Hydrogen-bonding catalysis of sulfonium salts†
Shiho
Kaneko
a,
Yusuke
Kumatabara
a,
Shoichi
Shimizu
b,
Keiji
Maruoka
c and
Seiji
Shirakawa
 *a
*a
aDepartment of Environmental Science, Graduate School of Fisheries and Environmental Sciences, Nagasaki University, 1-14, Bunkyo-machi, Nagasaki 852-8521, Japan. E-mail: seijishirakawa@nagasaki-u.ac.jp
bDepartment of Applied Molecular Chemistry, College of Industrial Technology, Nihon University, Izumi-cho, Narashino, Chiba 275-8575, Japan
cDepartment of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan
First published on 15th November 2016
Abstract
Although quaternary ammonium and phosphonium salts are known as important catalysts in phase-transfer catalysis, the catalytic ability of tertiary sulfonium salts has not yet been well demonstrated. Herein, we demonstrate the catalytic ability of trialkylsulfonium salts as hydrogen-bonding catalysts on the basis of the characteristic properties of the acidic α hydrogen atoms on alkylsulfonium salts.
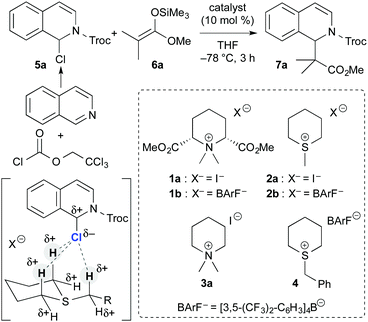
The importance of onium salt compounds has been established in the field of organic chemistry.1 Alkyl-ammonium, phosphonium, and sulfonium salts are some of the most important and reliable onium salt reagents in organic synthesis (Fig. 1). These compounds are often utilized as very useful reagents in the construction of organic building blocks, and the reactions using these reagents appear in textbooks of organic chemistry as important, named reactions.2,3 Furthermore, quaternary ammonium and phosphonium salts are also known as reliable catalysts, which are used to promote a wide variety of organic transformations as phase-transfer and/or base catalysts.4 Despite the wide synthetic utility of onium salt compounds as reagents and catalysts, the catalytic ability of tertiary sulfonium salts has not yet been demonstrated well in organic synthesis.5 The limitation of sulfonium salt catalysts has been attributed mainly to the high reactivity and instability of the compounds with acidic α hydrogen atoms. To create new possibilities for sulfonium salts as catalysts, we focused on the hydrogen-bonding abilities of α hydrogen atoms on alkylsulfonium salts when we reported the use of type 1 tetraalkylammonium salts as hydrogen-bonding catalysts on the basis of the characteristic properties of the α hydrogen atoms (Fig. 2).6,7 Herein, we demonstrate the catalytic ability of trialkylsulfonium salts in hydrogen-bonding catalysis.8
Based on the design of type 1 tetraalkylammonium salts as effective hydrogen-bonding catalysts,6 we focused on simple type 2 trialkylsulfonium salts (Fig. 2). The structures of the α hydrogen atoms that bound to the iodide anion compared favorably to the X-ray crystal structures of ammonium iodide 1a and sulfonium iodide 2a.9,10 Furthermore, we expected the acidity of the α hydrogen atoms of 2a to approximate the acidity of 1a, based on the reported pKa values.11
With the important structural information of the sulfonium salts of type 2 in hand, the catalytic ability of 2 as a hydrogen-bonding catalyst was investigated in a Mannich-type reaction of N-acylisoquinoline 5a, which was generated in situ from 2,2,2-trichloroethyl chloroformate (TrocCl) and isoquinoline, as a benchmark reaction (Table 1).12 As previously reported,6a the reaction of 5a with ketene silyl acetal 6a proceeded slowly in the absence of a catalyst at −78 °C for 3 h (7% yield; entry 1). The reaction with ammonium iodide catalyst 1a was promoted to a moderate extent (38% yield; entry 2). Sulfonium iodide 2a was then examined as a catalyst, and a moderate acceleration of the reaction was also observed (30% yield; entry 3). It should be noted that simple ammonium iodide catalyst 3a showed almost no acceleration of the reaction (9% yield; entry 4). These results clearly demonstrated the importance of the acidity of α hydrogen atoms on a sulfonium salt 2a to promote the reaction. The exchange of the counteranion in 2a to tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BArF−) as a non-coordinating counteranion (2b) improved the results (47% yield, entry 5). The reaction with benzylsulfonium barfate 4 further improved the results, and catalyst 4 exhibited a somewhat higher reactivity than that of ammonium barfate 1b (67 and 61% yields, respectively; entry 6 vs. 7).
