Fluoroalkyl-modified naphthodithiophene diimides†
Wei
Fan
ab,
Chunming
Liu
ab,
Yan
Li
*a and
Zhaohui
Wang
a
aBeijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yanli@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 25th November 2016
Abstract
Two kinds of fluoroalkyl-modified naphthodithiophene diimides (NDTI) were designed and synthesized. α-Modified NDTI could form favorable slipped one-dimensional (1D) stacking and N-modified NDTI shows a torsion cofacial stacking. Single-crystal transistors confirm that both fluoroalkyl-modified NDTI possess good electron transport ability with electron mobilities of 0.065 cm2 V−1 s−1 and 1.59 cm2 V−1 s−1, respectively.
In recent years, the design and synthesis of acene diimides and heteroacene diimides have attracted significant attention due to their unique optoelectronic properties, self-assembly behavior, and potential applications for organic electronics.1 In order to achieve high-performance organic semiconductors, extension of conjugated π-systems is a widely applied approach which is often accompanied by an enhanced intermolecular overlap,2 whereas introduction of fluorinated chains is considered as one of the most effective ways to improve the stability of devices.3 Since naphthalene diimides (NDIs) as high-performance n-type semiconductors were first reported by Katz in 2000,4 developing π-system extended acene and heteroacene diimides is always the research focus of chemical and materials communities.5 Recently, tetracene diimides were efficiently synthesized by us via direct double ring extension of NDIs involving metallacyclopentadienes.6 Quite recently, naphthodithiophene diimides (NDTI), which are heterotetracene diimides, were reported by Takimiya et al., and thin-film transistors showed an electron mobility up to 0.73 cm2 V−1 s−1via introducing chlorine groups into α-positions.7 Despite the great progress, organic field-effect transistors (OFETs) based on fluoroalkyl-modified NDTI and the structure–property relationships are rarely reported.
We are particularly interested in fluoroalkyl-modified rylene dyes. In 2008, we first introduced fluorinated chains into perylene diimides (PDIs) by copper-mediated perfluoroalkylation of dibrominated PDIs, which was later achieved via direct functionalization of PDIs.8 OFETs based on fluoroalkyl-modified PDI films and single crystals, and the structure–property relationships were also systematically studied, which concludes that the introduction of fluorinated chains not only makes the device have good stability under ambient conditions but also impacts the molecular packing arrangements, thus leading to different device performance.9 Herein, we designed and synthesized two kinds of fluoroalkyl-modified NDTI, namely α-modified NDTI and N-modified NDTI. Single-crystal packing arrangement shows that α-modified NDTI could form favorable slipped one-dimensional (1D) stacking even though there are large bulky groups in the imide positions, whereas N-modified NDTI shows a torsion cofacial stacking due to the electrostatic repulsive-force of the fluoroalkyl chains. Single-crystal transistors confirm that both fluoroalkyl-modified NDTI possess good electron transport ability under ambient conditions with electron mobilities of 0.065 cm2 V−1 s−1 and 1.59 cm2 V−1 s−1, respectively.
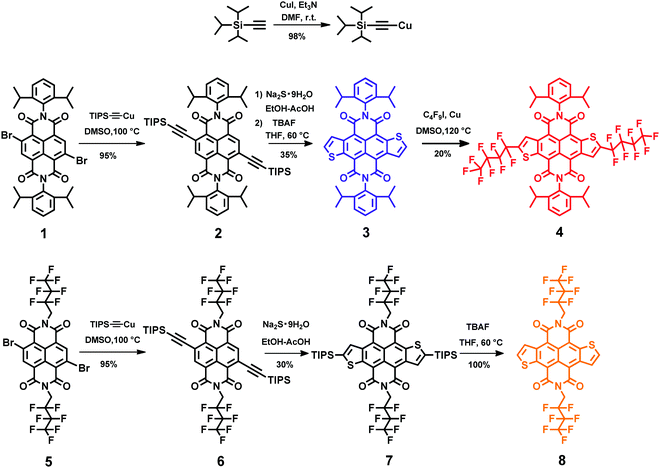
The synthetic route to fluoroalkyl-modified NDTI is shown in Scheme 1. The starting materials 2Br-NDIs 1 and 5 are prepared according to known procedures.10 There are two methods to prepare bis-(trimethylsilyl)ethynyl NDI. The first one is Suzuki–Miyaura cross-coupling reaction of 2Br-NDI with (trimethylsilyl)acetylene under a palladium catalyst.11 However, this method has poor repeatability. The second one is Stille coupling reaction of 2Br-NDI with stannylated (trimethylsilyl)acetylene, which involves poisonous organotin reagent and shows a relatively low yield.12 Herein, we synthesized a stable and solidified molecule [(triisopropylsilyl)ethynyl]copper(I) using a simple and green method, which is more stable in air compared with [(trimethylsilyl)ethynyl]copper(I). The reaction between 2Br-NDI and [(triisopropylsilyl)ethynyl]copper(I) in DMSO solvent at 100 °C has a very high reactivity to produce bis-(triisopropylsilyl)ethynyl NDIs 2 and 6 in 95% yield. Moreover, this step has a very short reaction time of 30 minutes without any catalyst. The intramolecular thiophene annulation of compounds 2 and 6 and cleavage of TIPS groups for compounds 3 and 8 were according to the previous literature.7a It is worth mentioning that compound 8 has poor solubility in common organic solvents, whereas intermediate 7 has good solubility and is very easy to purify. Thus, it is necessary to get the pure intermediate 7 first before cleaving off TIPS groups in order to get purified N-modified NDTI 8 more easily. α-Modified NDTI 4 was synthesized via direct functionalization of NDTI with 1-iodoperfluorobutane in the Cu powder and DMSO system at 120 °C.
The UV-vis absorption spectra and cyclic voltammograms of 3, 4 and 8 are shown in Fig. 1, and the opto-electronic properties and energy levels are summarized in Table 1. Compound 8 with fluorinated chains in the imide groups has almost the same absorption bands as those of diisopropylbenzene substituted NDTI 3 with absorbance maxima at 560 nm, implying that the different imide groups have limited effect on the optical properties of NDTI. In contrast, compound 4 with fluorinated chains in the thiophene α-positions shows a clear hypsochromic shift which results from a lower HOMO energy level and a larger HOMO–LUMO gap. The LUMO level of N-modified NDTI 8 is 0.1 eV lower than that of 3 though they have the same energy gap Eg. α-Modified NDTI 4 has a much lower LUMO level than that of 3, indicating that the introduction of fluorinated chains onto the conjugated backbones could effectively modulate the energy levels. The LUMO levels of the three compounds are all below −4.0 eV, indicating they are stable OFET materials under ambient conditions.
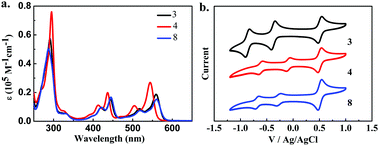 | ||
| Fig. 1 (a) UV-vis absorption spectra of 3, 4, and 8 in CHCl3 (1 × 10−5 M) and (b) cyclic voltammograms in CH2Cl2 with a scan rate of 0.1 V s−1. | ||
| λ abs | E 1r | E 2r | LUMOc | HOMOd | E g | |
|---|---|---|---|---|---|---|
| a Measurement performed in CHCl3 solution (nm). b Half-wave reductive potentials (in V vs. Fc/Fc+) measured in CH2Cl2 at a scan rate of 0.1 V s−1 with ferrocene as an internal potential marker. c Estimated from the onset potential of the first reduction wave (in eV). d Estimated from LUMO levels and Eg (in eV). e Obtained from the edge of the absorption spectra (in eV). | ||||||
| 3 | 559 | −0.38 | −0.87 | −4.05 | −6.20 | 2.15 |
| 4 | 544 | −0.09 | −0.62 | −4.32 | −6.53 | 2.21 |
| 8 | 560 | −0.27 | −0.68 | −4.16 | −6.31 | 2.15 |
The transfer integral and reorganization energy are the two critical factors for the transport properties of organic semiconductors, and the two factors depend on the molecular packing modes to a great extent. Thus, the single crystal packing modes of the three compounds are studied, which are obtained by slow solvent vapor diffusion. The crystallographic data are summarized in Table S1 (ESI†), and the solid packing motifs are shown in Fig. 2.13 Compound 3 has no π–π stacking because of bulky 2,6-diisopropylbenzene groups at imide positions, which are perpendicular to the NDTI core with an intermolecular distance of 6.96 Å. Besides, the adjacent molecules have little overlap with a large longitudinal shift of 4.81 Å and transverse shift of 2.66 Å. When the fluorinated chains were introduced into the thiophene α-positions, it turned out that α-modified NDTI 4 has a favorable molecule packing mode: the distance was decreased from 6.96 Å to 3.63 Å and the longitudinal shift shrank from 4.81 Å to 0.01 Å compared with parent molecule 3. Compound 4 has π–π stacking as well as small portion overlap between adjacent molecules in spite of the bulky groups at imide positions. When fluorinated chains are introduced instead of 2,6-diisopropylbenzene groups at imide positions of 3, N-modified NDTI 8 has a torsion angle of about 77.88° between adjacent molecules, which both avoided the steric hindrance and produced a large-area overlap. Compound 8 has a shorter distance of 3.40 Å between adjacent molecules resulting in a torsion cofacial stacking in crystal packing modes. Considering the larger area overlap and closer π–π stacking, N-modified NDTI 8 with a torsion cofacial stacking may have higher electron transport ability.
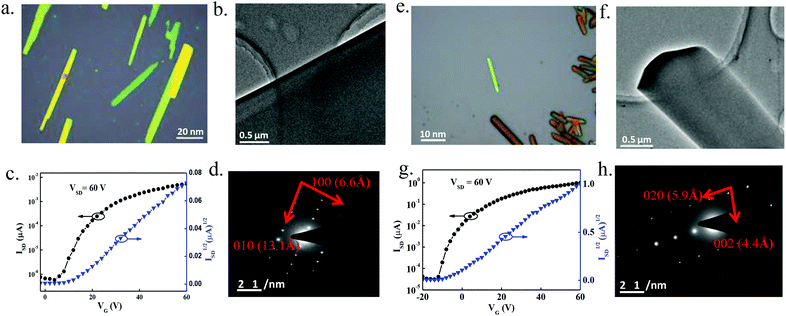
The performances of top-contact bottom-gate field-effect transistors based on micrometer-sized single crystals were measured under ambient conditions. Micro/nanometer-sized single crystal OFETs (SC-OFETs) of the three compounds were prepared by solution drop-casting. The optical microscopy (OM) and transmission electron microscopy (TEM) images, selected area electron diffraction (SAED) patterns, and transfer curves of the SC-OFET devices are shown in Fig. 3. The SC-OFET characteristics of 4 and 8 are summarized in Table 2. Because of the loose arrangement, effective charge-transporting channels could not be formed in single crystals. So SC-OFET devices based on 3 exhibited no transfer performance. SC-OFET devices based on 4 exhibited n-type semiconducting behaviors, with an average electron mobility of 0.037 cm2 V−1 s−1 and the highest value of 0.065 cm2 V−1 s−1. Compound 8-based SC-OFETs exhibited a better n-type performance, with an average electron mobility of 1.27 cm2 V−1 s−1 and the highest value of 1.59 cm2 V−1 s−1 for ribbon crystals based on 30 devices.
| Molecule | μ (cm2 V−1 s−1) | V th (V) | I on/Ioff |
|---|---|---|---|
| 4 | 0.065 | 13.2 | 9.12 × 103 |
| 8 | 1.59 | −2.06 | 3.28 × 104 |
In summary, two kinds of fluoroalkyl-modified naphthodithiophene diimides were designed and synthesized. Single-crystal packing arrangement shows that α-modified NDTI could form favorable slipped 1D stacking even though there are large bulky groups in the imide positions, whereas N-modified NDTI shows a torsion cofacial stacking due to the electrostatic repulsive-force of the fluoroalkyl chains. Single-crystal transistors confirm that both fluoroalkyl-modified NDTI possess good electron transport ability with electron mobilities of 0.065 cm2 V−1 s−1 and 1.59 cm2 V−1 s−1, which raises hope for the realization of high-performance complementary organic circuits under ambient conditions.
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 21225209, 91427303, 21190032), and the Chinese Academy of Sciences (XDB12010100).
Notes and references
- (a) S.-L. Suraru and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 7428–7448 CrossRef CAS PubMed; (b) K. Cai, Q. Yan and D. Zhao, Chem. Sci., 2012, 3, 3175–3182 RSC; (c) C. Li, Z. Lin, Y. Li and Z. Wang, Chem. Rec., 2016, 16, 873–885 CrossRef CAS PubMed; (d) F. Zhang, Y. Hu, T. Schuettfort, C. Di, X. Gao, C. R. McNeill, L. Thomsen, S. C. B. Mannsfeld, W. Yuan, H. Sirringhaus and D. Zhu, J. Am. Chem. Soc., 2013, 135, 2338–2349 CrossRef CAS PubMed.
- (a) S. Katsuta, K. Tanaka, Y. Maruya, S. Mori, S. Masuo, T. Okujima, H. Uno, K. Nakayama and H. Yamada, Chem. Commun., 2011, 47, 10112–10114 RSC; (b) K. Cai, J. Xie and D. Zhao, J. Am. Chem. Soc., 2014, 136, 28–31 CrossRef CAS PubMed; (c) L. E. Polander, L. Pandey, A. Romanov, A. Fonari, S. Barlow, B. M. Seifried, T. V. Timofeeva, J.-L. Brédas and S. R. Marder, J. Org. Chem., 2012, 77, 5544–5551 CrossRef CAS PubMed.
- (a) R. Schmidt, J.-H. Oh, Y.-S. Sun, M. Deppisch, A.-M. Krause, K. Radacki, H. Braunschweig, M. Könemann, P. Erk, Z. Bao and F. Würthner, J. Am. Chem. Soc., 2009, 131, 6215–6228 CrossRef CAS PubMed; (b) H. Usta, C. Kim, Z. Wang, S. Lu, H. Huang, A. Facchetti and T. J. Marks, J. Mater. Chem., 2012, 22, 4459–4472 RSC.
- H. E. Katz, A. J. Lovinger, J. Johnson, C. Kloc, T. Siegrist, W. Li, Y.-Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478–481 CrossRef CAS PubMed.
- (a) J. Xie, K. Shi, K. Cai, D. Zhang, J.-Y. Wang, J. Pei and D. Zhao, Chem. Sci., 2016, 7, 499–504 RSC; (b) W. Yue, A. Lv, J. Gao, W. Jiang, L. Hao, C. Li, Y. Li, L. E. Polander, S. Barlow, W. Hu, S. Di Motta, F. Negri, S. R. Marder and Z. Wang, J. Am. Chem. Soc., 2012, 134, 5770–5773 CrossRef CAS PubMed; (c) L. Zou, X. Wang, X. Zhang, Y. Dai, Y. Wu, J. Wang and J. Pei, Chem. Commun., 2015, 51, 12585–12588 RSC.
- W. Yue, J. Gao, Y. Li, W. Jiang, S. Di Motta, F. Negri and Z. Wang, J. Am. Chem. Soc., 2011, 133, 18054–18057 CrossRef CAS PubMed.
- (a) Y. Fukutomi, M. Nakano, J.-Y. Hu, I. Osaka and K. Takimiya, J. Am. Chem. Soc., 2013, 135, 11445–11448 CrossRef CAS PubMed; (b) M. Nakano, I. Osaka, D. Hashizume and K. Takimiya, Chem. Mater., 2015, 27, 6418–6425 CrossRef CAS.
- (a) Y. Li, L. Tan, Z. Wang, H. Qian, Y. Shi and W. Hu, Org. Lett., 2008, 10, 529–532 CrossRef CAS PubMed; (b) Y. Li, C. Li, W. Yue, W. Jiang, R. Kopecek, J. Qu and Z. Wang, Org. Lett., 2010, 12, 2374–2377 CrossRef CAS PubMed.
- C. Liu, C. Xiao, Y. Li, W. Hu, Z. Li and Z. Wang, Chem. Commun., 2014, 50, 12462–12464 RSC.
- (a) S. Chopin, F. Chaignon, E. Blart and F. Odobel, J. Mater. Chem., 2007, 17, 4139–4146 RSC; (b) Z. Yuan, Y. Ma, T. Geßner, M. Li, L. Chen, M. Eustachi, R. T. Weitz, C. Li and K. Müllen, Org. Lett., 2016, 18, 456–459 CrossRef CAS PubMed.
- D. Buckland, S. V. Bhosale and S. J. Langford, Tetrahedron Lett., 2011, 52, 1990–1992 CrossRef CAS.
- (a) M. W. Logue and K. Teng, J. Org. Chem., 1982, 47, 2549–2553 CrossRef CAS; (b) M. Nakano, M. Sawamoto, M. Yuki and K. Takimiya, Org. Lett., 2016, 18, 3770–3773 CrossRef CAS PubMed.
- Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 1494663 (3), 1494664 (4), 1494665 (8).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the synthesis, X-ray crystallographic analysis, and OFET measurements. CCDC 1494663–1494665. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc07102c |
| This journal is © The Royal Society of Chemistry 2017 |

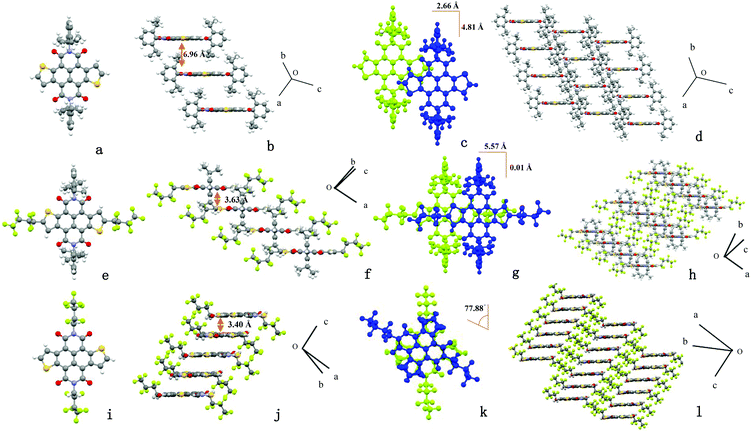
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, the neighbouring molecular distance is 6.96 Å); (f) 4 (
space group, the neighbouring molecular distance is 6.96 Å); (f) 4 (