Dendron engineering in self-host blue iridium dendrimers towards low-voltage-driving and power-efficient nondoped electrophosphorescent devices†
Yang
Wang
ab,
Shumeng
Wang
ab,
Junqiao
Ding
*a,
Lixiang
Wang
*a,
Xiabin
Jing
a and
Fosong
Wang
a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: junqiaod@ciac.ac.cn; lixiang@ciac.ac.cn
bUniversity of the Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 25th November 2016
Abstract
Dendron engineering in self-host blue Ir dendrimers is reported to develop power-efficient nondoped electrophosphorescent devices for the first time, which can be operated at low voltage close to the theoretical limit (Eg/e: corresponding to the optical bandgap divided by the electron charge). With increasing dendron's HOMO energy levels from B-POCz to B-CzCz and B-CzTA, effective hole injection is favored to promote exciton formation, resulting in a significant reduction of driving voltage and improvement of power efficiency. Consequently, the nondoped device of B-CzTA achieves extremely low driving voltages of 2.7/3.4/4.4 V and record high power efficiencies of 30.3/24.4/16.3 lm W−1 at 1, 100 and 1000 cd m−2, respectively. We believe that this work will pave the way to the design of novel power-efficient self-host blue phosphorescent dendrimers used for energy-saving displays and solid-state lightings.
Besides small molecules and polymers, nowadays transition-metal-containing phosphorescent dendrimers have become a third class of triplet emitters used for low-cost solution-processed phosphorescent organic light-emitting diodes (s-PhOLEDs) because they have both the well-defined structures of small molecules and the excellent solution processibility of polymers. Most importantly, due to the self-host feature that the peripheral dendrons can act as the host of the emissive core by themselves, such phosphorescent dendrimers are suitable for the fabrication of high-performance nondoped s-PhOLEDs.1–3 Compared with the conventional doped devices,4–11 an additional host matrix is not required any more in the emitting layer (EML). Hence, the tedious doping technology and inherent phase segregation could be avoided to improve device performance including efficiency, lifetime and reproducibility.12–14
Inspired by this advantage, a series of self-host iridium (Ir) dendrimers with blue,15–19 green,2,3,20–28 yellow29 and red30,31 emission colors have already been reported based on phenylene,15,16 arylamine,29 carbazole,17,21,23–25 Fréchet31 and Müllen22 dendrons. Among them, the blue-emitting ones usually show poor power efficiency induced by large driving voltage, limiting their potential applications in energy-saving displays and solid-state lightings. For example, a promising blue dendrimer B-G2 with a p-type oligocarbazole as the dendron has been recently developed for nondoped devices without any efficiency loss.17 By incorporating an additional n-type dendron to tune the charge balance at a single dendritic molecule, a negligible efficiency roll-off at high luminance is achieved.19 However, large driving voltages always accompany in these reported cases. We note that the voltage at 1 cd m−2 (3.4–3.6 V) is about 0.72–0.92 V away from the theoretical limit of driving voltage, which corresponds to the optical bandgap divided by the electron charge (Eg/e = 2.68 V).32 So there is still much room left for further decreasing the driving voltage of such self-host blue Ir dendrimers, and realizing power-efficient nondoped s-PhOLEDs.
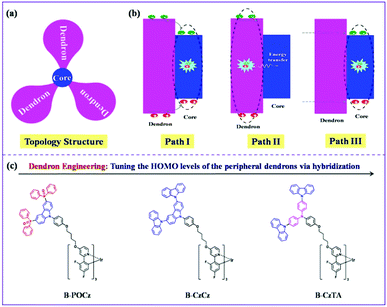
When a self-host dendrimer is utilized alone as the EML in a nondoped device, there may exist three possible pathways for exciton generation (Fig. 1a and b):33,34 (I) carriers (holes and/or electrons) are firstly injected into the dendron and then transported to the core, resulting in exciton formation directly on the core; (II) the injected carriers on the dendron are firstly recombined to produce excitons, whose energy is subsequently transferred to the core; (III) excitons are generated on the core after a direct carrier injection rather than a cascade one through the dendron bridge. On one hand, given the characteristic topology structure that the inner emissive core is densely encapsulated by the outer dendrons, the contribution from path III could be ignored because carriers are difficult to be directly injected into the core.22 On the other hand, as for paths I and II involving the initial carrier injection into the dendron, realization of high power efficiency is within our expectation if the outer dendron has a suitable energy level to facilitate this procedure so as to reduce the driving voltage. With this idea in mind, here we propose a strategy towards power-efficient nondoped s-PhOLEDs operating at a driving voltage close to the theoretical limit (Eg/e = 2.68 V) by using dendron engineering in self-host blue Ir dendrimers.