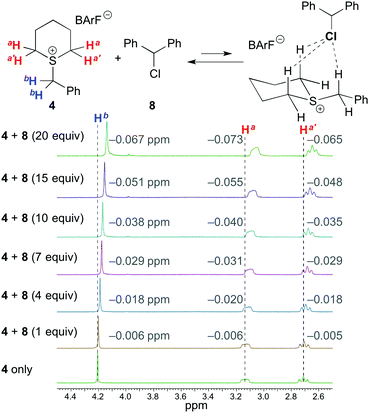
To further investigate the hydrogen-bonding interaction between the α hydrogen atoms of sulfonium salts and the chloride substrates of type 5, we performed NMR titration studies of benzylsulfonium salt 4 with chlorodiphenylmethane 8 as a relatively stable chloride compound (Fig. 3). As a result of the titration of 8, the clear upfield chemical shifts (0.065–0.073 ppm after adding 20 equiv. of 8) of the α hydrogen atoms of 4 were observed in 1H NMR measurements (0.010 M in CDCl3). This result strongly supported the proposition that sulfonium salts could activate chloride substrates of type 5via hydrogen bonding to promote the Mannich-type reaction. A titration experiment with ammonium salt 1b was also performed with the same concentration (0.010 M in CDCl3), and the degrees of chemical shifts (0.041–0.048 ppm after adding 20 equiv. of 8) were smaller than those for the titration with 4 (Fig. S2 in the ESI†).
Benzylsulfonium salt catalyst 4 could accelerate the reaction of N-acylisoquinoline 5a with ketone-derived silyl enol ether 6b to give the corresponding product 7b in a moderate yield (Scheme 1). The reaction of 3-methylisoquinoline derivative 5b with 6a was also accelerated by catalyst 4 to obtain product 7c in a moderate yield. We also examined sulfonium salt-catalyzed regioselective reactions with quinolines 9.13 The reaction of quinoline 9a was efficiently promoted by catalyst 4 to give product 10a in a good yield and regioselectivity (10a/11a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Furthermore, the reaction with 6-chloroquinoline 9b gave product 10b in a good yield with an almost perfect level of regioselectivity (10b/11b >30
1). Furthermore, the reaction with 6-chloroquinoline 9b gave product 10b in a good yield with an almost perfect level of regioselectivity (10b/11b >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
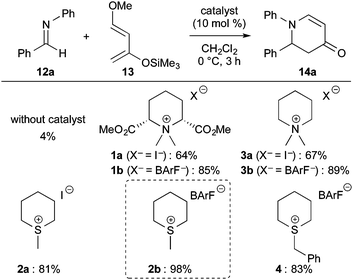
To expand the utility of sulfonium salt catalysts, we were next interested in the activation of imines 12. An aza Diels–Alder reaction of N-phenylbenzaldimine 12a with Danishefsky diene 13 was employed as a model reaction to evaluate the ability of sulfonium salt catalysts for the activation of imines (Scheme 2).14,15 In this reaction, sulfonium iodide 2a showed higher reactivity than ammonium iodides 1a and 3a. Also, sulfonium barfate 2b was a more effective catalyst than ammonium barfates 1b and 3b, and the reaction with catalyst 2b gave the highest yield of product 14a (98% yield). It should be noted that the more sterically hindered benzylsulfonium barfate 4 showed reactivity that was inferior to that of 2b. These results completely agree with our previous observations that sterically less-hindered catalysts are more effective in activating imines 12.6b
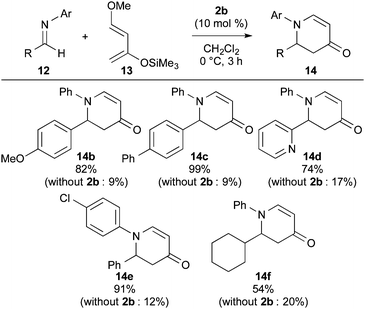
The substrate generality for the aza Diels–Alder reaction of imines 12 was examined with catalyst 2b (Scheme 3). Not only substituted aromatics but also heteroaromatic and alkyl group-introduced imines could be employed for the reaction to obtain products 14b–14f in moderate to high yields (54–99% yields). It should be noted that the addition of catalyst 2b produced clear accelerations of each reaction.
