly
a,
Peter
Kiraly
 a,
Andrew R.
Phillips
a,
Andrew R.
Phillips
 b,
Steven R.
Coombes
b,
Steven R.
Coombes
 c,
Mathias
Nilsson
c,
Mathias
Nilsson
 a and
Gareth A.
Morris
a and
Gareth A.
Morris
 *a
*a
aSchool of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: g.a.morris@manchester.ac.uk
bPharmaceutical Sciences, AstraZeneca, Silk Road Business Park, Macclesfield, SK10 2NA, UK
cPharmaceutical Technology and Development, AstraZeneca, Silk Road Business Park, Macclesfield, SK10 2NA, UK
First published on 30th August 2017
Abstract
“Pure shift” methods can greatly improve the resolution of proton NMR spectra. However, current pure shift spectra show small periodic artefacts that prevent their use for studying dilute mixture components. A new technique, compatible with all current pure shift methods, is presented that suppresses such sidebands to arbitrary order, allowing ultraclean spectra to be obtained.
The proton is the most frequently studied nucleus in NMR because of its chemical ubiquity and high sensitivity. 1H spectra are rich in chemical information, but their narrow chemical shift range and extensive overlap of multiplet structure complicate spectral analysis and interpretation. Pure shift NMR1–3 has proven to be a valuable tool for the analysis of crowded spectra,4–6 improving resolution by suppressing the effects of homonuclear couplings J while retaining the chemical shift. However, all current direct domain pure shift methods (as opposed to experiments suppressing couplings in an indirect domain of a multidimensional spectrum) suffer from weak periodic artefacts. Such experiments are based on the periodic refocusing of the scalar coupling evolution, using either 1D7 (real-time) or pseudo-2D (interferogram) data acquisition.8 The artefacts arise because for sensitivity reasons the pure shift FID is respectively acquired or constructed in chunks of a few tens of ms duration (1/sw1) each, while the scalar couplings are only perfectly refocused at the midpoint of each chunk.8–12 The small amount of J evolution during the rest of the chunk causes a weak modulation of the signal envelope with period 1/sw1 (as do a variety of other perturbations, including instrumental imperfections). On Fourier transformation this modulation leads to each peak acquiring small periodic sidebands at intervals sw1. These “chunking sidebands” are typically at a level less than 2–4% of the parent pure shift signal, and in most cases can be ignored. However, in mixture analysis these artefacts can cause serious problems, obscuring the real signals of minor mixture components. Here, we show that by systematically manipulating the timing of pure shift experiments during normal time averaging it is possible to suppress chunking sidebands to arbitrary order, at negligible cost in sensitivity. This new method (SAPPHIRE, Sideband Averaging by Periodic PHase Incrementation of Residual J Evolution) will allow the measurement of ultraclean pure shift spectra and facilitate the analysis of low-level mixture components.
The most challenging NMR samples are mixtures; those with a wide range of signal intensities are particularly challenging. Fig. 1 shows 1H 1D and pure shift spectra of the anti-cholesterol active pharmaceutical ingredient rosuvastatin (1, Scheme 1), spiked with 2.8% of its precursor BEM (2, Scheme 1) (see assignment of the pure compounds and their individual 1H 1D spectra in Fig. S3 and S4 of the ESI†). The conventional 1H pure shift spectrum of Fig. 1b/c, acquired using the interferogram-based Zangger–Sterk method8,13 (Fig. S1, ESI†), is complicated by the presence of chunking sidebands. At the level of 2–4% of the parent signals, these obscure the BEM signals and severely complicate their analysis. In contrast, the spectrum of Fig. 1d, which was acquired with the new technique described here, almost completely suppresses the sidebands, making the BEM signals clearly visible. The fact that the low-level signals and chunking sidebands are similar in intensity and that the sidebands appear here mostly as negative signals means that overlap between a resonance of interest and a sideband can lead to cancellation. For example, in Fig. 1c the BEM signal resonating at 6.58 ppm is completely suppressed because of its accidental overlap with a negative sideband. In general, the presence of chunking sidebands causes problems with both identification and quantification of low-level signals in the presence of large signals.
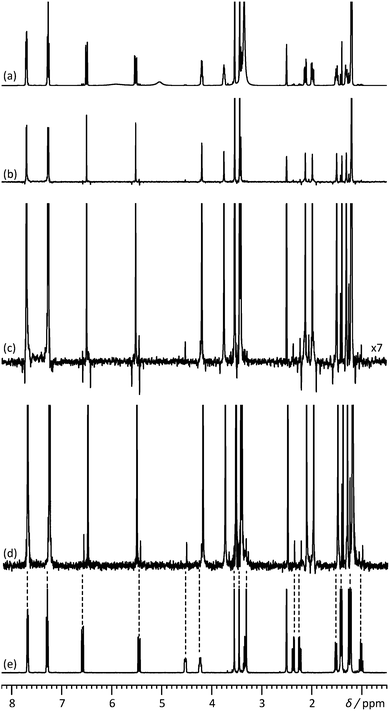 | ||
| Fig. 1 500 MHz (a) 1H 1D spectrum, (b) 1H pure shift spectrum recorded using the conventional pulse sequence of Fig. S1 (ESI†), (c) vertical expansion of the spectrum (b), and (d) 1H pure shift spectrum acquired with the pulse sequence of Fig. 2, for a mixture containing rosuvastatin (1) and 2.8% of its precursor BEM (2). (e) 1H 1D spectrum of BEM. Spectra c and d were acquired in approximately 9 h with an sw1 of 39.0625 Hz and 16 chunks using an RSNOB spatially selective spin inversion element with a peak RF amplitude of 47 Hz. | ||
There already exists one method for reducing the impact of chunking sidebands, which is simply to average the results of multiple experiments using different values of sw1, spreading the sidebands out over a range of frequencies, as shown in Fig. S5 of the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 This method has not yet found significant application, perhaps because it only reduces the peak amplitude of sidebands, but can in favourable cases give sufficient improvement to allow low-level signals to be identified, if not quantified. The new SAPPHIRE method, in contrast, gives essentially complete suppression of sidebands.
14 This method has not yet found significant application, perhaps because it only reduces the peak amplitude of sidebands, but can in favourable cases give sufficient improvement to allow low-level signals to be identified, if not quantified. The new SAPPHIRE method, in contrast, gives essentially complete suppression of sidebands.
Here, we propose a different method that can completely suppress sidebands, by manipulating the phase of the residual modulation through small (ms) timing changes. The classic Zangger–Sterk sequence8 in the commonly used form13 of Fig. S1 in the ESI† refocuses J modulation at a time 1/(2sw1) after the onset of acquisition, at the midpoint of the chunk of data acquired. Suppose that two experiments are carried out, one with the conventional timing and one in which J is refocused immediately before acquisition. If the results are averaged, the sum of the two modulation patterns will give a pattern in which the peak-to-peak modulation has been reduced and in which the periodicity has halved, from 1/sw1 to 1/(2sw1). Fourier transformation of the averaged signal will then yield a spectrum in which the first sidebands appear at ±2 sw1, rather than ±sw1. As shown in Fig. S8 (ESI†), extending this to four steps will cancel sidebands up to third order, and so on.
Fig. 2 shows a slightly modified sequence that incorporates a brief extra echo to allow the point of J refocusing to be shifted forwards or backwards in time, so that the J modulation phase can be varied over a complete cycle. The net J evolution time τJ is 2(τ1 − τ3), so in the conventional sequence τJ = −1/(2sw1). (There is an analogy here with the suppression of sidebands in bilevel heteronuclear decoupling15). To suppress sidebands to order N − 1, a series of pure shift interferograms is constructed with N different modulation time shifts ΔτJ, and subsequently averaged. In acquiring the first chunk of each interferogram, the delays τ1 are of duration Min(τmin + 1/(4sw1) − ΔτJ/2, τmin), the delays τ3 are of duration Max(τmin − 1/(4sw1) + ΔτJ/2, τmin), τ2 = τmin + 1/(4sw1) − |τ1 − τ3|, and the duration of data acquisition (i.e. the size of the first chunk) is 1/sw1 − ΔτJ, where τmin is the minimum time needed for a gradient pulse and subsequent recovery, and ΔτJ is incremented from 0 to (N − 1)/(Nsw1) in steps of 1/(Nsw1). Subsequent chunks of data in each interferogram are acquired with τ1 = τmin + 1/(4sw1) and τ3 = τmin, but with the evolution time t1 reduced by ΔτJ to allow for the duration of the first chunk acquired, and τ2 = τmin + ΔτJ/2. In this way all N interferograms experience the same T2 weighting. The process is summarised in Fig. S6 of the ESI,† which shows the 8 different modulation patterns for N = 8. The corresponding experimental data are shown in Fig. 3a to h; averaging the 8 spectra leads to a clean spectrum with sidebands suppressed to order 7. Since the sidebands decrease rapidly in amplitude with increasing order,16 it is rarely necessary to go beyond N = 3.
