Sulfoxidation of alkenes and alkynes with NFSI as a radical initiator and selective oxidant†
Yuexia
Zhang‡
a,
Zeng Rong
Wong‡
a,
Xingxing
Wu
a,
Sherman J. L.
Lauw
a,
Xuan
Huang
a,
Richard D.
Webster
a and
Yonggui Robin
Chi
*ab
aDivision of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: robinchi@ntu.edu.sg
bLaboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Huaxi District, Guiyang 550025, China
First published on 29th November 2016
Abstract
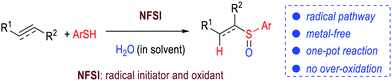
Sulfoxides are important functional molecules. We develop a short-route (one-pot) synthesis of this class of molecules by reacting thiols with alkenes or alkynes under mild and metal-free conditions. N-Fluorobenzenesulfonimide (NFSI) is used to play dual roles: as a radical initiator for a thiol–ene/–yne reaction to form sulfide adducts, and as efficient oxidant for conversion of the sulfides formed in situ to sulfoxides. Over-oxidation of the sulfoxides to sulfones is avoided in our approach.
The sulfoxide moiety is a functional group often found in pharmaceuticals and natural products, such as esomeprazole,1 armodafinil2 and alliin.3 Sulfoxides are also used as ligands for metal catalysts with applications in a large set of reactions, including Pummerer4 and Mislow–Evans rearrangements.5 To date, the synthesis of sulfoxides typically involves oxidation of sulfides using peroxides6 and hypervalent iodine reagent7 as the oxidants with the assistance of transition metal catalysts.8 One challenge of this otherwise widely used two-step protocol lies with over-oxidation of sulfoxides to sulfones.6 Here we report a new method that selectively converts alkenes and alkynes to sulfoxides in a one-pot metal-free operation (Fig. 1). N-Fluorobenzenesulfonimide (NFSI), a shelf-stable reagent,9 is used as a radical initiator and a selective oxidant. No over-oxidation of sulfoxides to sulfones was observed (Fig. 1).
 | ||
| Fig. 1 Our strategy of NFSI-enabled selective one-pot access to sulfoxides from alkenes/alkynes and thiols. | ||
Notably, Yadav10 and Lei11 have recently reported reactions of alkenes and thiols under dioxygen to form β-keto sulfoxides and β-oxy sulfoxides. In their reactions, oxidation of the alkene carbon by oxygen occurred concurrently. The NFSI reagent used in our reactions has often been employed as electrophilic fluorinating agent,12 aminating reagent,13 or a combined amino and fluorine source.14 In radical reactions (SET reactions), it can generate aminyl radical for Cu-catalyzed radical amination of olefins, as reported by Kanai,15 Zhang16 and Studer.17 Zhang and co-workers also developed radical aminofluorination of styrenes using NFSI.18 In addition, Ritter reported Pd-catalyzed direct radical arene amination with NFSI,19 and Studer developed radical aminooxygenation of alkenes with NFSI in combination with TEMPONa.20
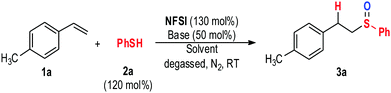
Our initial design was to combine NFSI and thiol to generate thiyl and aminyl radicals for aminosulfidation of alkenes. We first studied reactions between alkene 1a and thiol 2a with NFSI as a potential radical generator in the presence of K2CO3 or K3PO4 as the base (Table 1, entry 1). The expected alkene aminosulfidation adduct was not formed. Instead, we found alkene sulfoxidation adduct 3a as the product in 11% yield (entry 1). Additional evaluation of the base effects (Table 1, entries 2–6) indicated that 1,8-diazabicycloundec-7-ene (DBU) performed the best, giving 3a in 72% yield (Table 1, entry 6). Several other solvents studied here did not work as well as toluene (Table 1, entries 7–10). The yield of 3a (based on 1a) could be further improved by increasing the loadings of thiol 2a and NFSI (Table 1, entry 11).