As depicted in Fig. 1c, three kinds of self-host blue Ir dendrimers (B-POCz, B-CzCz and B-CzTA) have been designed, which bear the same blue emissive core but different functional dendrons. In this case, the chemical structure of the dendron is delicately modified via hybridization among diphenylphosphine oxide, carbazole and triphenylamine taking several concerns into account. Firstly, the triplet energy of the dendron should be higher than that of the core to avoid triplet energy back transfer.35,36 Therefore, except for the low-triplet-energy dendron TACz (2.63 eV), the other hybrids POCz, CzCz and CzTA (2.85–2.92 eV) can be used as the peripheral dendrons for the central core B-G0 with a triplet energy of 2.69 eV (Fig. S4, ESI†). Secondly, all hybridized dendrons are constructed by the second generation not only to effectively inhibit the luminescence quenching between the Ir cores13,32 but also to endow the final Ir dendrimers with similar molecular diameters (R = 23 Å), ruling out the influence of size on device performance (Fig. S5, ESI†).22,28 Thirdly, owing to the elevated electron donating ability in the order diphenylphosphine oxide < carbazole < triphenylamine, the highest occupied molecular orbital (HOMO) level is found to be increased gradually from −5.74 eV for POCz to −5.40 eV for CzCz and −5.13 eV for CzTA (Fig. S6 and Table S1, ESI†). This enables us to evaluate whether the dendron's energy level can be tuned to improve the power efficiency of the self-host blue Ir dendrimer as anticipated.
The detailed synthetic route of the dendrimers is plotted in Schemes S1–S3 (ESI†). A previously reported post-dendronization18 is adopted because this method does provide a high degree of freedom for further dendron engineering. That is, the alkyl bromide dendrons (POCz-O-C4H8Br, CzCz-O-C4H8Br and CzTA-O-C4H8Br) were prepared independently, which then reacted with the para-hydroxyl-substituted blue Ir core (p-HO-dfppyIr) under Williamson conditions to afford dendrimers B-POCz, B-CzCz and B-CzTA in good yields. The resultant self-host blue Ir dendrimers exhibit good solubility in common organic solvents, such as dichloromethane (CH2Cl2), chlorobenzene, toluene and tetrahydrofuran etc., ensuring their capability to form high quality films by a solution process (Fig. S7, ESI†). Additionally, they are all thermally stable with high decomposition temperatures above 400 °C and high glass transition temperatures above 190 °C (Fig. S8, ESI†). Among them, B-CzCz displays the highest glass transition temperature of 243 °C. In fact, the carbazole moiety seems to be more rigid than diphenylphosphine oxide and triphenylamine due to its planar structure and less freedom of rotation. Therefore, the molecular movement and relaxation in B-CzCz would become more difficult than those in B-POCz and B-CzTA, leading to the increased glass transition temperature.
