Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxygen-17 dynamic nuclear polarisation enhanced solid-state NMR spectroscopy at 18.8 T†
Nick J.
Brownbill
a,
David
Gajan
b,
Anne
Lesage
b,
Lyndon
Emsley
c and
Frédéric
Blanc
 *ad
*ad
aDepartment of Chemistry, University of Liverpool, Crown Street, Liverpool, L69 7ZD, UK. E-mail: frederic.blanc@liverpool.ac.uk
bCentre de RMN à Très Hauts Champs, Institut de Sciences Analytiques, Université de Lyon (CNRS/ENS Lyon/UCB Lyon 1), 69100 Villeurbanne, France
cInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
dStephenson Institute for Renewable Energy, University of Liverpool, Crown Street, Liverpool, L69 7ZD, UK
First published on 26th January 2017
Abstract
We report 17O dynamic nuclear polarisation (DNP) enhanced solid-state NMR experiments at 18.8 T. Several formulations were investigated on the Mg(OH)2 compound. A signal enhancement factor of 17 could be obtained when the solid particles were incorporated into a glassy o-terphenyl matrix doped with BDPA using the Overhauser polarisation transfer scheme whilst the cross effect mechanism enabled by TEKPol yielded a slightly lower enhancement but more time efficient data acquisition.
Solid-state nuclear magnetic resonance (NMR) spectroscopy involving quadrupolar nuclei, which account for more than 75% of all active nuclei, has become an essential analytical technique for the atomic-scale characterization of a wide range of inorganic, hybrid and organic materials such as glasses, zeolites, surface catalysts, metal–organic frameworks, battery materials or pharmaceuticals to name but a few.1–4 While for spin I = 1/2 nuclei, the dipolar and chemical shift anisotropy (CSA) interactions are fully averaged out by magic angle spinning (MAS),5 producing sharp lines positioned at the isotropic chemical shifts, the second-order quadrupolar interaction observed for spin I > 1/2 is not fully removed by MAS,6–8 yielding field-dependent shifts and broadened NMR lines. These are some of the major limitations to the widespread applicability of quadrupolar NMR.7–10 The second-order quadrupolar broadening is inversely proportional to the strength of the external field B0 and therefore very high magnetic fields allow high spectral resolution.11 In parallel, a range of NMR experiments have been designed to completely average out the second-order broadenings. They are based on technically challenging NMR probes permitting sample double rotation (DOR)12 or dynamic angle spinning (DAS),13 or consist of combining conventional MAS with the manipulation of the different nuclear spin transitions in techniques such as multiple quantum MAS (MQMAS)14 and satellite transition MAS (STMAS).15 Despite these sophisticated approaches and the increasing availability of high magnetic fields, the sensitivity of quadrupolar NMR remains often low for nuclei of poor natural abundance and/or of low gyromagnetic ratio γ, requiring prohibitively long experimental times (days) and/or expensive isotopic enrichment.
A spectacular approach to increase the solid-state NMR sensitivity is dynamic nuclear polarisation (DNP),16,17 which involves the microwave-driven transfer of the large polarisation of unpaired electrons18–21 (e.g. added to the samples as paramagnetic polarising agents)22–28 to the surrounding nuclei in a glass-forming matrix at cryogenic temperatures, typically 100 K or below.29,30 The drastic signal enhancements permitted by DNP have opened up ground breaking applications on an ever increasing range of systems31–37 and the approach has been reviewed recently.38–42 Recent developments have extended the temperature range in which the experiments can be conducted (>200 K).24,43,44 One of the biggest drawbacks to MAS DNP under high magnetic fields results from the unfavorable evolution of the NMR signal enhancements with B0 which scales as B0−1 and B0−2 for the two most common polarization transfer mechanisms, the cross effect (CE) and solid effect, respectively. However, large signal enhancements (>80) have recently been obtained at 18.8 T using the Overhauser effect (OE) DNP mechanism16,44,45 and the narrow-line 1,3-bisdiphenylene-2-phenylallyl (BDPA) radical.46 The apparent linear scaling of the OE signal enhancement with B0 currently represents one of the most attractive approaches for DNP under very high fields and is an exciting opportunity for quadrupolar nuclei.