| Entry | Base | Solvent | Yieldb [%] |
|---|---|---|---|
| a Conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.24 mmol, 1.2 equiv.), NFSI (130 mol%) and base (50 mol%) in 3.0 mL of solvent at rt for 2–5 h. b Isolated yield based on 1a. c 2a (0.4 mmol, 2.0 equiv.), NFSI (0.36 mmol, 1.8 equiv.). | |||
| 1 | K2CO3 or K3PO4 | Toluene | 11 |
| 2 | KOMe | Toluene | 23 |
| 3 | NaOtBu | Toluene | 29 |
| 4 | DIPEA | Toluene | 43 |
| 5 | DABCO | Toluene | 66 |
| 6 | DBU | Toluene | 72 |
| 7 | DBU | THF | 52 |
| 8 | DBU | Dioxane | 31 |
| 9 | DBU | CH3CN | 26 |
| 10 | DBU | EA | 48 |
| 11 | DBU | Toluene | 87 |
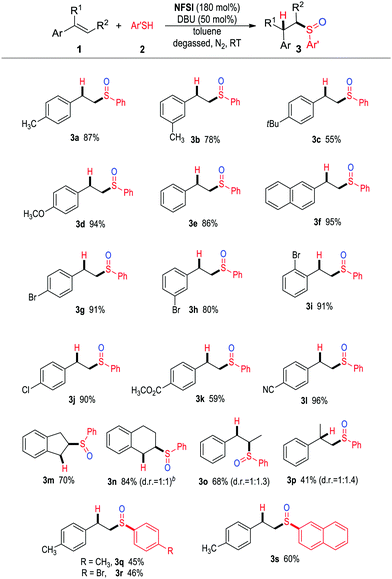
With an acceptable condition (Table 1, entry 11) on hand, we proceeded to explore the scope of the reaction (Table 2). We first examined the reaction between various alkenes and thiol 2a (3a–3p). Styrenes with different substituents or substitution patterns on the phenyl ring worked effectively (3a–3l). Internal alkenes, such as indene (3m), 1,2-dihydronaphthalene (3n), trans-β-methylstyrene (3o) were also effective substrates. Notably, no sulfoxidation products were obtained when aliphatic alkenes were used. Substituents (e.g., CH3 and Br) could be placed on the phenyl unit of thiol 2a (3q, 3r), while 2-napthyl thiol (3s) was also tolerated.
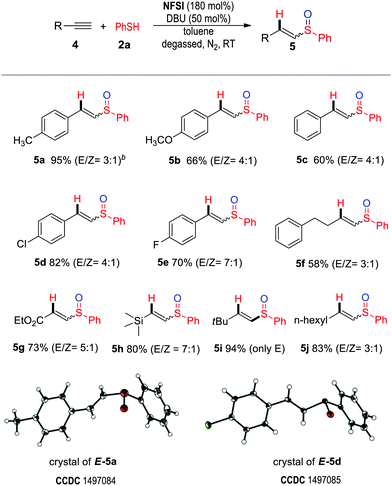
We next found that alkynes could be used as substrates (to replace alkenes) under the same condition, affording unsaturated sulfoxides as the products (Table 3). The reactions proceeded smoothly for aryl alkynes with either electron-withdrawing or electron-donating substituents present on the aromatic ring (4a–4e) to afford the desired products (5a–5e) in moderate to excellent yields. Alkyl alkynes (4f, 4i–j) and alkynes bearing an ester (4g) or silyl (4h) moiety were also effective substrates.21 The structures of two vinylsulfoxide products (E-5a, E-5d) were unambiguously assigned via X-ray crystallography.
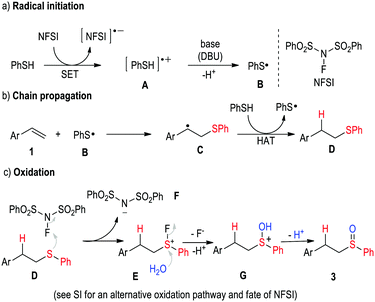
The proposed reaction pathway is illustrated in Scheme 1. Initially, removal of one electron from thiol 2a by oxidant NFSI12a,b generates radical cation A that can be subsequently transformed to thiyl radical B after a proton transfer (Scheme 1a). Thereafter, addition of radical B to alkene 1 forms radical intermediate C. A hydrogen atom transfer (HAT) from a molecule of 2a converts C to sulfide D with the regeneration of the thiyl radical intermediate B which returns back into the propagation step (Scheme 1b). Finally, the sulfide D is oxidized to sulfoxide 3 by NFSI. One possible pathway (see ESI† for another possibility) for the oxidation of sulfide D involves nucleophilic addition of the sulfide to the electrophilic fluorine atom of NFSI to afford intermediate E (Scheme 1c). Substitution of the fluorine atom of E by water forms G that then transforms to sulfoxide 3 after a proton transfer process.