Fig. 2 shows the UV-vis absorption spectra of the dendrimers in CH2Cl2 as well as their photoluminescence (PL) spectra in toluene, and the corresponding data are also summarized in Table S2 (ESI†). The intense absorption bands below 350 nm and weak bands in the region of 350–450 nm are observed for dendrimers B-POCz, B-CzCz and B-CzTA. Based on the absorption spectrum of B-G0 (Fig. S9, ESI†), these absorptions can be tentatively attributed to the spin-allowed ligand-centered (LC) transition together with the 1π–π* transition from the dendritic wedge and the metal-to-ligand charge-transfer (MLCT) transition from the central Ir core, respectively. In solutions, dendrimers B-POCz, B-CzCz and B-CzTA display the same PL spectra with an emission peak at 466 nm, corresponding to a triplet energy of 2.69 eV determined by the central Ir core. Meanwhile, their solution PL quantum yields (0.79–0.84) and lifetimes (0.75–0.80 μs) are close to those of B-G0.17 These observations indicate that the photophysical properties of the self-host blue Ir dendrimers are almost independent of the incorporated different dendrons due to the non-conjugated alkyl spacer between the core and the dendron. However, in solid state, B-POCz and B-CzTA seem to decay a little faster than B-CzCz associated with shorter lifetimes of 0.28–0.34 μs (Fig. S10, ESI†). Consistent with the variation of the glass transition temperatures, the flexibility of diphenylphosphine oxide and triphenylamine with respect to carbazole may contribute to stronger intermolecular interactions and thus more severe luminance quenching in B-POCz and B-CzTA than in B-CzCz.
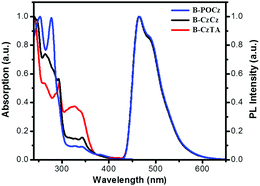 | ||
| Fig. 2 Absorption spectra in CH2Cl2 and PL spectra in toluene of the dendrimers at a concentration of 10−5 M. | ||
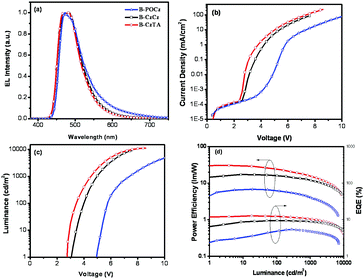
To explore the electroluminescence (EL) properties of the dendrimers, nondoped s-PhOLEDs were fabricated with a configuration of ITO/PEDOT:PSS (45 nm)/B-POCz, B-CzCz or B-CzTA (40 nm)/TPCz (8 nm)/Tm3PyPB (42 nm)/LiF (0.5 nm)/Al (100 nm) (Fig. S11a, ESI†). Herein, TPCz (3,6-bis(diphenylphosphoryl)-9-(4′-(diphenylphosphoryl)phenyl)-carbazole)37 is used as the exciton-blocking layer due to its high triplet energy of 3.0 eV, and Tm3PyPB (1,3,5-tri(m-pyrid-3-yl-phenyl)benzene)38 is used as the electron-transporting layer due to its excellent electron mobility of 10−3 cm2 V−1 s−1. Similar to their PL counterparts, all the dendrimers emit bright blue EL solely from the central Ir core (Fig. 3a), and the corresponding Commission Internationale de L’Eclairage (CIE) coordinates are (0.19, 0.34), (0.17, 0.32) and (0.16, 0.32) for B-POCz, B-CzCz and B-CzTA, respectively. Furthermore, no emission residues either from the outer dendron or from the Tm3PyPB electron-transporting layer are observed, indicative of the efficient energy transfer from the dendron to the core and the triplet exciton confinement mainly in the EML.
As can be clearly seen in Fig. 3b and c, both the current density–voltage (J–V) and luminance–voltage (L–V) plots are found to move toward a negative voltage following the sequence B-POCz, B-CzCz and B-CzTA. The maximum brightness increases accordingly from 7030 cd m−2 for B-POCz to 9360 cd m−2 for B-CzCz and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 cd m−2 for B-CzTA. Correspondingly, the driving voltage at 1, 100 and 1000 cd m−2 is greatly decreased from 4.9/5.8/7.4 V to 3.2/4.0/5.0 V and 2.7/3.4/4.4 V, respectively. It should be noted that the driving voltage of B-CzTA (2.7 V for 1 cd m−2) is the lowest ever reported for self-host blue Ir dendrimers, which nearly approaches the limit determined by Eg/e (2.68 V).32 Benefiting from such an extremely low driving voltage, the nondoped device of B-CzTA reveals record high power efficiencies of 30.3 lm W−1 for 1 cd m−2, 24.4 lm W−1 for 100 cd m−2 and 16.3 lm W−1 for 1000 cd m−2 (Fig. 3d and Table S3, ESI†), far exceeding those of B-CzCz (15.6/16.1/11.9 lm W−1) and B-POCz (5.6/6.1/4.1 lm W−1). Moreover, compared to the previously developed blue Ir dendrimer B-G2 (3.6/6.2/7.4 V and 25.3/13.3/8.9 lm W−1 at 1, 100 and 1000 cd m−2), the superior high-brightness performance including low driving voltage and high power efficiency of B-CzTA implies that B-CzTA other than B-G2 would be more suitable for the fabrication of white s-PhOLEDs since the general illumination requires high luminance above 1000 cd m−2. To the best of our knowledge, power-efficient nondoped electrophosphorescent devices, which can be operated at low voltage close to the theoretical limit, have been successfully realized based on self-host blue Ir dendrimers for the first time.
260 cd m−2 for B-CzTA. Correspondingly, the driving voltage at 1, 100 and 1000 cd m−2 is greatly decreased from 4.9/5.8/7.4 V to 3.2/4.0/5.0 V and 2.7/3.4/4.4 V, respectively. It should be noted that the driving voltage of B-CzTA (2.7 V for 1 cd m−2) is the lowest ever reported for self-host blue Ir dendrimers, which nearly approaches the limit determined by Eg/e (2.68 V).32 Benefiting from such an extremely low driving voltage, the nondoped device of B-CzTA reveals record high power efficiencies of 30.3 lm W−1 for 1 cd m−2, 24.4 lm W−1 for 100 cd m−2 and 16.3 lm W−1 for 1000 cd m−2 (Fig. 3d and Table S3, ESI†), far exceeding those of B-CzCz (15.6/16.1/11.9 lm W−1) and B-POCz (5.6/6.1/4.1 lm W−1). Moreover, compared to the previously developed blue Ir dendrimer B-G2 (3.6/6.2/7.4 V and 25.3/13.3/8.9 lm W−1 at 1, 100 and 1000 cd m−2), the superior high-brightness performance including low driving voltage and high power efficiency of B-CzTA implies that B-CzTA other than B-G2 would be more suitable for the fabrication of white s-PhOLEDs since the general illumination requires high luminance above 1000 cd m−2. To the best of our knowledge, power-efficient nondoped electrophosphorescent devices, which can be operated at low voltage close to the theoretical limit, have been successfully realized based on self-host blue Ir dendrimers for the first time.
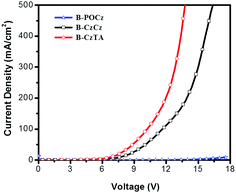
The decreased driving voltage and enhanced power efficiency of B-CzTA relative to B-POCz and B-CzCz are reasonably ascribed to the difference between the varied dendrons when considering the same used Ir complex core. As mentioned above, on going from B-POCz to B-CzCz and B-CzTA, their dendron's HOMO energy levels can be tailored in the range of −5.74 to −5.13 eV through hybridization. Consequently, the HOMO level (−5.13 eV) of the corresponding dendron in dendrimer B-CzTA matches well with the work function (−5.1 eV) of the ITO anode modified by PEDOT:PSS (Fig. S11b, ESI†). This means that, before exciton formation via path I and path II, holes can be injected into the dendron more effectively in B-CzTA than in B-POCz and B-CzCz, leading to a significant reduction of driving voltage and thereby enhancement of power efficiency. Hole-only devices of the dendrimers are further prepared to verify this point. As illustrated in Fig. 4, the hole current at the same driving voltage is observed to be obviously increased from B-POCz to B-CzCz and B-CzTA. The trend is in good agreement with the J–V characteristics of the nondoped devices shown in Fig. 3b, implying favored hole injection with increasing HOMO levels of the dendrons.
In summary, by engineering the dendritic wedge of self-host blue Ir dendrimers, we have successfully developed power-efficient nondoped electrophosphorescent devices that can be operated at a driving voltage close to the theoretical limit (Eg/e = 2.68 V). It is demonstrated that when the HOMO energy level of the outer dendron is tuned to favor effective hole injection, a significant reduction of driving voltage and improvement of power efficiency could be realized on going from B-POCz to B-CzCz and B-CzTA. As a result, dendrimer B-CzTA with the raised-HOMO-level dendrons achieves an ever-reported best nondoped device performance, giving extremely low driving voltages of 2.7/3.4/4.4 V and record high power efficiencies of 30.3/24.4/16.3 lm W−1 at 1, 100 and 1000 cd m−2, respectively. This work, we believe that, will pave the way to the design of novel power-efficient self-host blue phosphorescent dendrimers used for energy-saving displays and solid-state lightings.
The authors acknowledge the financial support from the 973 Project (2015CB655001), the National Key Research and Development Program (2016YFB0401301), and the National Science Foundation of China (No. 51322308, 51573183, 91333205 and 21174144).
Notes and references
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS.
- S.-C. Lo, T. D. Anthopoulos, E. B. Namdas, P. L. Burn and I. D. W. Samuel, Adv. Mater., 2005, 17, 1945 CrossRef CAS.
- J. Ding, B. Wang, Z. Yue, B. Yao, Z. Xie, Y. Cheng, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2009, 48, 6664 CrossRef CAS PubMed.
- T. Giridhar, T.-H. Han, W. Cho, C. Saravanan, T.-W. Lee and S.-H. Jin, Chem. – Eur. J., 2014, 20, 8260 CrossRef CAS PubMed.
- S. M. Wang, X. D. Wang, B. Yao, B. H. Zhang, J. Q. Ding, Z. Y. Xie and L. X. Wang, Sci. Rep., 2015, 5, 12487 CrossRef CAS PubMed.
- N. Aizawa, Y. J. Pu, M. Watanabe, T. Chiba, K. Ideta, N. Toyota, M. Igarashi, Y. Suzuri, H. Sasabe and J. Kido, Nat. Commun., 2014, 5, 5756 CrossRef PubMed.
- K. S. Yook and J. Y. Lee, Org. Electron., 2011, 12, 1711 CrossRef CAS.
- B. Liu, F. L. Huang, Y. F. Shi, Z. Q. Ni, Y. Lin and S. C. Yin, Chin. J. Polym. Sci., 2015, 33, 1133 CrossRef CAS.
- F. F. Wang, Y. T. Tao and W. Huang, Acta Chim. Sin., 2015, 73, 9 CrossRef CAS.
- M. M. Sun, W. Wang, L. Y. Liang, S. H. Yan, M. L. Zhou and Q. D. Ling, Chin. J. Polym. Sci., 2015, 33, 783 CrossRef CAS.
- Q. Cao, Q. Chen and B. H. Han, Acta Chim. Sin., 2015, 73, 541 CrossRef CAS.
- Y.-Y. Noh, C.-L. Lee, J.-J. Kim and K. Yase, J. Chem. Phys., 2003, 118, 2853 CrossRef CAS.
- H. A. A. Attar and A. P. Monkman, Adv. Funct. Mater., 2006, 16, 2231 CrossRef.
- S. Reineke, K. Walzer and K. Leo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125328 CrossRef.
- S.-C. Lo, R. N. Bera, R. E. Harding, P. L. Burn and I. D. W. Samuel, Adv. Funct. Mater., 2008, 18, 3080 CrossRef CAS.
- S.-C. Lo, R. E. Harding, C. P. Shipley, S. G. Stevenson, P. L. Burn and I. D. W. Samuel, J. Am. Chem. Soc., 2009, 131, 16681 CrossRef CAS PubMed.
- D. Xia, B. Wang, B. Chen, S. Wang, B. Zhang, J. Ding, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 1048 CrossRef CAS PubMed.
- Y. Wang, S. Wang, S. Shao, J. Ding, L. Wang, X. Jing and F. Wang, Dalton Trans., 2015, 44, 1052 RSC.
- Y. Wang, Y. Lu, B. Gao, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, Chem. Commun., 2016, 52, 11508 RSC.
- J. P. J. Markham, S.-C. Lo, S. W. Magennis, P. L. Burn and I. D. W. Samuel, Appl. Phys. Lett., 2002, 80, 2645 CrossRef CAS.
- J. Ding, J. Gao, Y. Cheng, Z. Xie, L. Wang, D. Ma, X. Jing and F. Wang, Adv. Funct. Mater., 2006, 16, 575 CrossRef CAS.
- T. Qin, J. Ding, L. Wang, M. Baumgarten, G. Zhou and K. Müllen, J. Am. Chem. Soc., 2009, 131, 14329 CrossRef CAS PubMed.
- L. Chen, Z. Ma, J. Ding, L. Wang, X. Jing and F. Wang, Chem. Commun., 2011, 47, 9519 RSC.
- L. Chen, Z. Ma, J. Ding, L. Wang, X. Jing and F. Wang, Org. Electron., 2012, 13, 2160 CrossRef CAS.
- J. Ding, J. Lü, Y. Cheng, Z. Xie, L. Wang, X. Jing and F. Wang, J. Organomet. Chem., 2009, 694, 2700 CrossRef CAS.
- W. Tian, C. Yi, B. Song, W. Jiang, Q. Qi, Y. Zheng, Z. Qi and Y. Sun, J. Mater. Chem. C, 2014, 2, 1104 RSC.
- W. Tian, Q. Qi, B. Song, C. Yi, W. Jiang, X. Cui, W. Shen, B. Huang and Y. Sun, J. Mater. Chem. C, 2015, 3, 981 RSC.
- Y. Wang, S. Wang, N. Zhao, B. Gao, S. Shao, J. Ding, L. Wang, X. Jing and F. Wang, Polym. Chem., 2015, 6, 1180 RSC.
- M. Zhu, J. Zou, X. He, C. Yang, H. Wu, C. Zhong, J. Qin and Y. Cao, Chem. Mater., 2012, 24, 174 CrossRef CAS.
- J. Ding, J. Lü, Y. Cheng, Z. Xie, L. Wang, X. Jing and F. Wang, Adv. Funct. Mater., 2008, 18, 2754 CrossRef CAS.
- K. M. Jung, K. H. Kim, J. I. Jin, M. J. Cho and D. H. Choi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7517 CrossRef CAS.
- H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y.-J. Pu and J. Kido, Adv. Mater., 2012, 24, 3212 CrossRef CAS PubMed.
- J. P. J. Markham, I. D. W. Samuel, S.-C. Lo, P. L. Burn, M. Weiter and H. Bässler, J. Appl. Phys., 2004, 95, 438 CrossRef CAS.
- S. Gambino, S. G. Stevenson, K. A. Knights, P. L. Burn and I. D. W. Samuel, Adv. Funct. Mater., 2009, 19, 317 CrossRef CAS.
- N. R. Evans, L. S. Devi, C. S. K. Mak, S. E. Watkins, S. I. Pascu, A. Köhler, R. H. Friend, C. K. Williams and A. B. Holmes, J. Am. Chem. Soc., 2006, 128, 6647 CrossRef CAS PubMed.
- J. C. Ribierre, A. Ruseckas, K. Knights, S. V. Staton, N. Cumpstey, P. L. Burn and I. D. W. Samuel, Phys. Rev. Lett., 2008, 100, 017402 CrossRef CAS PubMed.
- J. Ding, Q. Wang, L. Zhao, D. Ma, L. Wang, X. Jing and F. Wang, J. Mater. Chem., 2010, 20, 8126 RSC.
- S.-J. Su, T. Chiba, T. Takeda and J. Kido, Adv. Mater., 2008, 20, 2125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc08722a |
| This journal is © The Royal Society of Chemistry 2017 |