A quadrupolar nucleus of particular interest is 17O due to the ubiquity of oxygen in materials chemistry and biochemistry. However, its extremely low natural abundance (0.037%) makes its NMR detection near impossible unless samples are 17O-enriched.47 The feasibility of MAS DNP on 17O has been recently reported.48–52 In particular, we demonstrated that high S/N natural abundance 17O cross polarization (CP) MAS NMR spectra could be obtained on nanoparticles,49 while more recently, the detection of 17O DNP NMR spectra of surface hydroxyl sites on mesoporous silica in natural abundance was reported.52 All these experiments were recorded at 9.4 T and relied on the CE mechanism with bTbk23 or TEKPol24 radicals in tetrachloroethane (TCE)53 as a polarising agent.
Here, we show that DNP can be applied to efficiently enhance the 17O MAS NMR signal of Mg(OH)2 in a glassy matrix at 18.8 T, via both the CE and OE mechanisms. We show that CE DNP (using TEKPol/TCE) gives enhancements up to εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 14, and allows for the fast acquisition of the 17O spectrum of Mg(OH)2 at natural abundance. Similarly, using the OE scheme, we demonstrate that polarisation can be transferred from BDPA in a glassy matrix (10% o-terphenyl (OTP), 90% o-terphenyl-d14 (OTP-d14)) to Mg(OH)2 with excellent efficiency, giving enhancements of εO
CP = 14, and allows for the fast acquisition of the 17O spectrum of Mg(OH)2 at natural abundance. Similarly, using the OE scheme, we demonstrate that polarisation can be transferred from BDPA in a glassy matrix (10% o-terphenyl (OTP), 90% o-terphenyl-d14 (OTP-d14)) to Mg(OH)2 with excellent efficiency, giving enhancements of εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 17. Both CP13,54 and PRESTO-II55 (herein referred to as PRESTO) experiments are reported.
CP = 17. Both CP13,54 and PRESTO-II55 (herein referred to as PRESTO) experiments are reported.
Fig. 1a shows the 18.8 T DNP enhanced 17O MAS NMR spectrum of 17O enriched Mg(OH)2 (i.e. Mg(17OH)2), impregnated with TEKPol in TCE under the CE condition at ν0(1H) = 800.130 MHz. The 1H enhancement obtained on TCE (εH = 23) is transferred to the protons of Mg(17OH)2via spin diffusion and subsequently to the 17O nuclei via a selective CP Hahn echo to yield a maximum signal enhancement of εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 14. In an attempt to be more representative in evaluating the increase in the NMR signal with DNP, we have estimated an overall DNP gain
CP = 14. In an attempt to be more representative in evaluating the increase in the NMR signal with DNP, we have estimated an overall DNP gain 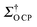 (see Section S2 of the ESI† for calculation details)29,41,56–58 to compare the benefit of 18.8 T DNP with standard NMR at 18.8 and 9.4 T. The εO
(see Section S2 of the ESI† for calculation details)29,41,56–58 to compare the benefit of 18.8 T DNP with standard NMR at 18.8 and 9.4 T. The εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 14 of TEKPol in a TCE matrix translates into a
CP = 14 of TEKPol in a TCE matrix translates into a 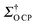 of 72 at 18.8 T (Table 1), demonstrating substantial CE DNP efficiency despite the B0−1 dependency of the CE. It is worth noting that on freshly prepared samples lower enhancements were observed (εH = 16, εO
of 72 at 18.8 T (Table 1), demonstrating substantial CE DNP efficiency despite the B0−1 dependency of the CE. It is worth noting that on freshly prepared samples lower enhancements were observed (εH = 16, εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 9) and that the maximum values reported above were obtained after holding the sample at 253 K for 20 h before freeze–thaw cycling (see the ESI†) and inserting it into the probe (Fig. 1 and Fig. S4, ESI†).
CP = 9) and that the maximum values reported above were obtained after holding the sample at 253 K for 20 h before freeze–thaw cycling (see the ESI†) and inserting it into the probe (Fig. 1 and Fig. S4, ESI†).
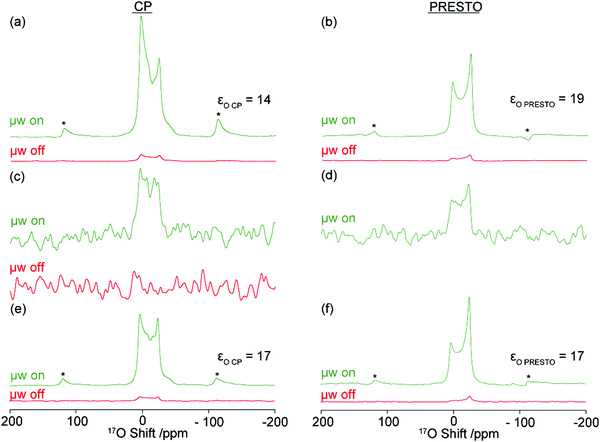 | ||
Fig. 1 (a–d) 17O CE and (e and f) 17O OE MAS DNP at 18.8 T. CP (left column) and PRESTO (right column) spectra of Mg(17OH)2 (a, b, e and f) and natural abundance Mg(OH)2 (c and d) have been recorded at ν0(1H) = 800.130 MHz for the CE and at ν0(1H) = 800.215 MHz for the OE with microwave irradiation (μw) at ν0(e−) = 527 GHz (green) at T = 131 K and without μw (red) at T = 115 K. For the CE, both samples were impregnated with 16 mM TEKPol in TCE and paramagnetic O2 removed by freeze–thaw cycles (see Section S1 in the ESI† for details). For the OE, the sample was prepared with a matrix containing 1.4 wt% BPDA in 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 OTP-d14 10 OTP-d14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OTP and five freeze–melt cycles (see the ESI†). Spectra (a and b), (c and d) and (e and f) are plotted in absolute intensity. The μw off PRESTO spectrum of Mg(OH)2 was not recorded. Asterisks (*) denote spinning sidebands. OTP and five freeze–melt cycles (see the ESI†). Spectra (a and b), (c and d) and (e and f) are plotted in absolute intensity. The μw off PRESTO spectrum of Mg(OH)2 was not recorded. Asterisks (*) denote spinning sidebands. | ||
It was recently shown that the use of a PRESTO (phase-shifted recoupling effects a smooth transfer of order) sequence55 is more efficient for the 1H–17O heteronuclear polarization transfer and yields for Mg(OH)2, Ca(OH)2 and silica surfaces52 line-shapes closer to simulations than with CP, including those in the context of MAS DNP.59Fig. 1b shows the corresponding CE DNP 17O PRESTO spectra on the same Mg(17OH)2 sample at 18.8 T. A maximum signal enhancement of εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PRESTO = 19 was obtained and is slightly higher than εO
PRESTO = 19 was obtained and is slightly higher than εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP (14). However, in our hands and at 18.8 T, the overall signal intensity is lower than with 17O CP as predicted.55 We also note that the asymmetric line shape and dephased spinning side bands are exacerbated by the addition of microwave irradiation (Fig. S3, ESI†). The lower intensity of PRESTO vs. CP and line shape disparity is also observed using the OE (Fig. 1e and f). The 17O signal enhancement factors obtained open the way to obtain natural abundance spectra using the CE at 18.8 T, and we were able to record 17O CP and PRESTO MAS NMR spectra of Mg(OH)2 relatively quickly in 82 minutes (Fig. 1c and d), while the corresponding microwave off spectra show no signal (Fig. 1c).
CP (14). However, in our hands and at 18.8 T, the overall signal intensity is lower than with 17O CP as predicted.55 We also note that the asymmetric line shape and dephased spinning side bands are exacerbated by the addition of microwave irradiation (Fig. S3, ESI†). The lower intensity of PRESTO vs. CP and line shape disparity is also observed using the OE (Fig. 1e and f). The 17O signal enhancement factors obtained open the way to obtain natural abundance spectra using the CE at 18.8 T, and we were able to record 17O CP and PRESTO MAS NMR spectra of Mg(OH)2 relatively quickly in 82 minutes (Fig. 1c and d), while the corresponding microwave off spectra show no signal (Fig. 1c).
Sample formulation is essential to achieve high DNP enhancement factors, and in particular the choice of the solvent is often critical.60 It has been shown that OTP forms a highly effective glassy matrix61 and could be polarized by CE DNP with TEKPol and even more efficiently by OE DNP with BDPA.44,45,61,62 In the next paragraphs, we report 17O enhancement factors using OTP with both TEKPol and BDPA as the polarising matrix. The Mg(17OH)2 samples were prepared by grinding them with a mixture of protonated and fully deuterated OTP of various ratios containing TEKPol or BDPA radicals (1.3–1.4 wt% equivalent to 34 mM electron spins) followed by multiple cycles (typically 5) between a melt at ∼343 K61 and a frozen state at 77 K (in liquid N2) before the melt was inserted into the precooled NMR probe at ∼115 K (see the ESI,† Section S1, for detailed sample preparation).
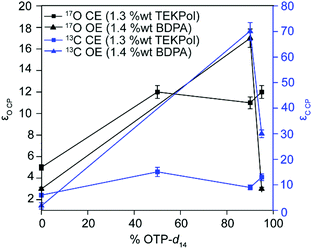
Fig. 2 plots the 13C and 17O CPMAS CE DNP signal enhancements of the Mg(17OH)2/TEKPol/OTP glass matrix as a function of the percentage of OTP-d14. The 13C and 17O enhancements correspond to the NMR signal amplification of, respectively, the OTP matrix and the Mg(17OH)2 particles. The data show that with a fully protonated OTP matrix, the signal enhancements are similar (εC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 8 and εO
CP = 8 and εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 6), revealing that the polarisation is efficiently transferred from the glassy matrix to Mg(17OH)2 and that the polarisation is evenly distributed across the particles by 1H–1H spin diffusion mechanisms.63 However, the enhancements are lower than with >50% OTP-d14 matrices (vide infra) and are due to both the large size of the 1H bath and the short 1H polarisation time τDNP (∼3 s), yielding a fast decay of the enhanced polarization by relaxation before it can be transferred to Mg(17OH)2. Increasing the content of OTP-d14 in the matrix improves the signal enhancements of the sample (Table 1 and Fig. S2, ESI†), in agreement with the previous literature showing that deuteration improves DNP enhancements,60,64 due to the decrease in 1H–1H spin diffusion and associated longer 1H τDNP (∼31 s). This increase in enhancement plateaued by εO
CP = 6), revealing that the polarisation is efficiently transferred from the glassy matrix to Mg(17OH)2 and that the polarisation is evenly distributed across the particles by 1H–1H spin diffusion mechanisms.63 However, the enhancements are lower than with >50% OTP-d14 matrices (vide infra) and are due to both the large size of the 1H bath and the short 1H polarisation time τDNP (∼3 s), yielding a fast decay of the enhanced polarization by relaxation before it can be transferred to Mg(17OH)2. Increasing the content of OTP-d14 in the matrix improves the signal enhancements of the sample (Table 1 and Fig. S2, ESI†), in agreement with the previous literature showing that deuteration improves DNP enhancements,60,64 due to the decrease in 1H–1H spin diffusion and associated longer 1H τDNP (∼31 s). This increase in enhancement plateaued by εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP after 50% OTP-d14 and dropped off beyond 90% OTP-d14 in
CP after 50% OTP-d14 and dropped off beyond 90% OTP-d14 in 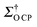 , suggesting that for the CE with TEKPol/OTP at 18.8 T, a 90
, suggesting that for the CE with TEKPol/OTP at 18.8 T, a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixture of OTP-d14 and OTP may be optimal for the signal to noise ratio per unit time per unit weight.
10 mixture of OTP-d14 and OTP may be optimal for the signal to noise ratio per unit time per unit weight.
Fig. 2 also displays the 13C and 17O DNP signal enhancements under the OE condition of Mg(17OH)2 prepared in an OTP matrix (with various concentrations of OTP-d14) and using monoradical BDPA as a polarising agent, providing a comparison between CE and OE mechanisms at 18.8 T. At 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 OTP-d14
10 OTP-d14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OTP, large matrix enhancements are observed (εH = 44, εC
OTP, large matrix enhancements are observed (εH = 44, εC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 71), and subsequently the 17O enhancement of Mg(17OH)2 (εO
CP = 71), and subsequently the 17O enhancement of Mg(17OH)2 (εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 17, Fig. 1e and 2) is substantially larger than with BDPA in a TCE matrix (εO
CP = 17, Fig. 1e and 2) is substantially larger than with BDPA in a TCE matrix (εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 5, Table 1 and Fig. S5, ESI†). The multiple freeze–melt cycles appear to improve the quality of the glassy matrix, and thus improve the enhancement values on the OTP matrix and sample, compared to directly inserting the sample into the probe (Fig. S5, ESI†). Contrary to the results observed under the CE condition with TEKPol, a further increase in OTP-d14 concentration to 95% yields a decrease in matrix enhancement factors (εH = 21, εC
CP = 5, Table 1 and Fig. S5, ESI†). The multiple freeze–melt cycles appear to improve the quality of the glassy matrix, and thus improve the enhancement values on the OTP matrix and sample, compared to directly inserting the sample into the probe (Fig. S5, ESI†). Contrary to the results observed under the CE condition with TEKPol, a further increase in OTP-d14 concentration to 95% yields a decrease in matrix enhancement factors (εH = 21, εC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP = 30), and very limited transfer of polarisation to the 17O of Mg(17OH)2.
CP = 30), and very limited transfer of polarisation to the 17O of Mg(17OH)2.
Despite the maximum εO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP reported in Table 1 being for Mg(17OH)2/BDPA/90
CP reported in Table 1 being for Mg(17OH)2/BDPA/90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 OTP-d14
10 OTP-d14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OTP, the corresponding
OTP, the corresponding 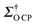 value is still lower than the maximum
value is still lower than the maximum 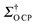 values for 17O obtained with the CE (using the same matrix). This is due to the much shorter τDNP in the CE system (∼11 s) than that of the OE in OTP-d14 (∼31 s), allowing for more scans to be accumulated per unit time.
values for 17O obtained with the CE (using the same matrix). This is due to the much shorter τDNP in the CE system (∼11 s) than that of the OE in OTP-d14 (∼31 s), allowing for more scans to be accumulated per unit time.
In conclusion, we have shown that it is possible to transfer the OE DNP enhanced polarisation of OTP doped with BDPA to the 17O spins of Mg(17OH)2 hydroxides at 18.8 T with good efficiency by mixing both solids together. We have also demonstrated that despite the lower ε values obtained with the CE, TEKPol/OTP and TEKPol/TCE provide time efficient signal enhancement. This has enabled a large gain in absolute sensitivity, permitting the challenging detection of natural abundance 17O NMR spectra. This study paves the way to a wider application of 17O DNP enhanced NMR under a high magnetic field and its transposition to other quadrupolar nuclei in a variety of crystalline and amorphous inorganic materials with strong second-order quadrupolar broadenings hampering the spectral resolution under a low field.
Financial support from the EPSRC for a DTA studentship for N. J. B. and grant EP/M00869X/1 to F. B., ERC Advanced Grant No. 320860 for L. E. and EQUIPEX contract ANR-10-EQPX-47-01 for A. L. and L. E. is acknowledged. We thank Dr Sachin R. Chaudhari (CRMN Lyon), Kenneth K. Inglis (University of Liverpool), and Dr O. Ouari and Prof. P. Tordo (Aix-Marseille Université, CNRS) for valuable technical assistance, sample preparation, and radicals, respectively. F. B. thanks the TGIR-RMN-THC Fr3050 CNRS for access to the 18.8 T DNP NMR facility at the CRMN. The experimental data are provided as a supporting dataset from the University of Liverpool Data Catalogue portal at http://datacat.liverpool.ac.uk/245/.
Notes and references
- D. D. Laws, H.-M. L. Bitter and A. Jerschow, Angew. Chem., Int. Ed., 2002, 41, 3096 CrossRef CAS PubMed.
- F. Blanc, C. Copéret, A. Lesage and L. Emsley, Chem. Soc. Rev., 2008, 37, 518 RSC.
- S. P. Brown and H. W. Spiess, Chem. Rev., 2001, 101, 4125 CrossRef CAS PubMed.
- J. Gath, G. L. Hoaston, R. L. Vold, R. Berthoud, C. Copéret, M. Grellier, S. Sabo-Etienne, A. Lesage and L. Emsley, Phys. Chem. Chem. Phys., 2009, 11, 6962 RSC.
- E. R. Andrew, A. Bradbury and R. G. Eades, Nature, 1958, 182, 1659 CrossRef CAS.
- M. E. Smith and E. R. H. van Eck, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 159 CrossRef CAS.
- S. E. Ashbrook, Phys. Chem. Chem. Phys., 2009, 11, 6892 RSC.
- NMR of Quadrupolar Nuclei in Solid Materials, ed. R. E. Wasylishen, S. E. Ashbrook and S. Wimperis, Wiley, 2012, pp. 3–17 Search PubMed.
- S. E. Ashbrook and D. M. Dawson, Acc. Chem. Res., 2013, 46, 1964 CrossRef CAS PubMed.
- S. E. Ashbrook and S. Sneddon, J. Am. Chem. Soc., 2014, 136, 15440 CrossRef CAS PubMed.
- Z. Gan, P. Gor'kov, T. A. Cross, A. Samoson and D. Massiot, J. Am. Chem. Soc., 2002, 124, 5634 CrossRef CAS PubMed.
- A. Samoson, E. Lippmaa and A. Pines, Mol. Phys., 1988, 65, 1013 CrossRef CAS.
- K. T. Mueller, B. Q. Sun, G. C. Chingas, J. W. Zwanziger, T. Terao and A. Pines, J. Magn. Reson., 1990, 86, 470 CAS.
- A. Medek, J. S. Harwood and L. Frydman, J. Am. Chem. Soc., 1995, 117, 12779 CrossRef CAS.
- Z. Gan, J. Am. Chem. Soc., 2000, 122, 3242 CrossRef CAS.
- A. W. Overhauser, Phys. Rev., 1953, 92, 411 CrossRef CAS.
- T. R. Carver and C. P. Slichter, Phys. Rev., 1956, 102, 975 CrossRef CAS.
- D. A. Hall, D. C. Maus, G. J. Gerfen, S. J. Inati, L. R. Becerra, F. W. Dahlquist and R. G. Griffin, Science, 1997, 276, 930 CrossRef CAS PubMed.
- T. Maly, G. T. Debelouchina, V. S. Bajaj, K.-N. Hu, C.-G. Joo, M. L. Mak-Jurkauskas, J. R. Sirigiri, P. C. A. van der Wel, J. Herzfeld, R. J. Temkin and R. G. Griffin, J. Chem. Phys., 2008, 128, 52211 CrossRef PubMed.
- M. Rosay, L. Tometich, S. Pawsey, R. Bader, R. Schauwecker, M. Blank, P. M. Borchard, S. R. Cauffman, K. L. Felch, R. T. Weber, R. J. Temkin, R. G. Griffin and W. E. Maas, Phys. Chem. Chem. Phys., 2010, 12, 5850 RSC.
- M. Rosay, M. Blank and F. Engelke, J. Magn. Reson., 2016, 264, 88 CrossRef CAS PubMed.
- K.-N. Hu, Solid State Nucl. Magn. Reson., 2011, 40, 31 CrossRef CAS PubMed.
- Y. Matsuki, T. Maly, O. Ouari, H. Karoui, F. Le Moigne, E. Rizzato, S. Lyubenova, J. Herzfeld, T. Prisner, P. Tordo and R. G. Griffin, Angew. Chem., Int. Ed., 2009, 48, 4996 CrossRef CAS PubMed.
- A. Zagdoun, G. Casano, O. Ouari, M. Schwarzwälder, A. J. Rossini, F. Aussenac, M. Yulikov, G. Jeschke, C. Copéret, A. Lesage, P. Tordo and L. Emsley, J. Am. Chem. Soc., 2013, 135, 12790 CrossRef CAS PubMed.
- C. Sauvée, M. Rosay, G. Casano, F. Aussenac, R. T. Weber, O. Ouari and P. Tordo, Angew. Chem., Int. Ed., 2013, 125, 11058 CrossRef.
- B. Corzilius, A. A. Smith, A. B. Barnes, C. Luchinat, I. Bertini and R. G. Griffin, J. Am. Chem. Soc., 2011, 133, 5648 CrossRef CAS PubMed.
- Q. Z. Ni, E. Daviso, T. V. Can, E. Markhasin, S. K. Jawla, T. M. Swager, R. J. Temkin, J. Herzfeld and R. G. Griffin, Acc. Chem. Res., 2013, 46, 1933 CrossRef CAS PubMed.
- D. J. Kubicki, G. Casano, M. Schwarzwälder, S. Abel, C. Sauvée, K. Ganesan, M. Yulikov, A. J. Rossini, G. Jeschke, C. Copéret, A. Lesage, P. Tordo, O. Ouari and L. Emsley, Chem. Sci., 2016, 7, 550 RSC.
- K. R. Thurber, W.-M. Yau and R. Tycko, J. Magn. Reson., 2010, 204, 303 CrossRef CAS PubMed.
- E. Bouleau, P. Saint-Bonnet, F. Mentink-Vigier, H. Takahashi, J.-F. Jacquot, M. Bardet, F. Aussenac, A. Purea, F. Engelke, S. Hediger, D. Lee and G. De Paëpe, Chem. Sci., 2015, 6, 6806 RSC.
- A. Lesage, M. Lelli, D. Gajan, M. A. Caporini, V. Vitzthum, P. Miéville, J. Alauzun, A. Roussey, C. Thieuleux, A. Mehdi, G. Bodenhausen, C. Copéret and L. Emsley, J. Am. Chem. Soc., 2010, 132, 15459 CrossRef CAS PubMed.
- M. Lelli, D. Gajan, A. Lesage, M. A. Caporini, V. Vitzthum, P. Miéville, F. Héroguel, F. Rascón, A. Roussey, C. Thieuleux, M. Boualleg, L. Veyre, G. Bodenhausen, C. Copéret and L. Emsley, J. Am. Chem. Soc., 2011, 133, 2104 CrossRef CAS PubMed.
- D. Lee, H. Takahashi, A. S. L. Thankamony, J.-P. Dacquin, M. Bardet, O. Lafon and G. De Paëpe, J. Am. Chem. Soc., 2012, 134, 18491 CrossRef CAS PubMed.
- F. Blanc, S. Y. Chong, T. O. McDonald, D. J. Adams, S. Pawsey, M. A. Caporini and A. I. Cooper, J. Am. Chem. Soc., 2013, 135, 15290 CrossRef CAS PubMed.
- F. Ziarelli, M. Casciola, M. Pica, A. Donnadio, F. Aussenac, C. Sauvée, D. Capitani and S. Viel, Chem. Commun., 2014, 50, 10137 RSC.
- F. Blanc, L. Sperrin, D. Lee, R. Dervişoğlu, Y. Yamazaki, S. M. Haile, G. De Paëpe and C. P. Grey, J. Phys. Chem. Lett., 2014, 5, 2431 CrossRef CAS PubMed.
- T.-C. Ong, W.-C. Liao, V. Mougel, D. Gajan, A. Lesage, L. Emsley and C. Copéret, Angew. Chem., Int. Ed., 2016, 55, 4743 CrossRef CAS PubMed.
- A. J. Rossini, A. Zagdoun, M. Lelli, A. Lesage, C. Copéret and L. Emsley, Acc. Chem. Res., 2013, 46, 1942 CrossRef CAS PubMed.
- Ü. Akbey and H. Oschkinat, J. Magn. Reson., 2016, 269, 213 CrossRef PubMed.
- T. Polenova, R. Gupta and A. Goldbourt, Anal. Chem., 2015, 87, 5458 CrossRef CAS PubMed.
- D. Lee, S. Hediger and G. De Paëpe, Solid State Nucl. Magn. Reson., 2015, 66–67, 6 CrossRef CAS PubMed.
- J.-H. Ardenkjaer-Larsen, G. S. Boebinger, A. Comment, S. Duckett, A. S. Edison, F. Engelke, C. Griesinger, R. G. Griffin, C. Hilty, H. Maeda, G. Parigi, T. Prisner, E. Ravera, J. van Bentum, S. Vega, A. Webb, C. Luchinat, H. Schwalbe and L. Frydman, Angew. Chem., Int. Ed., 2015, 54, 9162 CrossRef CAS PubMed.
- Ü. Akbey, A. H. Linden and H. Oschkinat, Appl. Magn. Reson., 2012, 43, 81 CrossRef.
- M. Lelli, S. R. Chaudhari, D. Gajan, G. Casano, A. J. Rossini, O. Ouari, P. Tordo, A. Lesage and L. Emsley, J. Am. Chem. Soc., 2015, 137, 14558 CrossRef CAS PubMed.
- T. V. Can, M. A. Caporini, F. Mentink-Vigier, B. Corzilius, J. J. Walish, M. Rosay, W. E. Maas, M. Baldus, S. Vega, T. M. Swager and R. G. Griffin, J. Chem. Phys., 2014, 141, 64202 CrossRef CAS PubMed.
- C. F. Koelsch, J. Am. Chem. Soc., 1957, 79, 4439 CrossRef CAS.
- S. E. Ashbrook and M. E. Smith, Chem. Soc. Rev., 2006, 35, 718 RSC.
- V. K. Michaelis, E. Markhasin, E. Daviso, J. Herzfeld and R. G. Griffin, J. Phys. Chem. Lett., 2012, 3, 2030 CrossRef CAS PubMed.
- F. Blanc, L. Sperrin, D. A. Jefferson, S. Pawsey, M. Rosay and C. P. Grey, J. Am. Chem. Soc., 2013, 135, 2975 CrossRef CAS PubMed.
- V. K. Michaelis, B. Corzilius, A. A. Smith and R. G. Griffin, J. Phys. Chem. B, 2013, 117, 14894 CAS.
- F. A. Perras, T. Kobayashi and M. Pruski, J. Am. Chem. Soc., 2015, 137, 8336 CrossRef CAS PubMed.
- F. A. Perras, U. Chaudhary, I. I. Slowing and M. Pruski, J. Phys. Chem. C, 2016, 120, 11535 CAS.
- A. Zagdoun, A. J. Rossini, D. Gajan, A. Bourdolle, O. Ouari, M. Rosay, W. E. Maas, P. Tordo, M. Lelli, L. Emsley, A. Lesage and C. Copéret, Chem. Commun., 2012, 48, 654 RSC.
- A. Pines, M. G. Gibby and J. S. Waugh, Chem. Phys. Lett., 1972, 15, 373 CrossRef CAS.
- X. Zhao, W. Hoffbauer, J. Schmedt auf der Günne and M. H. Levitt, Solid State Nucl. Magn. Reson., 2004, 26, 57 CrossRef CAS PubMed.
- A. J. Rossini, A. Zagdoun, M. Lelli, D. Gajan, F. Rascón, M. Rosay, W. E. Maas, C. Copéret, A. Lesage and L. Emsley, Chem. Sci., 2012, 3, 108 RSC.
- A. J. Rossini, A. Zagdoun, F. Hegner, M. Schwarzwälder, D. Gajan, C. Copéret, A. Lesage and L. Emsley, J. Am. Chem. Soc., 2012, 134, 16899 CrossRef CAS PubMed.
- H. Takahashi, D. Lee, L. Dubois, M. Bardet, S. Hediger and G. De Paëpe, Angew. Chem., Int. Ed., 2012, 51, 11766 CrossRef CAS PubMed.
- F. A. Perras, T. Kobayashi and M. Pruski, Phys. Chem. Chem. Phys., 2015, 17, 22616 RSC.
- Ü. Akbey, W. T. Franks, A. Linden, S. Lange, R. G. Griffin, B.-J. van Rossum and H. Oschkinat, Angew. Chem., Int. Ed., 2010, 49, 7803 CrossRef PubMed.
- K. A. Earle, J. K. Moscicki, A. Polimeno and J. H. Freed, J. Chem. Phys., 1997, 106, 9996 CrossRef CAS.
- T.-C. Ong, M. L. Mak-Jurkauskas, J. J. Walish, V. K. Michaelis, B. Corzilius, A. A. Smith, A. M. Clausen, J. C. Cheetham, T. M. Swager and R. G. Griffin, J. Phys. Chem. B, 2013, 117, 3040 CrossRef CAS PubMed.
- M. Negoro, K. Nakayama, K. Tateishi, A. Kagawa, K. Takeda and M. Kitagawa, J. Chem. Phys., 2010, 133, 154504 CrossRef CAS PubMed.
- A. Zagdoun, A. J. Rossini, M. P. Conley, W. R. Grüning, M. Schwarzwälder, M. Lelli, W. T. Franks, H. Oschkinat, C. Copéret, L. Emsley and A. Lesage, Angew. Chem., Int. Ed., 2013, 52, 1222 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, DNP sensitivity gain and additional figures. See DOI: 10.1039/c6cc09743j |
| This journal is © The Royal Society of Chemistry 2017 |