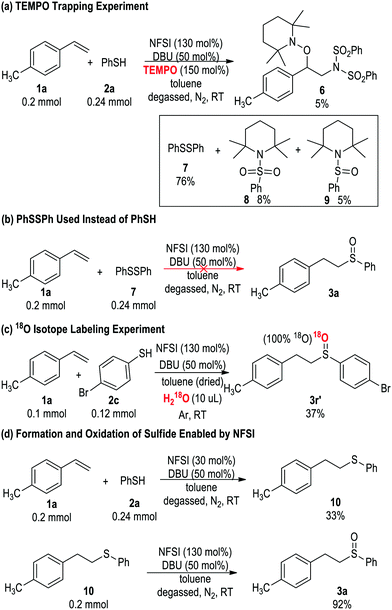
To investigate the reaction mechanism, we performed multiple experiments (Scheme 2). The addition of TEMPO inhibited the formation of 3a (Scheme 2a). In the presence of 150 mol% TEMPO, the formation of sulfoxide product 3a was completely suppressed. The aminooxygenation adduct 6 previously reported by Studer20 was observed in about 5% yield. Adducts 7, 8 and 9 (89% of combined yields based on thiol 2a) were previously reported by Greci and co-workers in a study on the reaction between 2a and TEMPO.22 The use of diphenyl disulfide 7 in place of thiophenol 2a did not lead to desired product 3a (Scheme 2b), suggesting that under the oxidative condition of NSFI the disulphide adduct did not convert to thiol. The oxygen atom in the sulfoxide product came from the trace amount of H2O present in the solvent, as suggested by an 18O isotope labelling experiment (Scheme 2c). Additional experiments by adjusting the loadings of NFSI (Scheme 2d) suggest that both the thiol–ene reaction and oxidation reaction are enabled by NFSI. When 30 mol% of NFSI was added, only sulfide adduct 10 was observed. Sulfide 10 could then be transformed under standard conditions to sulfoxide 3a.23 These experiments (Scheme 2a and b) support the formation of radical intermediate as proposed in Scheme 1a and b. The results (Scheme 2c and d) showed that the formation of sulfide (D, Scheme 1c) and its oxidation occurs in a stepwise process.
In summary, we have developed a facile synthesis of sulfoxides from alkenes and alkynes in the presence of NFSI. Sulfoxides are useful structural moieties for construction of natural products and other functional molecules. Our approach provides a metal-free and concise synthesis of this class of molecules. The substrates and reagents used in our approach are either inexpensive or readily available with reasonable cost. NFSI is used as a radical initiator (to initiate the sulfide formation) and a selective oxidant (to transform sulfides to sulfoxides). The oxygen atom in the sulfoxide products was determined to originate from trace water in the reaction mixture, providing a simple way for the incorporation of 18O isotope to sulfoxide-containing molecules. Further development of related radical reactions, including catalytic versions mediated by carbene organic catalysts, is in progress.
We thank Dr Yongxin Li (NTU) and Dr Rakesh Ganguly for assistance with X-ray structure analysis; We acknowledge financial support by the Singapore National Research Foundation (NRF-NRFI2016-06), the Ministry of Education of Singapore (MOE2013-T2-2-003), Nanyang Technological University (NTU, Singapore), China's Ministry of Education, National Key program for Basic Research (No. 2010CB 126105), Thousand Talent Plan (700059143302), National Natural Science Foundation of China (No. 21132003 and 21472028). Guizhou Province Returned Oversea Student Science and Technology Activity Program, Science and Technology of Guizhou Province, and Guizhou University.
Notes and references
- C. M. Spencer and D. Faulds, Drugs, 2000, 60, 321–329 CrossRef CAS PubMed.
- K. P. Garnock-Jones, S. Dhillon and L. J. Scott, CNS Drugs, 2009, 23, 793–803 CrossRef CAS PubMed.
- A. Stoll and E. Seebeck, Adv. Enzymol. Relat. Areas Mol. Biol., 2006, 11, 377–400 Search PubMed.
- For selected reviews on Pummer rearrangments, see (a) S. K. Bur and A. Padwa, Chem. Rev., 2004, 104, 2401–2432 CrossRef CAS PubMed; (b) K. S. Feldman, Tetrahedron, 2006, 62, 5003–5034 CrossRef CAS; (c) L. H. Smith, S. C. Coote, H. F. Sneddon and D. J. Procter, Angew. Chem., Int. Ed., 2010, 49, 5832–5844 CrossRef CAS PubMed.
- C. M. Rojas, Molecular Rearrangements in Organic Synthesis, John Wiley & Sons, Inc., 2015, pp. 569–626 Search PubMed.
- See the review: K. Kaczorowska, Z. Kolarska, K. Mitka and P. Kowalski, Tetrahedron, 2005, 61, 8315–8327 CrossRef CAS.