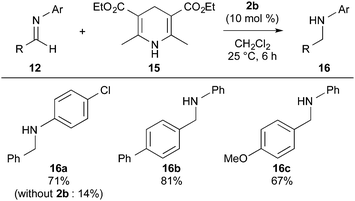
Furthermore, sulfonium barfate 2b efficiently promoted the reduction of imines 12 with Hantzsch ester 15 (Scheme 4).16 The reactions with catalyst 2b gave secondary amine products 16a–16c in good yields (67–81% yields).17
In summary, we have successfully demonstrated that trialkylsulfonium salts can function as hydrogen-bonding catalysts. The structure and binding abilities of sulfonium salts were discussed in this study based on the X-ray crystal structures and 1H NMR titration studies. The catalytic ability of trialkylsulfonium salts was superior to that of the related tetraalkylammonium salts in both the Mannich-type reaction and the aza Diels–Alder reaction. This report revealed a new dimension of sulfonium salt chemistry in organic synthesis on the basis of the characteristic properties of the acidic α hydrogen atoms on alkylsulfonium salts. Further applications of sulfonium salts as catalysts are currently underway in our laboratory.
This work was partially supported by a Grant-in-Aid for Scientific Research(C) from JSPS, the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”, and The Naito Foundation.
Notes and references
- (a) G. A. Olah, Aldrichimica Acta, 1973, 6, 7 CAS; (b) E. Vedejs and F. G. West, Chem. Rev., 1986, 86, 941 CrossRef CAS; (c) G. K. S. Prakash, J. Org. Chem., 2006, 71, 3661 CrossRef CAS PubMed; (d) Recent Developments in Carbocation and Onium Ion Chemistry, ed. K. K. Laali, ACS Symposium Series 965, American Chemical Society, Washington DC, 2007 Search PubMed; (e) J. B. Sweeney, Chem. Soc. Rev., 2009, 38, 1027 RSC; (f) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS PubMed; (g) S. Preshlock, M. Tredwell and V. Gouverneur, Chem. Rev., 2016, 116, 719 CrossRef CAS PubMed.
- (a) L. Kürti and B. Czakó, Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, San Diego, 2005 Search PubMed; (b) J. J. Li, Name Reactions, Springer, Berlin, 2006 Search PubMed; (c) Name Reactions for Functional Group Transformations, ed. J. J. Li, John Wiley & Sons, Hoboken, 2007 Search PubMed; (d) Comprehensive Organic Name Reactions and Reagents, ed. Z. Wang, John Wiley & Sons, Hoboken, 2009 Search PubMed.
- For recent examples of sulfonium salt reagents in organic synthesis, see: (a) R. K. Kunz and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 3240 CrossRef CAS PubMed; (b) E. M. McGarrigle, E. L. Myers, O. Illa, M. A. Shaw, S. L. Riches and V. K. Aggarwal, Chem. Rev., 2007, 107, 5841 CrossRef CAS PubMed; (c) S. Lakhdar, R. Appel and H. Mayr, Angew. Chem., Int. Ed., 2009, 48, 5034 CrossRef CAS PubMed; (d) S. Lin and E. N. Jacobsen, Nat. Chem., 2012, 4, 817 CrossRef CAS PubMed; (e) J. Novacek, L. Roiser, K. Zielke, R. Robiette and M. Waser, Chem. – Eur. J., 2016, 22, 11422 CrossRef CAS PubMed; (f) A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 9842 CrossRef CAS PubMed.
- (a) E. V. Dehmlow and S. S. Dehmlow, Phase Transfer Catalysis, VCH, Weinheim, 3rd edn, 1993 Search PubMed; (b) C. M. Starks, C. L. Liotta and M. Halpern, Phase-Transfer Catalysis, Chapman & Hall, New York, 1994 Search PubMed; (c) Handbook of Phase-Transfer Catalysis, ed. Y. Sasson and R. Neumann, Blackie Academic & Professional, London, 1997 Search PubMed; (d) in Phase-Transfer Catalysis, ed. M. E. Halpern, ACS Symposium Series 659, American Chemical Society, Washington DC, 1997 Search PubMed; (e) M. J. O'Donnell, Aldrichimica Acta, 2001, 34, 3 Search PubMed; (f) T. Ooi and K. Maruoka, Acc. Chem. Res., 2004, 37, 526 CrossRef CAS PubMed; (g) T. Ooi and K. Maruoka, Angew. Chem., Int. Ed., 2007, 46, 4222 CrossRef CAS PubMed; (h) Asymmetric Phase-Transfer Catalysis, ed. K. Maruoka, Wiley-VCH, Weinheim, 2008 Search PubMed; (i) S.-s. Jew and H.-g. Park, Chem. Commun., 2009, 7090 RSC; (j) T. Werner, Adv. Synth. Catal., 2009, 351, 1469 CrossRef CAS; (k) D. Enders and T. V. Nguyen, Org. Biomol. Chem., 2012, 10, 5327 RSC; (l) J. Novacek and M. Waser, Eur. J. Org. Chem., 2013, 637 CrossRef CAS; (m) S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 4312 CrossRef CAS PubMed; (n) S. Kaneko, Y. Kumatabara and S. Shirakawa, Org. Biomol. Chem., 2016, 14, 5367 RSC; (o) S. Liu, Y. Kumatabara and S. Shirakawa, Green Chem., 2016, 18, 331 RSC.
- For trial application of tertiary sulfonium salts as phase-transfer catalysts, see: (a) S. Kondo, Y. Takeda and K. Tsuda, Synthesis, 1988, 403 CrossRef CAS; (b) X.-Z. Zhang, Y.-H. Deng, X. Yan, K.-Y. Yu, F.-X. Wang, X.-Y. Ma and C.-A. Fan, J. Org. Chem., 2016, 81, 5655 CrossRef CAS PubMed.
- (a) S. Shirakawa, S. Liu, S. Kaneko, Y. Kumatabara, A. Fukuda, Y. Omagari and K. Maruoka, Angew. Chem., Int. Ed., 2015, 54, 15767 CrossRef CAS PubMed; (b) Y. Kumatabara, S. Kaneko, S. Nakata, S. Shirakawa and K. Maruoka, Chem. – Asian J., 2016, 11, 2126 CrossRef CAS PubMed.
- For related studies, see: (a) M. T. Reetz, Angew. Chem., Int. Ed. Engl., 1988, 27, 994 CrossRef; (b) M. T. Reetz, S. Hütte and R. Goddard, J. Am. Chem. Soc., 1993, 115, 9339 CrossRef CAS; (c) M. T. Reetz, S. Hütte and R. Goddard, J. Phys. Org. Chem., 1995, 8, 231 CrossRef CAS; (d) M. T. Reetz, S. Hütte, R. Goddard and C. Robyr, Chem. – Eur. J., 1996, 2, 382 CrossRef CAS; (e) M. T. Reetz, S. Hütte and R. Goddard, J. Prakt. Chem., 1999, 341, 297 CrossRef CAS; (f) R. Goddard, H. M. Herzog and M. T. Reetz, Tetrahedron, 2002, 58, 7847 CrossRef CAS; (g) C. E. Cannizzaro and K. N. Houk, J. Am. Chem. Soc., 2002, 124, 7163 CrossRef CAS PubMed; (h) T. Ohshima, T. Shibuguchi, Y. Fukuta and M. Shibasaki, Tetrahedron, 2004, 60, 7743 CrossRef CAS; (i) T. C. Cook, M. B. Andrus and D. H. Ess, Org. Lett., 2012, 14, 5836 CrossRef CAS PubMed; (j) C. Thomas, A. Milet, F. Peruch and B. Bibal, Polym. Chem., 2013, 4, 3491 RSC; (k) C. Thomas, S. Brut and B. Bibal, Tetrahedron, 2014, 70, 1646 CrossRef CAS; (l) T. Kamachi and K. Yoshizawa, Org. Lett., 2014, 16, 472 CrossRef CAS PubMed; (m) C. P. Johnston, A. Kothari, T. Sergeieva, S. I. Okovytyy, K. E. Jackson, R. S. Paton and M. D. Smith, Nat. Chem., 2015, 7, 171 CrossRef CAS PubMed; (n) Q. Peng and R. S. Paton, Acc. Chem. Res., 2016, 49, 1042 CrossRef CAS PubMed; (o) J. Novacek, J. A. Izzo, M. J. Vetticatt and M. Waser, Chem. – Eur. J., 2016, 22, 17339 CrossRef CAS PubMed.
- For reviews on hydrogen-bonding catalysis, see: (a) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289 RSC; (b) P. M. Pihko, Angew. Chem., Int. Ed., 2004, 43, 2062 CrossRef CAS PubMed; (c) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS PubMed; (d) T. J. Auvil, A. G. Schafer and A. E. Mattson, Eur. J. Org. Chem., 2014, 2633 CrossRef CAS.