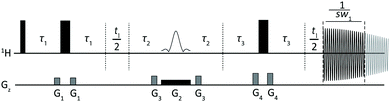 | ||
| Fig. 2 Modified Zangger–Sterk pulse sequence used here for SAPPHIRE acquisition of clean, sideband-free 1H pure shift spectra. Closed narrow and wide rectangles in the 1H trace represent 90° and 180° hard RF pulses respectively, while the shaped pulse represents a soft 180° pulse. The incremented delays for the J-evolution and for the reconstruction of the pure shift interferogram are denoted as τ1/τ3 and t1 respectively. G1, G3 and G4 are CTP field gradient pulses and G2 is a weaker gradient pulse for spatial encoding. Full experimental details are given in the experimental section of the ESI.† | ||
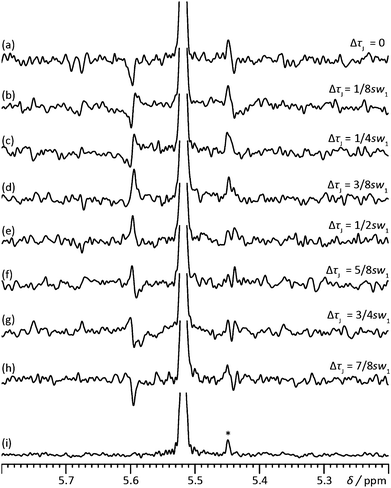 | ||
| Fig. 3 Illustration of the cyclic phase rotation of the sidebands for H6 of rosuvastatin in the sample used for Fig. 1. Here, the right hand first-order side sideband overlaps with a BEM signal (*). ΔτJ indicates the incremented J evolution time. The modulation phase changes in spectra (a) to (h) in steps of +45°, starting from −180°. The sum of spectra (a–h) is shown in (i). | ||
At first sight the new method would appear to be very time-consuming, but in practice this is not the case. The sensitivity of the new method is almost identical to that of the parent experiment, the only difference is a small extra penalty incurred in T2 relaxation during the period 2τ2. To acquire a pure shift spectrum in which chunking sidebands are visible above the noise will almost always require significant time averaging, so the minimum experiment duration is unchanged, and even the simplest possible implementation (a 2-step SAPPHIRE suppression) will yield 90% of the benefit (Fig. S8, ESI†). Apart from the very small extra T2 weighting, under high resolution conditions the quantitative character of SAPPHIRE is essentially the same as that of the parent pure shift method (Zangger–Sterk in the example shown): the intensity of the centreband is unaffected by the suppression of the sidebands.
The extra echo in Fig. 2 can be dispensed with if desired, reverting to the basic framework of the standard Zangger–Sterk sequence of Fig. S1 (ESI†). Instead of respectively decrementing and incrementing the delays τ1 and τ3 of the sequence of Fig. 2, the delay τ1 of Fig. S1 (ESI†) is decremented until it reaches zero, at which point the hard 180° pulse echo and the soft pulse echo are interchanged and τ1 is incremented until the modulation cycle is complete. If the sequence of Fig. S1 (ESI†) is used it is necessary to adjust the phase of the soft 180° pulse to match exactly that of the hard 180° pulse, in order to avoid a phase discontinuity between the two sequence variants.8
The results presented here were acquired using the original Zangger–Sterk J-refocusing method. Exactly the same approach can be used with other J-refocusing sequence elements such as PSYCHE11 or band-selection9,17 as shown in Fig. S10 and S11 of the ESI.† The logic of the SAPPHIRE method should also be independent of whether data are acquired in interferogram or real-time mode.
In direct-domain pure shift experiments there is a tension between sensitivity and spectral purity when choosing the parameter sw1, which determines the data chunk duration 1/sw1. Residual J modulation sidebands increase rapidly as sw1 decreases, so it is usual to choose a value of sw1 large compared with the maximum multiplet width of interest. Practical experiments typically use sw1 values of the same order as the 39 Hz used for Fig. 1 and 3. With the SAPPHIRE method it is possible to reduce sw1 substantially, to values that would normally give rise to unacceptably strong chunking sidebands, as illustrated by the results in Fig. S9 of the ESI† with sw1 values of 10 and 20 Hz. At first sight it is very tempting to make use of the two- or four-fold time saving this would allow. Unfortunately while the sidebands are very efficiently suppressed, a price is paid for the time saving in distorted signal intensities, wide multiplets giving slightly weaker pure shift singlets.
One welcome by-product of chunking sideband suppression is a reduction in strong coupling artefacts. All pure shift methods fail to a greater or lesser extent when spins are strongly coupled.18 Where this failure leads to signal discontinuities between data chunks, the artefacts that result are periodic, as for example around 2 ppm in Fig. 1b and c and in Fig. S12 of the ESI.† SAPPHIRE averaging suppresses these periodic artefacts, leading to the much cleaner result in Fig. 1d. A further improvement could result if this is combined with the recently-published triple spin echo PSYCHE sequence.19
Over 70 papers have been published to date on direct-domain pure shift methods. In every case, the use of chunked data acquisition, whether in real time or in pseudo-2D (interferogram) mode, leads to periodic artefacts. These chunking sidebands can cause significant problems in the analysis of low-level components of mixtures (and indeed of other low-level signals such as those of end groups and branch points in polymers). The novel approach introduced here offers the possibility of acquiring ultraclean, high dynamic range 1H pure shift spectra by suppressing these artefacts, whether in interferogram or in real-time pure shift experiments, without affecting the quantitative character of the measurement.
This work was supported by AstraZeneca and by the Engineering and Physical Sciences Research Council (grant numbers EP/M013820 and EP/N033949). All raw experimental data, pulse sequence code and processing software can be downloaded from DOI: http://10.15127/1.309229.
Notes and references
- R. W. Adams, eMagRes, John Wiley & Sons, Ltd, 2007 DOI:10.1002/9780470034590.emrstm1362.
- L. Castañar and T. Parella, Magn. Reson. Chem., 2015, 53, 399–426 CrossRef PubMed.
- K. Zangger, Prog. Nucl. Magn. Reson. Spectrosc., 2015, 86–87, 1–20 CrossRef CAS PubMed.
- G. Dal Poggetto, L. Castañar, G. A. Morris and M. Nilsson, RSC Adv., 2016, 6, 100063 RSC.
- M. Foroozandeh, L. Castañar, L. G. Martins, D. Sinnaeve, G. Dal Poggetto, C. F. Tormena, R. W. Adams, G. A. Morris and M. Nilsson, Angew. Chem., Int. Ed., 2016, 55, 15579–15582 CrossRef CAS PubMed.
- M. Nilsson and G. A. Morris, Chem. Commun., 2007, 933–935, 10.1039/B617761A.
- A. Lupulescu, G. L. Olsen and L. Frydman, J. Magn. Reson., 2012, 218, 141–146 CrossRef CAS PubMed.
- K. Zangger and H. Sterk, J. Magn. Reson., 1997, 124, 486–489 CrossRef CAS.
- R. W. Adams, L. Byrne, P. Kiraly, M. Foroozandeh, L. Paudel, M. Nilsson, J. Clayden and G. A. Morris, Chem. Commun., 2014, 50, 2512–2514 RSC.
- L. Castañar, P. Nolis, A. Virgili and T. Parella, Chem. – Eur. J., 2013, 19, 15472–15475 CrossRef PubMed.
- M. Foroozandeh, R. W. Adams, N. J. Meharry, D. Jeannerat, M. Nilsson and G. A. Morris, Angew. Chem., Int. Ed., 2014, 53, 6990–6992 CrossRef CAS PubMed.
- A. J. Pell, R. A. E. Edden and J. Keeler, Magn. Reson. Chem., 2007, 45, 296–316 CrossRef CAS PubMed.
- J. A. Aguilar, S. Faulkner, M. Nilsson and G. A. Morris, Angew. Chem., Int. Ed., 2010, 49, 3901–3903 CrossRef CAS PubMed.
- J. Mauhart, S. Glanzer, P. Sakhaii, W. Bermel and K. Zangger, J. Magn. Reson., 2015, 259, 207–215 CrossRef CAS PubMed.
- E. Kupče, R. Freeman, G. Wider and K. Wüthrich, J. Magn. Reson., Ser. A, 1996, 122, 81–84 CrossRef.
- L. Kaltschnee, K. Knoll, V. Schmidts, R. W. Adams, M. Nilsson, G. A. Morris and C. M. Thiele, J. Magn. Reson., 2016, 271, 99–109 CrossRef CAS PubMed.
- L. Castañar, M. Pérez-Trujillo, P. Nolis, E. Monteagudo, A. Virgili and T. Parella, ChemPhysChem, 2014, 15, 854–857 CrossRef PubMed.
- M. J. Thrippleton, R. A. E. Edden and J. Keeler, J. Magn. Reson., 2005, 174, 97–109 CrossRef CAS PubMed.
- M. Foroozandeh, R. W. Adams, P. Kiraly, M. Nilsson and G. A. Morris, Chem. Commun., 2015, 51, 15410–15413 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc04423b |
| This journal is © The Royal Society of Chemistry 2017 |