- (a) M. Xia and Z. C. Chen, Synth. Commun., 1997, 27, 1315–1320 CrossRef CAS; (b) B. Yu, C. X. Guo, C. L. Zhong, Z. F. Diao and L. N. He, Tetrahedron Lett., 2014, 55, 1818–1821 CrossRef CAS.
- (a) M. Matteucci, G. Bhalay and M. Bradley, Org. Lett., 2003, 5, 235–237 CrossRef CAS PubMed; (b) W. Dai, J. Li, B. Chen, G. Li, Y. Lv, L. Wang and S. Gao, Org. Lett., 2013, 15, 5658–5661 CrossRef CAS PubMed; (c) X. F. Wu, Tetrahedron Lett., 2012, 53, 4328–4331 CrossRef CAS; (d) M. Bagherzadeh, M. M. Haghdoost and A. Shahbazirad, J. Coord. Chem., 2012, 65, 591–601 CrossRef CAS.
- X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826–870 CrossRef CAS PubMed.
- T. Keshari, V. K. Yadav, V. P. Srivastava and L. D. S. Yadav, Green Chem., 2014, 16, 3986–3992 RSC.
- H. Wang, Q. Lu, C. Qian, C. Liu, W. Liu, K. Chen and A. Lei, Angew. Chem., Int. Ed., 2016, 55, 1094–1097 CrossRef CAS PubMed.
- (a) X. Dong, W. Yang, W. Hu and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 660–663 CAS; (b) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236–10254 CrossRef CAS PubMed; (c) D. D. Steiner, N. Mase and C. F. Barbas, 3rd, Angew. Chem., Int. Ed., 2005, 44, 3706–3710 CrossRef CAS PubMed; (d) T. D. Beeson and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 8826–8828 CrossRef CAS PubMed.
- (a) K. Sun, Y. Li, T. Xiong, J. Zhang and Q. Zhang, J. Am. Chem. Soc., 2011, 133, 1694–1697 CrossRef CAS PubMed; (b) J. Trenner, C. Depken, T. Weber and A. Breder, Angew. Chem., Int. Ed., 2013, 52, 8952–8956 CrossRef CAS PubMed; (c) T. Xiong, Y. Li, Y. Lv and Q. Zhang, Chem. Commun., 2010, 46, 6831–6833 RSC.
- (a) Y. Li, N. Lou and L. Gan, Org. Lett., 2015, 17, 524–527 CrossRef CAS PubMed; (b) S. Qiu, T. Xu, J. Zhou, Y. Guo and G. Liu, J. Am. Chem. Soc., 2010, 132, 2856–2857 CrossRef CAS PubMed.
- K. Kaneko, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2013, 15, 2502–2505 CrossRef CAS PubMed.
- J. Sun, G. Zheng, T. Xiong, Q. Zhang, J. Zhao, Y. Li and Q. Zhang, ACS Catal., 2016, 6, 3674–3678 CrossRef CAS.
- B. Zhang and A. Studer, Org. Lett., 2014, 16, 1790–1793 CrossRef CAS PubMed.
- H. Zhang, Y. Song, J. Zhao, J. Zhang and Q. Zhang, Angew. Chem., Int. Ed., 2014, 53, 11079–11083 CrossRef CAS PubMed.
- G. B. Boursalian, M. Y. Ngai, K. N. Hojczyk and T. Ritter, J. Am. Chem. Soc., 2013, 135, 13278–13281 CrossRef CAS PubMed.
- Y. Li, M. Hartmann, C. G. Daniliuc and A. Studer, Chem. Commun., 2015, 51, 5706–5709 RSC.
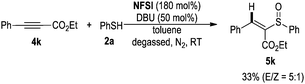
- When internal alkyne 4k was used as the substrate, product 5k was isolated in low yield.
. - P. Carloni, E. Damiani, M. Iacussi, L. Greci, P. Stipa, D. Cauzi, C. Rizzoli and P. Sgarabotto, Tetrahedron, 1995, 51, 12445–12452 CrossRef CAS.
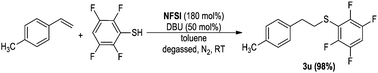
- When the electron-deficient aryl thiol 2,3,5,6-tetrafluorobenzenethiol was used, no desired sulfoxide was isolated, instead sulfide 3u was isolated in 98% yield. This might be due to the electron-deficient sulfide not being easy to oxidize.
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, mechanistic details, spectroscopic data and copies of 1H and 13C NMR spectra. CCDC 1497084 and 1497085. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc08631d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |