Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Prebiotic synthesis of aminooxazoline-5′-phosphates in water by oxidative phosphorylation†
C.
Fernández-García‡
,
N. M.
Grefenstette‡
and
M. W.
Powner
 *
*
Department of Chemistry, UCL, 20 Gordon St., London, WC1H 0AJ, UK. E-mail: matthew.powner@ucl.ac.uk
First published on 5th April 2017
Abstract
RNA is essential to all life on Earth and is the leading candidate for the first biopolymer of life. Aminooxazolines have recently emerged as key prebiotic ribonucleotide precursors, and here we develop a novel strategy for aminooxazoline-5′-phosphate synthesis in water from prebiotic feedstocks. Oxidation of acrolein delivers glycidaldehyde (90%), which directs a regioselective phosphorylation in water and specifically affords 5′-phosphorylated nucleotide precursors in upto 36% yield. We also demonstrated a generational link between proteinogenic amino acids (Met, Glu, Gln) and nucleotide synthesis.
Phosphorylation is a key step in nucleotide synthesis, however, elucidating the chemical origins of biological phosphorylation remains an unmet challenge.1 Biology universally requires nucleotide-5′-phosphates but known prebiotic nucleotide syntheses yield 2′,3′-cyclic phosphates.2 This dichotomy between prebiotic synthesis and biochemical structure warrants investigation of prebiotic nucleotide-5′-phosphate synthesis.
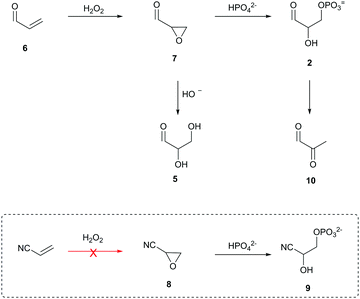
Stepwise nucleotide synthesis from ribose-5-phosphate (1) or glyceraldehyde-3-phosphate (2) furnishes the key intermediate ribose aminooxazoline-5′-phosphate (3) (Scheme 1),3 which can be converted into the corresponding pyrimidine β-ribonucleotides.3,4 However, neither 1 nor 2 are prebiotically plausible substrates.5–7 Ribose (4) is notoriously unstable and is only synthesised as a minor component of complex mixtures.8 Additionally, prebiotic phosphorylations of 4 afford 1-, 2-, or 3-phosphates but not 5-phosphate 1.5 Equally, the highly facile (E1cB) elimination of phosphate hinders the prebiotic synthesis and the utility of 2,6,9 and prebiotic phosphorylation of glyceraldehyde (5) yields the 2-phosphate isomer rather than the desired 3-phosphate isomer 2.5,7 Accordingly, the synthesis of 3 remains a key unmet goal for origins of life research. Therefore, we set out to develop a selective prebiotic synthesis of 3 in water.
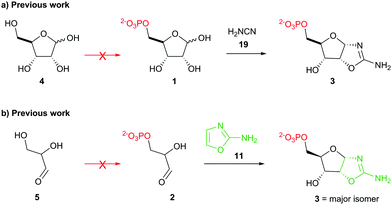 | ||
| Scheme 1 (a) Synthesis of ribose aminooxazoline-5′-phosphate (3) from prebiotically inaccessible (red arrow) pentose-5-phosphate (1).3a (b) Synthesis of 3 from prebiotically inaccessible (red arrow) glyceraldehyde-3-phosphate (2).3b | ||
We recently developed a prebiotically plausible reaction network to access all the key intermediates of the core metabolic pathway of triose glycolysis.7 Importantly our strategy bypassed the unstable aldehyde-3-phosphate 2, which suggested that prebiotic nucleotide-5′-phosphate syntheses employing related strategies should be investigated. Accordingly, we turned our attention to oxidative phosphorylation and hypothesised that the constitutional simplicity and prebiotic versatility of acrolein (6)10,11 presented a novel solution to the phosphorylation of nucleotide precursor 3.
Acrolein (6) is readily oxidised to glycidaldehyde (7) by treatment with hydrogen peroxide12—a prebiotically simple and robust oxidant.7,13 Hydrolysis of 7 yields glyceraldehyde (5) and provides a direct generational link to sugars, but we suspected that the reaction of 7 in phosphate solution would lead to direct nucleophilic phosphorylation and transient access to 2 (Scheme 2). Eschenmoser and co-workers previously reported that oxirane 8 reacts with 2 M phosphate (pH 10.5) to afford glycolaldehyde-2-phosphate cyanohydrin (9) in 70% yield.14 Consequently, we suspected that 7—which, unlike 8, can be facilely prepared under prebiotically plausible conditions—would be an ideal prebiotic substrate to direct phosphorylation in water.