- R. Gerdil, Helv. Chim. Acta, 1974, 57, 489 CrossRef CAS.
- Another binding mode was also observed in the X-ray crystal structure of 2a (Fig. S1 in the ESI†).
- (a) X.-M. Zhang and F. G. Bordwell, J. Am. Chem. Soc., 1994, 116, 968 CrossRef CAS; (b) J.-P. Cheng, B. Liu and X.-M. Zhang, J. Org. Chem., 1998, 63, 7574 CrossRef CAS.
- (a) M. S. Taylor, N. Tokunaga and E. N. Jacobsen, Angew. Chem., Int. Ed., 2005, 44, 6700 CrossRef CAS PubMed; (b) A. G. Schafer, J. M. Wieting, T. J. Fisher and A. E. Mattson, Angew. Chem., Int. Ed., 2013, 52, 11321 CrossRef CAS PubMed; (c) J. M. Wieting, T. J. Fisher, A. G. Schafer, M. D. Visco, J. C. Gallucci and A. E. Mattson, Eur. J. Org. Chem., 2015, 525 CrossRef CAS; (d) M. Zurro, S. Asmus, J. Bamberger, S. Beckendorf and O. García Mancheño, Chem. – Eur. J., 2016, 22, 3785 CrossRef CAS PubMed see also: ; (e) S. E. Reisman, A. G. Doyle and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 7198 CrossRef CAS PubMed; (f) F. Kniep, S. H. Jungbauer, Q. Zhang, S. M. Walter, S. Schindler, I. Schnapperelle, E. Herdtweck and S. M. Huber, Angew. Chem., Int. Ed., 2013, 52, 7028 CrossRef CAS PubMed; (g) A. Berkessel, S. Das, D. Pekel and J. M. Neudörfl, Angew. Chem., Int. Ed., 2014, 53, 11660 CrossRef CAS PubMed; (h) S. H. Jungbauer and S. M. Huber, J. Am. Chem. Soc., 2015, 137, 12110 CrossRef CAS PubMed.
- (a) T. Itoh, M. Miyazaki, K. Nagata and A. Ohsawa, Heterocycles, 1997, 46, 83 CrossRef CAS; (b) M. Takamura, K. Funabashi, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2000, 122, 6327 CrossRef CAS; (c) H. Rudler, B. Denise, Y. Xu, A. Parlier and J. Vaissermann, Eur. J. Org. Chem., 2005, 3724 CrossRef CAS; (d) Y. Yamaoka, H. Miyabe and Y. Takemoto, J. Am. Chem. Soc., 2007, 129, 6686 CrossRef CAS PubMed; (e) M. Zurro, S. Asmus, S. Beckendorf, C. Mück-Lichtenfeld and O. García Mancheño, J. Am. Chem. Soc., 2014, 136, 13999 CrossRef CAS PubMed.
- (a) Y. Yuan, X. Li and K. Ding, Org. Lett., 2002, 4, 3309 CrossRef CAS PubMed; (b) C. Loncaric, K. Manabe and S. Kobayashi, Chem. Commun., 2003, 574 RSC; (c) Y. Park, E. Park, H. Jung, Y.-J. Lee, S.-s. Jew and H.-g. Park, Tetrahedron, 2011, 67, 1166 CrossRef CAS; (d) Y. Takeda, D. Hisakuni, C.-H. Lin and S. Minakata, Org. Lett., 2015, 17, 318 CrossRef CAS PubMed.
- Other control experiments were also performed (Scheme S1 in the ESI†).
- (a) M. Rueping, C. Azap, E. Sugiono and T. Theissmann, Synlett, 2005, 2367 CrossRef CAS; (b) D. Menche, J. Hassfeld, J. Li, G. Menche, A. Ritter and S. Rudolph, Org. Lett., 2006, 8, 741 CrossRef CAS PubMed; (c) D. Menche and F. Arikan, Synlett, 2006, 841 CrossRef CAS; (d) A. Kumar, S. Sharma and R. A. Maurya, Adv. Synth. Catal., 2010, 352, 2227 CrossRef CAS; (e) P. Bachu, C. Zhu and T. Akiyama, Tetrahedron Lett., 2013, 54, 3977 CrossRef CAS; (f) W. He, Y.-C. Ge and C.-H. Tan, Org. Lett., 2014, 16, 3244 CrossRef CAS PubMed.
- No catalyst decomposition was observed after the reaction.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data for new compounds. See DOI: 10.1039/c6cc08411g |
| This journal is © The Royal Society of Chemistry 2017 |