 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A simple route to complex materials: the synthesis of alkaline earth – transition metal sulfides†
Mundher
Al-Shakban
 a,
Peter D.
Matthews
a,
Peter D.
Matthews
 b and
Paul
O'Brien
b and
Paul
O'Brien
 *ab
*ab
aSchool of Materials, University of Manchester, Oxford Road, Manchester M13 9PL, UK. E-mail: paul.o’brien@manchester.ac.uk; Fax: +44 (0)161 275 4616; Tel: +44 (0)161 275 4653
bSchool of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK
First published on 18th August 2017
Abstract
A simple, low-temperature synthesis of a family of alkaline earth metal chalcogenide thin films is reported. These materials have previously only been produced from demanding, high temperature, high pressure reactions. The decomposition of calcium, barium and copper xanthates leads to the clean formation of CaS, BaS, CaCu2S2, β-BaCu2S2 and β-BaCu4S3.
The synthesis of potentially useful complex materials is a key area for research, and one class of these, metal chalcogenides, are of particular interest to the semiconductor industry. Transition metal chalcogenides have been shown to make promising photovoltaic devices, energy storage, electronics and lubricants.1–8 The introduction of an alkaline earth metal to make a mixed metal chalcogenides produces a broad variety of structures with distinctive properties.9–15
Metal chalcogenides often provide significant synthetic challenges and syntheses often require very high temperatures and/or pressures. Synthetic routes that involve more benign conditions will represent a major step forward. The mixed alkaline earth/transition metal compound CaCu2S2 has previously only been synthesized by a challenging ammonothermal process,16 whilst BaCu2S2 has been reported via a hydrothermal method or in a flux of potassium thiocyanate.17,18 Here we report a much simpler approach making use of metal xanthates in melt reactions.
There are numerous ways to deposit thin films of metal chalcogenides, though it is undoubtedly true that the simpler the better if industrial applications are to be realised. We have previously discussed chemical bath deposition (CBD),19,20 as well as aerosol-assisted chemical vapour deposition (AA-CVD)21,22 as being more general than metal–organic (MO-CVD) or other CVD techniques, as the precursor does not have to be volatile, which is a requirement for MO-CVD.23,24 Mitzi has previously proposed ‘dimensional reduction’, which involves dismantling an extended metal–anion framework by reacting it with an ionic reagent resulting in dissolution. The resulting solution may be deposited and annealed to generate a thin film. This method results in high quality films,25–27 but also relies extensively on toxic hydrazine, limiting its usefulness.7 The method that we have applied here is simpler in its elegance – we have spin coated single source precursors onto a substrate (a method that can be extended to any type of material, or size of substrate), and thermally decomposed it to generate clean metal sulfides.28,29
We, and others, have previously described the use of metal xanthates [M(S2COR)x] (M = transition metal, R = alkyl chain) to generate nanostructured metal sufides via AA-CVD, melt reactions and hot-injection syntheses.29–39 Metal xanthates are a good choice as single-source precursors to metal sulfides, as they decompose cleanly at low temperature. One potential decomposition pathway is the Chugaev elimination mechanism (Scheme 1), from which the only by-products are gases that are readily removed from the reaction system.40
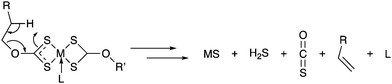 | ||
| Scheme 1 A generalised breakdown mechanism proposal for the xanthate precursors. M = Ca, Ba or Cu, L = iPrOH or PPh3, R = iPr or 2-methoxyethyl. | ||
We report here the simple, low-temperature synthesis of AS, ACu2S2 and ACu4S3 (A = Ca or Ba; Fig. 1) from [Ca(S2COiPr)2(iPrOH)3] (1) [Ba(S2COiPr)2] (2) and [(PPh3)2Cu(S2CO(CH2)2OMe)] (3). The calcium and copper xanthates were synthesized according to literature methods,41,42 whilst we report a novel barium xanthate (see ESI,† for full details).
 | ||
| Fig. 1 Schematic drawing of the unit cells of (a) CaCu2S2, (b) β-BaCu2S2 and (c) β-BaCu4S3. Teal = Ca, brown = Ba, blue = Cu, yellow = S. | ||
The Ca and Ba xanthates were synthesized from the insertion of CS2 into the metal–alkoxide bond, according to the procedure of Bezougli et al.41 (eqn (1)). The Cu xanthate was prepared from CuCl, PPh3 and potassium 2-methoxyethylxanthate by a method that we have previously reported.42
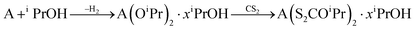 | (1) |
An assessment of the thermogravimetric profile of 1, 2 and 3 (ESI,† Fig. S1) suggests that the complexes breakdown cleanly in the region of 225–250 °C and 100–125 °C respectively. The final weight of the residue is in good agreement with the formation of CaS, BaS and CuS respectively. The suggested breakdown pathway is shown in Scheme 1.
Thin films of the metal sulfides were prepared by spin-coating a solution of the precursor xanthates in dry THF onto a clean glass slide, followed by thermolysis in a tube furnace under an inert nitrogen atmosphere (ESI,† Table S1).
1 decomposes cleanly at 300 °C to form cubic CaS, with the grazing incidence X-ray diffraction (GIXRD) pattern agreeing well with phase formed being that of oldhamite (JCPDS 00-08-0464, Fig. 2a). The unit cell of a = 5.699(2) Å is a close match to the literature (a = 5.694 Å). The decomposition of the barium xanthate 2 requires higher temperatures to form a pure phase. At 550 °C, cubic BaS is formed (Fig. 2b) – the unit cell is a = 6.392(6) Å, which is a good match with the literature (a = 6.388 Å). CaS and BaS both adopt the cubic structure of NaCl. 3 forms orthorhombic chalcocite Cu1.73S (JCPDS 00-009-0328, ESI,† Fig. S3) at 300 °C, with a unit cell of a = 11.810(1) Å, b = 27.020(7) Å and c = 13.435(1) Å.
 | ||
| Fig. 2 The grazing incidence X-ray diffraction (GIXRD) patterns of the thin films. (a) cubic CaS and reference pattern JCPDS 00-008-0464, (b) cubic BaS and reference pattern JCPDS 04-001-3579, (c) hexagonal CaCu2S2 and reference pattern generated from Purdy's work,16 (d) β-BaCu2S2 with reference pattern JCPDS 04-008-2860 and (e) orthorhombic β-BaCu4S3 and reference pattern generated from Iglesias's work.43 | ||
In order to generate the mixed alkaline earth/transition metal compounds the appropriate molar ratios of 1/2 and 3 were dissolved in dry THF and spin coated onto glass slides and annealed in a N2 atmosphere. For the Ca–Cu system an annealing temperature of 300 °C was used. The GIXRD pattern of the obtained films matches that of hexagonal CaCu2S2 as reported by Purdy16 (Fig. 2c) and the unit cell parameters of a = 3.949(3) Å, c = 6.520(3) Å are consistent with that data.16 Comparing the Raman spectra of this film with that of Cu1.73S (the structure produced from the decomposition of 3) indicates that there is no chalcocite contamination of the calcium copper sulfide film (ESI,† Fig. S10). In the CaCu2S2 structure, planes of double layer, puckered six member Cu–S rings are separated by Ca2+ (Fig. 1a). Energy-dispersive X-ray spectroscopy (EDX) analysis gives a formula consistent with that of CaCu2S2 and EDX mapping reveals a homogenous dispersion of the elements (ESI,† Fig. S5 and Table S4).
Unlike CaCu2S2, which exists as only one phase, there are two phases for BaCu2S2: an α (orthorhombic) and a β (tetragonal) phase. We find at 550 °C, the temperature required to breakdown 2, that we form a mixture of α- and β-BaCu2S2 from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3
1 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (ESI,† Fig. S2). Increasing the temperature to 650 °C leads to a loss of the orthorhombic phase, leaving pure tetragonal β-BaCu2S2 (Fig. 2d, JCPDS 04-008-2860) with a unit cell of a = 3.907(3) Å, c = 12.648(3) Å (literature values: a = 3.907 Å, c = 12.640 Å). Note that in this case we switched to a silicon substrate to reflect the high temperature of deposition. Compositional analysis by EDX indicates an appropriate ratio of Ba
2 (ESI,† Fig. S2). Increasing the temperature to 650 °C leads to a loss of the orthorhombic phase, leaving pure tetragonal β-BaCu2S2 (Fig. 2d, JCPDS 04-008-2860) with a unit cell of a = 3.907(3) Å, c = 12.648(3) Å (literature values: a = 3.907 Å, c = 12.640 Å). Note that in this case we switched to a silicon substrate to reflect the high temperature of deposition. Compositional analysis by EDX indicates an appropriate ratio of Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, which is evenly distributed across the sample (ESI,† Fig. S6 and Table S4).