To test our hypothesis, we incubated 6 with hydrogen peroxide (140 mM, pH 8.5) and obtained 7 in 90% yield. We then incubated 7 (250 mM) in 1 M phosphate (room temperature, pH 8.5) and were pleased to observe the formation of 2 (61% by NMR). However, as anticipated, only transient access to 2 was observed followed by rapid elimination to methyl glyoxal (10).
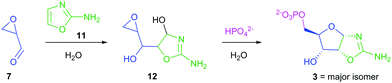
Intrigued by the predisposed chemical activation of 7 towards terminal phosphorylation in water and its simple prebiotically plausible synthesis, we set out to resolve the elimination problem encountered during synthesis of 2. Pleasingly, we found that incubation of 7 (86 mM) and 2-aminooxazole (11; 103 mM)15,16 for 18 h yielded 12 (78%) (Scheme 3).
Compound 12 retains its epoxide moiety, and therefore is an appropriate substrate for 5′-phosphorylation. Indeed, incubation of epoxide 12 in 1 M phosphate (pH 7.0) gave pentose aminooxazoline-5′-phosphate (36% over two steps from 7). This method bypasses both ribose 1 and aldehyde-phosphate 2, and provides a regio- and diastereo-selective prebiotic synthesis of oxazoline 3. This phosphorylation strategy exploits the intrinsic nucleophilicity of inorganic phosphate to achieve 5′-phosphorylation in water.
The diastereoselectivity of the reaction of 7 and 11 was observed to favour the ribo-stereochemistry of 3 (ribo/arabino 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) more strongly than the reaction of 5 with 11 (ribo/arabino 1.1
1) more strongly than the reaction of 5 with 11 (ribo/arabino 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
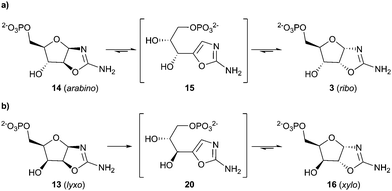
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).15 Additionally, lyxo-13 was not observed during the synthesis of 3 from 7. The propensity of 7 to yield the ribo-configuration was observed upon both alkaline hydrolysis of epoxide 12 at pH 12.0 (ESI,† Fig. S6) and nucleophilic phosphorylation of epoxide 12 at pH 7.0 (ESI,† Fig. S7). However the kinetic ribo selectivity, observed during alkaline hydrolysis, is further augmented by phosphate-catalysed stereochemical inversion of arabino-14 → ribo-3 (Scheme 4) during nucleophilic phosphorylation.17
1).15 Additionally, lyxo-13 was not observed during the synthesis of 3 from 7. The propensity of 7 to yield the ribo-configuration was observed upon both alkaline hydrolysis of epoxide 12 at pH 12.0 (ESI,† Fig. S6) and nucleophilic phosphorylation of epoxide 12 at pH 7.0 (ESI,† Fig. S7). However the kinetic ribo selectivity, observed during alkaline hydrolysis, is further augmented by phosphate-catalysed stereochemical inversion of arabino-14 → ribo-3 (Scheme 4) during nucleophilic phosphorylation.17
Incubation of 14 in 1 M phosphate (40 °C, pH 7.0, 5 d) gave 3 (58%) as the major aminooxazoline product, whereas incubation of 3 returned only a moderate amount of 14 (10%) after 5 d. Furthermore, at room temperature, incubation of 14 yielded 3 (50% after 20 d), whereas under comparable conditions incubation of 3 established an equilibrium with oxazole 15, but not 14, which allowed selective transformation of arabino → ribo stereochemistry.
It is of note that the free sugar ribose (4) isomerises within 13 d at 25 °C, pH 7.0 to yield 75% arabinose, 6% ribulose, and only 19% ribose (4);18 therefore, thermodynamic selection against the ribose is observed in the free sugar, whereas thermodynamic selection for ribo-stereochemistry is observed in aminooxazoline-5′-phosphates. Several groups have suggested mineral stabilisation of 4 under prebiotic constaints,19 therefore it is notable that ribose aminooxazolines are (at least) 70 times more stable than ribose-borate.16,20 These observations suggest that prebiotic nucleotide synthesis pathways that avoid the free sugars will be a key element in elucidating the chemical origins of life.8
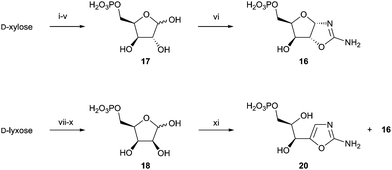
We suspected that the same C2′-epimerisation pattern would be reflected in the lyxo-13 and xylo-16,21 and was responsible for 13 not being observed during our synthesis of pentose aminooxazoline-5′-phosphates. To test this xylose-5-phosphate (17)22 and lyxose-5-phosphate (18) were synthesised (Scheme 5), and their reactivity with cyanamide (19) was investigated. xylo-16 was readily synthesised and incubation in 1 M phosphate (40 °C, pH 7.0) gave oxazole 20 by reversible dehydration, but lyxo-13 was not observed. The reaction of 18 with cyanamide (19) gave a mixture of oxazole 20 and xylo-16, but again lyxo-13 was not observed (ESI,† Fig. S21), which supported the observation that lyxo-13 was not obtained from the reaction of epoxide 12 and phosphate.
Finally, given the close biochemical relatioship between amino acids and nucleotides, we turned our attention to prebiotic amino acid synthesis. Analysis of the proteinogenic amino acids suggested a clear link between glycidaldehyde (7) and the amino acids methionine (Met), glutamic acid (Glu) and glutamine (Gln).
Met, Gln and Glu can be synthesised from 3-(methylthio)propanal (21) and 3-(cyano)propanal (22), which are in turn accessed from 6 by Michael addition of methanethiol (23) or cyanide, respectively (Scheme 6).10,11 Therefore, we incubated 6 (140 mM) in water at room temperature with 23 (saturated, pH 7.0) or potassium cyanide (700 mM, pH 9.2). Pleasingly, we observed smooth conversion of 6 to aldehyde 21 (94%) and the cyanohydrin of aldehyde 22 (95%), respectively. Subsequently, aldehydes 21 and 22 were readily converted to aminonitriles 24 and 25via Strecker reaction in water.
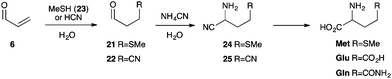 | ||
| Scheme 6 Prebiotic synthesis of amino acids methionine (Met), glutamine (Gln), and glutamic acid (Glu) from acrolein (6). | ||
These studies demonstrate the first prebiotically plausible synthesis of aminooxazoline-5′-phosphates in water by a facile nucleophilic phosphorylation. Importantly, we demonstrate that acrolein (6) provides a generational link between a key nucleotide precursor 3 and proteinogenic amino acids (Met, Gln and Glu), this new generational link supports the unified origins of these two distinct classes of metabolite.
The EPSRC (EP/K004980/1) and the Simons Foundation (318881) supported this work.
References
- (a) F. H. Westheimer, Science, 1987, 235, 1173–1178 CAS; (b) S. C. L. Kamerlin, P. K. Sharma, R. B. Prasad and A. Warshel, Q. Rev. Biophys., 2013, 46, 1–132 CrossRef CAS PubMed.
- (a) L. E. Orgel, Crit. Rev. Biochem. Mol. Biol., 2004, 39, 99–123 CrossRef CAS PubMed; (b) A. W. Schwartz, Philos. Trans. R. Soc. London, Ser. B, 2006, 361, 1743–1749 CrossRef CAS PubMed; (c) M. W. Powner, B. Gerland and J. D. Sutherland, Nature, 2009, 459, 239–242 CrossRef CAS PubMed; (d) K. Ruiz-Mirazo, C. Briones and A. de la Escosura, Chem. Rev., 2014, 114, 285–366 CrossRef CAS PubMed; (e) Y. Furukawa, H.-J. Kim, D. Hutter and S. A. Benner, Astrobiology, 2015, 4, 259–267 CrossRef PubMed; (f) M. Gull, M. A. Mojica, F. M. Fernández, D. A. Gaul, T. M. Orlando, C. L. Liotta and M. A. Pasek, Sci. Rep., 2015, 5, 17198 CrossRef CAS PubMed; (g) J. D. Sutherland, Angew. Chem., Int. Ed., 2016, 55, 104–121 CrossRef CAS PubMed; (h) B. Burcar, M. Pasek, M. Gull, B. J. Cafferty, F. Velasco, N. V. Hud and C. Menor-Salván, Angew. Chem., Int. Ed., 2016, 55, 13249–13253 CrossRef CAS PubMed; (i) J. Xu, M. Tsanakopoulou, C. J. Magnani, R. Szabla, J. E. Šponer, J. Šponer, R. W. Góra and J. D. Sutherland, Nat. Chem., 2017, 9, 303–309 CrossRef PubMed.