S, which is evenly distributed across the sample (ESI,† Fig. S6 and Table S4).
CaCu2S2 is the only known calcium copper sulfide, but for the barium family there is another: BaCu4S3. This also has an α and β phase, though in this case they are both orthorhombic.44 The structure consists of BaS6 triangular face sharing trigonal prisms (Fig. 1c). The key difference between the α and β phases is that S caps a rectangular face in α-BaCu4S3 and a triangular face in β-BaCu4S3.43,44 Previous work has focused on the synthesis of these materials through a vapour transport method, and Iglesias et al. noted an α to β phase transition at 640 ± 10 °C.43,44 We have successfully synthesized the high temperature phase, β-BaCu4S3, at 580 °C on glass (Fig. 2e). The unit cell of a = 4.053(1) Å, b = 13.848(3) Å and c = 10.377(2) Å agrees with the literature values of a = 4.058 Å, b = 13.863 Å and c = 10.373 Å and the EDX analysis is consistent with the target formula (ESI,† Fig. S7 and Table S4).
It is clear from the GIXRD patterns that there is a broad hump in the CaS, BaS and CaCu2S2 spectra in the 25–30° region. This is due to the glass signal showing through from beneath the film. We have calculated the penetration depth, which is the thickness of the sample contributing to 99% of the diffracted intensity for a given incident angle, for our samples in ESI,† Table S3. The penetration depths for the samples are larger than the thickness of the films (ESI,† Fig. S4), which explains why we see the broad glass signal.
There is little difference in the morphology of the films that can be discerned by scanning electron microscopy (SEM), other than that of CaS (Fig. 3). The alkaline earth metal–copper sulfides consist of conjoined spheres 0.1–0.5 μm in diameter, whereas CaS appears to be much smoother. Optical images of the films (ESI,† Table S1) indicate that the CaS/BaS films are a lighter brown colour, whilst the others are a dark black.
 | ||
| Fig. 3 SEM images of thin films of (a) CaS, (b) BaS, (c) hexagonal CaCu2S2 (d) β-BaCu2S2 and (e) β-BaCu4S3. | ||
The TGA data (ESI,† Fig. S1) indicates that there is unlikely to be much carbon residue left in the films, as the final decomposition percentage is in good agreement with the clean formation of CaS, BaS and CuS. We probed the samples for C inclusion through EDX by depositing the films on a Si substrate (as glass is known to contain carbon) and coated them with an Au/Pt target. The binary systems (CaS, BaS and CuS) all contain ∼0.1 at% C, whereas the ternary barium–copper films contain ∼0.3 at% C (ESI,† Table S5 and Fig. S9). The use of EDX to determine carbon content quantitatively is notoriously difficult, however, these values suggest that only a very small amount of C is included in the final film.
In summary, we have presented a simple, efficient route to three complex alkaline earth metal–copper sulfides; CaCu2S2, β-BaCu2S2 and β-BaCu4S3. These syntheses have been achieved by a melt reaction of calcium isopropylxanthate and bis(triphenylphosphine)copper 2-methoxyethylxanthate with the novel compound barium isopropylxanthate.
The authors would like to acknowledge the Iraqi Culture Attaché in London for financial support (M. A. S.) and the EPSRC (Doctoral Prize for P. D. M., grant EP/M507969/1). Some of the equipment used in this study were provided by the Engineering and Physical Sciences Research Council (Core Capability in Chemistry, EPSRC grant number EP/K039547/1). We thank Prof. D. Michael P. Mingos (University of Oxford) for helpful discussions.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- P. D. Matthews, P. D. McNaughter, D. J. Lewis and P. O’Brien, Chem. Sci., 2017, 8, 4177–4187 RSC.
- K. Woo, Y. Kim and J. Moon, Energy Environ. Sci., 2012, 5, 5340–5345 CAS.
- Y.-C. Wang, D.-Y. Wang, Y.-T. Jiang, H.-A. Chen, C.-C. Chen, K.-C. Ho, H.-L. Chou and C.-W. Chen, Angew. Chem., Int. Ed., 2013, 52, 6694–6698 CrossRef CAS PubMed.
- D. J. Lewis, P. Kevin, O. Bakr, C. A. Muryn, M. A. Malik and P. O’Brien, Inorg. Chem. Front., 2014, 1, 577–598 RSC.
- A. A. Tedstone, D. J. Lewis and P. O’Brien, Chem. Mater., 2016, 28, 1965–1974 CrossRef CAS.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- D. B. Mitzi, Adv. Mater., 2009, 21, 3141–3158 CrossRef CAS.
- S.-L. Li, K. Tsukagoshi, E. Orgiu and P. Samorì, Chem. Soc. Rev., 2015, 45, 118–151 RSC.
- S.-M. Kuo, Y.-M. Chang, I. Chung, J.-I. Jang, B.-H. Her, S.-H. Yang, J. B. Ketterson, M. G. Kanatzidis and K.-F. Hsu, Chem. Mater., 2013, 25, 2427–2433 CrossRef CAS.
- K. Feng, W. Yin, Z. Lin, J. Yao and Y. Wu, Inorg. Chem., 2013, 52, 11503–11508 CrossRef CAS PubMed.
- A. Mesbah, E. Ringe, S. Lebègue, R. P. Van Duyne and J. A. Ibers, Inorg. Chem., 2012, 51, 13390–13395 CrossRef CAS PubMed.
- A. Mesbah, S. Lebègue, J. M. Klingsporn, W. Stojko, R. P. Van Duyne and J. A. Ibers, J. Solid State Chem., 2013, 200, 349–353 CrossRef CAS.
- A. Mesbah, J. Prakasha and J. A. Ibers, Dalton Trans., 2016, 45, 16067–16080 RSC.
- M.-C. Chen, L.-M. Wu, H. Lin, L.-J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 6058–6060 CrossRef CAS PubMed.
- A. Assoud, N. Soheilnia and H. Kleinke, Chem. Mater., 2005, 17, 2255–2261 CrossRef CAS.
- A. P. Purdy, Chem. Mater., 1998, 10, 692–694 CrossRef CAS.
- Z. Ma, F. Weng, Q. Wang, Q. Tang, G. Zhang, C. Zheng, R. P. S. Han and F. Huang, RSC Adv., 2014, 3, 28937–28940 RSC.
- J. Huster and W. Bronger, Z. Anorg. Allg. Chem., 1999, 625, 2033–2040 CrossRef CAS.
- D. S. Boyle, P. O’Brien, D. J. Otway and O. Robbe, J. Mater. Chem., 1999, 9, 725–729 RSC.
- D. S. Boyle, O. Robbe, D. P. Halliday, M. R. Heinrich, A. Bayer, P. O’Brien, D. J. Otway and M. D. G. Potter, J. Mater. Chem., 2000, 10, 2439–2441 RSC.
- D. J. Lewis, A. A. Tedstone, X. L. Zhong, E. A. Lewis, A. Rooney, N. Savjani, J. R. Brent, S. J. Haigh, M. G. Burke, C. A. Muryn, J. M. Raftery, C. Warrens, K. West, S. Gaemers and P. O’Brien, Chem. Mater., 2015, 27, 1367–1374 CrossRef CAS.
- J. Akhtar, M. Afzaal, M. A. Vincent, N. A. Burton, I. H. Hillier and P. O’Brien, Chem. Commun., 2011, 47, 1991–1993 RSC.
- M. A. Malik, M. Afzaal and P. O’Brien, Chem. Rev., 2010, 110, 4417–4446 CrossRef CAS PubMed.
- P. Marchand, I. A. Hassan, I. P. Parkin and C. J. Carmalt, Dalton Trans., 2013, 42, 9406–9422 RSC.
- D. B. Mitzi, L. L. Kosbar, C. E. Murray, M. Copel and A. Afzali, Nature, 2004, 428, 299–303 CrossRef CAS PubMed.
- D. B. Mitzi, M. Copel and C. E. Murray, Adv. Mater., 2006, 18, 2448–2452 CrossRef CAS.
- D. J. Milliron, D. B. Mitzi, M. Copel and C. E. Murray, Chem. Mater., 2006, 18, 581–590 CrossRef.
- M. Al-Shakban, P. D. Matthews, N. Savjani, X. L. Zhong, Y. Wang, M. Missous and P. O’Brien, J. Mater. Sci., 2017, 52, 12761–12771 CrossRef CAS.
- E. A. Lewis, P. D. McNaughter, Z. Yin, Y. Chen, J. R. Brent, S. A. Saah, J. Raftery, J. A. M. Awudza, M. A. Malik, P. O’Brien and S. J. Haigh, Chem. Mater., 2015, 27, 2127–2136 CrossRef CAS.
- M. Afzaal, C. L. Rosenberg, M. A. Malik, A. J. P. White and P. O’Brien, New J. Chem., 2011, 35, 2773 RSC.
- P. S. Nair, T. Radhakrishnan, N. Revaprasadu, G. A. Kolawole and P. O’Brien, J. Mater. Chem., 2003, 22, 3129–3135 Search PubMed.
- M. Afzaal, M. A. Malik and P. O’Brien, J. Mater. Chem., 2010, 20, 4031–4040 RSC.
- E. A. Lewis, S. Haigh and P. O’Brien, J. Mater. Chem. A, 2014, 2, 570–580 CAS.
- P. D. McNaughter, S. A. Saah, M. Akhtar, K. Abdulwahab, M. A. Malik, J. Raftery, J. A. M. Awudza and P. O’Brien, Dalton Trans., 2016, 45, 16345–16353 RSC.
- P. D. Matthews, M. Akhtar, M. A. Malik, N. Revaprasadu and P. O’Brien, Dalton Trans., 2016, 45, 18803–18812 RSC.
- N. Alam, M. S. Hill, G. Kociok-Köhn, M. Zeller, M. Mazhar and K. C. Molloy, Chem. Mater., 2008, 20, 6157–6162 CrossRef CAS.
- J. M. Clark, G. Kociok-Köhn, N. J. Harnett, M. S. Hill, R. Hill, K. C. Molloy, H. Saponia, D. Stanton and A. Sudlow, Dalton Trans., 2011, 40, 6893–6900 RSC.
- T. Lutz, A. MacLachlan, A. Sudlow, J. Nelson, M. S. Hill, K. C. Molloy and S. A. Haque, Phys. Chem. Chem. Phys., 2012, 14, 16192–16196 RSC.
- T. Rath, A. J. MacLachlan, M. D. Brown and S. A. Haque, J. Mater. Chem. A, 2015, 3, 24155–24162 CAS.
- M. A. Buckingham, A. L. Catherall, M. S. Hill, A. L. Johnson and J. D. Parish, Cryst. Growth Des., 2017, 17, 907–912 CAS.
- I. K. Bezougli, A. Bashall, M. McPartlin and D. M. P. Mingos, J. Chem. Soc., Dalton Trans., 1998, 2671–2677 RSC.
- M. Al-Shakban, P. D. Matthews, G. Deogratias, P. D. McNaughter, J. Raftery, I. Vitorica-Yrezabal, E. B. Mubofu and P. O’Brien, Inorg. Chem., 2017, 56, 9247–9254 CrossRef CAS PubMed.
- J. E. Iglesias, K. E. Pachali and H. Steinfink, Mater. Res. Bull., 1972, 7, 1247–1258 CrossRef CAS.
- Z. Eliezer and H. Steinfink, Mater. Res. Bull., 1976, 11, 385–388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, TGA, GIXRD, Raman and SEM/EDX data. See DOI: 10.1039/c7cc05643e |
| This journal is © The Royal Society of Chemistry 2017 |