- (a) R. A. Sanchez and L. E. Orgel, J. Mol. Biol., 1970, 47, 531–543 CrossRef CAS PubMed; (b) C. Anastasi, M. A. Crowe and J. D. Sutherland, J. Am. Chem. Soc., 2007, 129, 24–25 CrossRef CAS PubMed.
- M. W. Powner, C. Anastasi, M. A. Crowe, A. L. Parkes, J. Raftery and J. D. Sutherland, ChemBioChem, 2007, 8, 1170–1179 CrossRef CAS PubMed.
- (a) M. Halmann, R. A. Sanchez and L. E. Orgel, J. Org. Chem., 1969, 34, 3702–3703 CrossRef CAS; (b) R. Krishnamurthy, S. Guntha and A. Eschenmoser, Angew. Chem., Int. Ed., 2000, 39, 2281–2285 CrossRef CAS.
- (a) J. P. Richard, J. Am. Chem. Soc., 1984, 106, 4926–4936 CrossRef CAS; (b) D. Gauss, B. Schoenenberger and R. Wohlgemuth, Carbohydr. Res., 2014, 389, 18–24 CrossRef CAS PubMed.
- A. J. Coggins and M. W. Powner, Nat. Chem., 2017, 9, 310–317 CrossRef PubMed.
- M. W. Powner, J. D. Sutherland and J. W. Szostak, Synlett, 2011, 1956–1964 CrossRef CAS.
- The stability of 4-3P increases at pH < 5,6b but phosphate (pKa = 1.5, 7.2, and 12.0) nucleophilicity is pH dependent and limited phosphorylation of 7 was observed at pH 4.0–5.0 (7%, 168 h).
- J. E. Van Trump and S. L. Miller, Science, 1972, 178, 859–860 CAS.
- Y. Wolman, W. J. Haverland and S. L. Miller, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 809–811 CrossRef CAS.
- G. B. Payne, J. Am. Chem. Soc., 1959, 81, 4901–4904 CrossRef CAS.
- J. F. Kasting, H. D. Holland and J. R. Pinto, J. Geophys. Res., 1987, 90, 10497–10510 CrossRef.
- S. Pitsch, E. Pombo-Villar and A. Eschenmoser, Helv. Chim. Acta, 1994, 77, 2251–2285 CrossRef CAS.
- C. Anastasi, M. A. Crowe, M. W. Powner and J. D. Sutherland, Angew. Chem., Int. Ed., 2006, 45, 6176–6179 CrossRef CAS PubMed.
- S. Islam, D.-K. Bucar and M. W. Powner, Nat. Chem., 2017 DOI:10.1038/nchem.2703.
- M. W. Powner and J. D. Sutherland, Angew. Chem., Int. Ed., 2010, 49, 4641–4643 CrossRef CAS PubMed.
- Y. B. Tewari and R. N. Goldberg, Biophys. Chem., 1985, 22, 197–204 CrossRef CAS PubMed.
- (a) H.-J. Kim, A. Ricardo, H. I. Illangkoon, M. J. Kim, M. A. Carrigan, F. Frye and S. A. Benner, J. Am. Chem. Soc., 2011, 133, 9457–9468 CrossRef CAS PubMed; (b) Á. Vázquez-Mayagoitia, S. R. Horton, B. G. Sumpter, J. Šponer, J. E. Šponer and M. Fuentes-Cabrera, Astrobiology, 2011, 11, 115–121 CrossRef PubMed; (c) Y. Furukawa, M. Horiuchi and T. Kakegawa, Origins Life Evol. Biospheres, 2013, 43, 353–361 CrossRef PubMed.
- G. Springsteen and G. F. Joyce, J. Am. Chem. Soc., 2004, 126, 9578–9583 CrossRef CAS PubMed.
- N. Saewan, M. A. Crowe, M. Helliwell, J. Raftery, K. Chantrapromma and J. D. Sutherland, Chem. Biodiversity, 2005, 2, 66–83 CAS.
- M. Ahn, F. C. Cochrane, M. L. Patchett and E. J. Parker, Bioorg. Med. Chem., 2008, 16, 9830–9836 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc02183f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |