Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review
Iman
Adipurnama
a,
Ming-Chien
Yang
 *a,
Tomasz
Ciach
b and
Beata
Butruk-Raszeja
b
*a,
Tomasz
Ciach
b and
Beata
Butruk-Raszeja
b
aDepartment of Materials Science and Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan. E-mail: myang@mail.ntust.edu.tw
bFaculty of Chemical and Process Engineering, Warsaw University of Technology, Warsaw, Poland
First published on 12th December 2016
Abstract
Cardiovascular implants, especially vascular grafts made of synthetic polymers, find wide clinical applications in the treatment of cardiovascular diseases. However, cases of failure still exist, notably caused by restenosis and thrombus formation. Aiming to solve these problems, various approaches to surface modification of synthetic vascular grafts have been used to improve both the hemocompatibility and long-term patency of artificial vascular grafts. Surface modification using hydrophilic molecules can enhance hemocompatibility, but this may limit the initial vascular endothelial cell adhesion. Therefore, the improvement of endothelialization on these grafts with specific peptides and biomolecules is now an exciting field of research. In this review, several techniques to improve surface modification and endothelialization on vascular grafts, mainly polyurethane (PU) grafts, are summarized, together with the recent development and evolution of the different strategies: from the use of PEG, zwitterions, and polysaccharides to peptides and other biomolecules and genes; from in vitro endothelialization to in vivo endothelialization; and from bio-inert and bio-active to bio-mimetic approaches.
Introduction
Major cardiovascular diseases (CVDs), including stroke and heart attack, are the leading cause of deaths worldwide. Mortality data show that CVDs as the listed underlying cause of death accounted for 31.9% (787![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650) of all 2
650) of all 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468
468![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435 deaths in 2010, or 1 of every 3 deaths in United States.1 The most notable cause of the cardiovascular disease is atherosclerosis, a build-up fatty plaques in the arteries.2 Atherosclerosis is a chronic inflammatory disease, producing arterial plaques characterized by inflammatory infiltrates, lipid accumulation, cell deaths, and fibrosis.3
435 deaths in 2010, or 1 of every 3 deaths in United States.1 The most notable cause of the cardiovascular disease is atherosclerosis, a build-up fatty plaques in the arteries.2 Atherosclerosis is a chronic inflammatory disease, producing arterial plaques characterized by inflammatory infiltrates, lipid accumulation, cell deaths, and fibrosis.3
The term percutaneous coronary intervention (PCI) is commonly used to describe procedures that can be used to mechanically improve myocardial perfusion without resorting to surgery.2 PCI is now carried out with the insertion of a stent. Stents made from metal are still mainstream and have been applied for a long time, since the 1980s. Although coronary artery infection after stent implantation is rare, the impact is a devastating and potentially life-threatening complication of PCI. Furthermore, the inflammatory response to this infection can contribute to restenosis in metal stents.2
Aside from the stent, the gold standard for a vascular replacement is using an autologous native vessel, which possesses the most physiological properties. The most common treatment is coronary artery bypass graft (CABG) surgery. In CABG surgery, the internal mammary artery and the radial artery are superior to a greater saphenous vein graft (SVG).4 However, the availability of native vein and artery segments is limited, which limits traditional transplantation surgeries.
An ideal vascular substitute is a major clinical essential in blood vessel-related surgery, such as CABG surgery. Blood vessels have a complex structure and functionally dynamic tissue, with minimal regeneration potential.5 The diverse function performed by blood vessels is maintained by the presence of the complex extracellular matrix (ECM) of blood vessels. The ECM varies in its composition, thickness, and overall architecture, differing between arteries, capillaries and veins.6 Thus, it poses a challenge for finding a consistent tissue engineering approach while choosing an appropriate material for tissue engineered vascular grafts (TEVG).
Boffito et al. summarized the requirements needed for the tissue engineering of vascular grafts,7 including biocompatibility, biodegradability, and biomimicry. In other words, it must replicate the properties of the cardiac extracellular matrix (ECM) in terms geometry, mechanical properties and interaction with cells, and degrade into non-toxic degradation products as the need for mechanical support decreases. Ideally, the scaffold should have an interconnected porous structure, a high surface-to-volume ratio and appropriate porosity and pore dimensions to favor cell homing and migration, vascularization and nutrient and oxygen diffusion.7–9
Several commercial artificial vascular grafts have been approved by the U.S. Food and Drug Administration and millions of patients have benefited from these products. Due to the ease of polymer synthesis, the controlled adjustment of their properties, high reproducibility and rapid availability of polymers such as polyethylene (PE), poly(methyl methacrylate) (PMMA), polyurethanes (PUs), polyglycolide (PGA), and polylactide (PLA), they have been selected for implants and other medical devices.10 Among the aforementioned polymers, the most common material in the production of blood-containing devices is PU.11
In general, vascular tissue engineering with the use of polymeric scaffolds represents a promising approach in meeting the growing demand for blood vessel replacements. To solve this cardiovascular problem, the most viable approach is by using small-diameter (<6 mm) autologous veins and arteries.12 Because of the limitation in providing this material and the increasing risk of thrombosis and occlusion in small-diameter vascular grafts,13 the need for a new strategy to improve compliance and hemocompatibility of synthetic vascular grafts has become important.10,14,15
The most common approaches used to resist the non-specific adhesion of proteins, improve hemocompatibility and enhance long-term patency of artificial vascular grafts are surface modifications, either physical or chemical, while some research also considered endothelialization of the inner surface of vascular biomaterials.10,15–17 Both of these approaches were used to overcome the problems that were mentioned in the previous section, mainly thrombogenicity and hemocompatibility.
This review will focus on the recent developments of polyurethanes and their surface modification. In the first part, we will briefly introduce several biomaterials used for artificial vascular grafts and describe this in more detail for polyurethanes. The second part will review the surface modification methods of polyurethanes to improve their surface properties. Such surface modifications involve various physical and chemical treatments, such as grafting hydrophilic polymers and zwitterionic polymers or groups, and also immobilization of bioactive molecules. The last part will review surface endothelialization on the inner surfaces of biomaterials.
Synthetic vascular grafts
Polymer-based synthetic vascular grafts are one of the options since they have been commercially available since the 1970s, often made of expanded polytetrafluoroethylene (ePTFE), Dacron (PET) or PU, in particular Dacron and ePTFE which have been used for more than 50 years.4,15,18 Currently, Dacron is the most commonly used for aortic replacement and to a lesser extent as a conduit for femoropopliteal bypass surgery.19Aside from Dacron and ePTFE, the most common material used in synthetic vascular grafts is polyurethane. Since the 1930s, after being first synthesized, PU has found many applications, such as in rigid foams, adhesives, resins, elastomers and coatings. Today, PU is known as one of the most biocompatible and blood compatible materials, with applications ranging from catheters to total artificial hearts.11
PU consists of three different monomer types: a diisocyanate hard domain, a chain extender, and a diol soft domain. Soft domains provide flexibility while hard domains impart strength. The most common medical grade polyurethanes are based on soft domains made from polyester, polyether or polycarbonate. With its potential to surpass the other materials, various components have been added to the graft design to improve synthetic graft function and yield biohybrid conduits.19
However, the general problems in all these polymers are that they have limited resistance to thrombus formation on the surface, which leads to the obstruction of the grafts. To further improve the potential of polyurethane, a strategic approach to modify its surface is needed.
Blood–material reaction
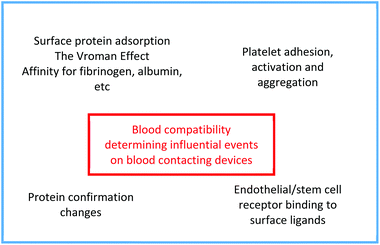
Host reactions to the vascular prosthesis start immediately after restoration of blood circulation. The tissue–prosthesis and blood–prosthesis interfaces are complex microenvironments, and several physicochemical properties such as charge, energy, wettability, and topography might hold responsibility for the graft patency and thus influence the interfacial behavior adjacent to the biomaterial.20–22The interfacial region at the blood–biomaterial surface will continue to alter and redistribute the protein/electrolyte/water layer, and the host cells and tissues react to changes in this layer. In addition, the adsorption of proteins will direct and aid the blood cell adhesion, especially platelets. The cellular components interact with the protein layer to guide migration, initiate blood coagulation, and stimulate cell proliferation and differentiation.23Fig. 1 summarizes the main mechanisms influencing blood compatibility.
One of the common and severe events, as mentioned in an earlier paragraph, is thrombus formation on the surface of the material, which leads to obstruction. To understand the thrombus formation, each step within the blood–material interactions needs to be observed.
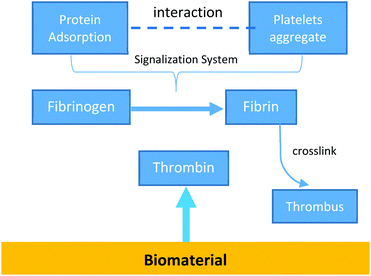
The first step of this reaction is the plasma protein adsorption/desorption process, which is commonly known as the Vroman effect, followed by platelet adhesion and eventually endothelial and smooth muscle cell migration.21,22
Once attached to the surface, platelets aggregate and become active. This activation leads to the release of several molecules that act as a signal. A series of complex and succeeding reactions takes place.14,22 During this stage, thrombin converts the soluble fibrinogen into insoluble fibrin strands. Those strands are then crosslinked, forming a thrombus.
The resultant thrombus may detach from the surface and get carried away, resulting in the blockage of a blood vessel, known as thromboembolism.22 This usually happens in the lungs, brain, gastrointestinal tract, kidneys, or legs. It is a significant cause of patient morbidity and mortality. Fig. 2 represents this process.
To minimize the inflammatory response and promote endothelialization, biodegradable materials have received particular attention as scaffolds. They generally demonstrate tailorable mechanical properties and high reproducibility and compared to natural polymers, can be produced in high amounts.24–26 There are several examples of these polymers, such as poly(ε-caprolactone) (PCL), PGA, and PLA.27–29
However, none of the biodegradable materials can usually be implanted for long-term bio-applications. Although the non-degradable polymers in the previous section have been described as being excellent for the preparation of artificial vascular grafts in long-term applications, there are concerns regarding their lack of good surface properties. For ideal artificial vascular grafts, their surface properties, such as hemocompatibility and anti-thrombotic and anti-infective activities, should be considered. Thereby, to achieve those goals, several strategies have been developed over the years.
The hemocompatibility of biomaterials is mainly dependent on their physical and chemical surface characteristics. Surface modification is one of the most direct and effective strategies to minimize thrombogenicity and to improve hemocompatibility.14,30 One remarkable advantage is that the intrinsic mechanical properties of the biomaterials and grafts are not significantly changed after the surface modification process takes place. In this review, the biomaterials we will focus on are polyurethanes.
Polyurethanes
Polyurethanes (PUs) are well known as a type of commercial biomaterial due to their biocompatibility and mechanical properties.11 They are a class of polymers with carbamate groups (–NHCOO–) on their backbones. They have recently gained substantial interest because of their tunable biological, biochemical and biomechanical properties and also various favorable properties.31The first biomedical grade polyether polyurethane was synthesized by Boretos and Pierce.32 Both of them introduced the biomedical application of segmented polyether polyurethanes containing hard segments of urea and soft segments of polyether linked by the urethane group. These materials sustained high modulus of elasticity, biocompatibility, resistance to flex-fatigue and good stability over long implantations.
Polyether-based polyurethanes are indeed more stable than polyester-based polyurethanes (PEU) and poly(carbonate urethanes) (PCU) in hydrolytic degradation tests in vitro. But they seem to degrade significantly faster under enzymatic attack and in oxidative environments in vivo, especially under high stress.10
Several researchers also described a failure mechanism affecting polyether PUs under strain with the term environmental stress cracking (ESC).33 This description implicated factors from in vivo environments such as oxidative processes, residual stress, ether content in the soft segment and the presence of cells associated with the foreign body response (FBR), as well as an unknown biological element. The factors affecting the performance of PU are summarized in Table 1.
| Parameter | Notes |
|---|---|
| Mechanical properties | Materials must be saturable, with elasticity depending on the application, and have sufficient modulus without being too stiff (e.g., cardiac tissue has a modulus of 10–50 kPa, tensile strength of 3–15 kPa, and strain of 22–90%).7,33 |
| Degradation | Slower-degrading materials have performed better than fast-degrading PUs. Degradation by-products should be non-toxic.33 |
| Porosity | Porosity must be sufficient to allow tissue infiltration, not compromise rate of degradation, and promote cell attachment and growth.34,35 |
| Non-activating chemistries | For blood-contacting PUs, chemistries that reduce platelet and white blood cell activation are essential.35,36 |
In conclusion, polyether-based polyurethanes cannot be used to prepare medical devices for long term applications. Therefore, PCUs have been developed to address the instability problem of PUs.10 In addition, PCUs are relatively stable comparing with PEUs.10,37
PCUs are considered to be stable and provide beneficial hemocompatibility and good mechanical properties. In accordance with their properties, PCUs have been widely used in catheters, vascular grafts, blood bags and artificial hearts. Most importantly, PCU artificial grafts show similarity in their mechanical properties and compliance with natural blood vessels.
However, the formation of thrombi remains the main cause of implant failure. Thrombus formation is caused by platelet adhesion and the failure of rapid endothelialization, which is an enormous challenge.10,22 Thus, a strategic approach is needed to inhibit the thrombus formation.
A novel nanocomposite polymer, POSS-PCU, has been developed by covalently attaching a chemically robust polyhedral oligomeric silsesquioxane (POSS) nanocage to the PCU backbone to improve the in vivo biostability of PUs.38–40
There are several molecules that can be used in modifying the PU surface, including PEG and peptides. A peptide sequence as a chain extender and PEG as a soft segment in the polymer backbone in PEG-modified PU were incorporated to reduce the thrombogenicity.11,41 Another explanation regarding surface modification of polyurethane will be reviewed in the next section.
Surface modification
The performance of a material in a range of biological environments is initially dependent on its surface properties.22 Most of the biological reactions occur at surfaces and interfaces. As described earlier, the performance of a blood-contacting device depends on the reaction with blood on its surface.Integration of materials or blood-contacting devices into a cardiovascular system leads to a number of unfavorable reactions, such as protein adsorption, initiation of the coagulation cascade with fibrin formation, activation of the complement cascade, and dysfunction of the endothelium caused by the damage of endothelial cells (ECs) and hyperplasia of smooth cells (SMCs).14
In order to overcome unexpected interactions between the surface and tissue, surface modification has been introduced.10,14,22 Understanding how tissue reacts to implanted devices is essential for developing new cardiovascular devices or surface modification methods.42 Moreover, this will enable the better design of the surfaces of blood-contacting materials in cardiovascular implants.23
The tissue reaction process can described as follows: (1) it begins with protein adsorption on exposure to the body fluids and a protein layer is formed on implant surfaces; (2) these proteins are followed by cells of the immune system where (3) macrophages recognize the layer and attempt to engulf what is presumed to be a foreign invader. (4) Because the size scale of most implants is an order of magnitude larger than the cells themselves, the cells fuse to form foreign-body giant cells (FBGCs). These FBGCs release chemical signals that attract fibroblasts to the region.42–44
Meyers et al. hypothetically concluded that there are two approaches that can be used. First, the surface must be treated so as to prevent the nonspecific protein coating from forming, with the effect of making the device “stealthy” by producing a non-fouling surface coating. Second, the incident cells of the immune system and cells surrounding the site of implantation must be provided with attached integrin ligands and cytokines so the biological–material interaction can occur properly. Such a coating can create a bioactive surface with which biology has an inherent communicative understanding. Both of these approaches are biomimetic by design.42
Surface modification plays an important role in biomedical applications since coatings can be used to adapt the surface to the desired properties without compromising its bulk properties. Such modification will be influenced by nanotechnology, which plays an important role through bio-functionalization of polymers and peptides in nanocomposites and through nanofabrication of polymers, which will pave the way for finding a closer blood match through hemostasis when developing cardiovascular implants with a greater degree of patency.23
Alves et al. summarized that there are several major classes of surface modification techniques that can be considered.22 The first concerns surface modification by physical agents. These include flame treatment, corona discharge treatment, plasma treatment, etching, ultraviolet (UV) radiation exposure, laser ablation treatment, gamma irradiation, and X-ray treatments. The second includes surface modification by using chemical agents and functional group incorporation, such as surface oxidation, hydrolysis, chemical grafting, and surface coating.22 The third class involves the use and immobilization of biological molecules or cells onto the surface of the materials.10 Thus, various biomolecules can be combined to be immobilized on the surface.
Several surface modification methods have been developed over the years. Some of them, as detailed by Alves et al.22 and Meyers et al.,42 are listed below:
1. The physical deposition of active compounds by coating.
2. The covalent immobilization of polymer chains onto a surface by chemical reactions, taking place after coating.
3. The grafting of polymer chains on the surface after and during plasma treatment by corona discharge, and grafting that has been initiated by γ-radiation treatment.
4. Bioactive surface coatings, modulating cell interactions with an implanted surface through providing signals that direct biological activities at the interface.
5. A biomimetic combined approach, combining non-fouling PEG and biomimetic sequences.
Qi et al. summarized the strategies developed during the past 30 years as shown in Fig. 3. Bioinert and bioactive coatings are based on the regulation of physical, chemical or biological properties. It is yet still difficult to prepare an ideal surface with anticoagulant properties, endothelialization promotion and SMC inhibition. With a deeper understanding of the EC microenvironment and the usage of bio-inspired materials, as well as an approach to mimicking a self-healing system to accelerate endothelialization, the “Biomimic” approach has been established. Thus, the surface modification techniques have made great progress over the years from the concept of bio-inertness to bio-activity and nowadays biomimetic strategies and in situ induced endothelialization are applied.14
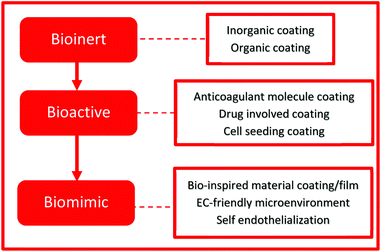 | ||
| Fig. 3 The strategies evolved over the years, shifting from bioinert to bioactive and lastly biomimic. | ||
Similar to Meyers et al., there are two approaches to the development of cardiovascular grafts described by de Mel et al.23 The first involves the design of a permanent vascular replacement, which is a non-adhesive, inert, non-biofouling surface. Physicochemical methods have been applied to achieve this aim as described in the previous section.
The second approach aims to functionalize the grafts, which facilitate a cascade of biological events that eventually regenerates or replaces functioning tissue. The term bio-functionalization is popularized for the achievement of this goal.
Polyurethane surface modification
Polyurethane can be surface modified by using either biological, chemical or physical methods. Surface modification can be carried out by radiation grafting of monomers, chemical modification, immobilizing biological molecules and silanization. With these techniques, PU surfaces have been modified using molecules such as PEG, sulfobetaine monomers and other molecules containing hydroxyl groups. This section will describe several modifications that have been widely used to modify PU surfaces and proved to increase its hemocompatibility.PEG and its derivatives
Poly(ethylene glycol) (PEG) has been widely used as a chain extender or additional material because of its hydrophilicity.10 It has good resistance against the adsorption of plasma proteins and platelet adhesion due to high surface mobility under physiological conditions.45 However, the grafting density of PEG was relatively low due to the lack of surface functionality on PUs.Jung et al. used the thiol–ene system to overcome the lack of surface functionality on PUs.45 A thiol–ene system employs the reaction between thiols and ene (vinyl) monomers, which proceeds via a step-growth radical addition mechanism. The thiolated surface of PU was developed from its surface with allyl groups, as the substrate for grafting the hydrophilic vinyl monomer, poly(ethylene glycol) methacrylate (PEGMA). PEGMA was then grafted onto the thiolated PU surface via thiol–ene polymerization. The schematic of the process of the thiol–ene system is described in Fig. 4.
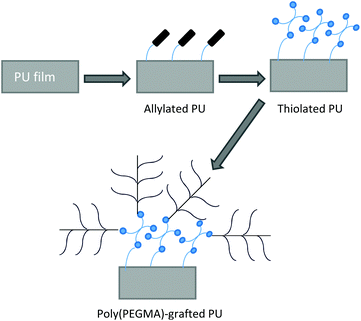 | ||
| Fig. 4 Schematic illustration of a thiol–ene system. The thiolated surface of PU is developed from the surface with allyl groups, acting as the substrate for grafting PEGMA. | ||
Qiu et al. used plasma-induced graft polymerization of poly(ethylene glycol) methacrylate (PEGMA) to introduce PEG graft chains onto the polyurethane surface.46 PEG chains were successfully grafted as indicated by the change of water contact angle on the surface.
The other approach to incorporate PEG is by using the electrospinning method. Wang et al. fabricated crosslinked PU/PEGMA directly from PU and PEGMA macromonomer solutions by the reactive electrospinning method.47 In the reactive electrospinning process, PEGMA molecules can react with the crosslinker to generate a network structure that could penetrate into PU macromolecules to form a semi-interpenetrating network.
Wang et al. confirmed the advantages offered by the crosslinked PU/PEGMA scaffolds in providing appropriate mechanical properties combined with good cytocompatibility.47 Combining PU with the PEGMA polymer through the reactive electrospinning process could provide a potential substitute for artificial vascular scaffolds.
On the other hand, another approach was introduced by Yuan et al., combining the electrospinning process with the surface-initiated atom transfer radical polymerization (SI-ATRP) method. The fibrous PEGMA molecules were grafted to the fiber surface by the SI-ATRP method.48 There are three steps for this surface modification, as described in Fig. 5.
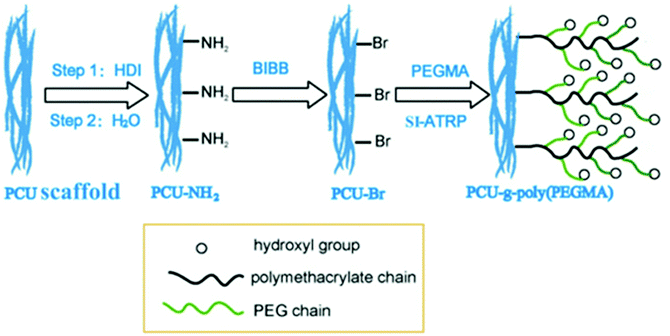 | ||
| Fig. 5 Schematic illustration of introducing PEGMA onto PCU using a two-step process of chemical reaction and SI-ATRP.48 Reprinted with permission from Elsevier © 2013. | ||
First, amino groups were introduced to PCU by immersing PCU into the reactive solution containing hexamethylene diisocyanate (HDI). Second, Br groups were introduced by immersing PCU-NH2 in n-hexane and triethylamine (TEA) solution while 2-bromoisobutyryl bromide (BiBB) was slowly dropped in. Lastly, a solution containing CuBr2 and 2,2-bypiridine (Bipy) were prepared, and the PCU-Br scaffold submerged into the solution while CuBr was added to form a CuBr–Bipy complex, which acts as an ATRP catalyst. The PEGMA molecules were then incorporated as the polymerization was performed at 50 °C with stirring for 24 h under nitrogen gas atmosphere.
These two studies by Wang et al. and Yuan et al. indicated that the increase in the hydrophilic properties of PUs also increases their biocompatibility and hemocompatibility, due to better cell attachment to the hydrophilic surface, which influences their growth. It is an advantage to use PEGMA molecules to form a porous PU scaffold, in comparison with the traditional method.49
Zwitterionic polymers
Zwitterionic polymers are well known as hydrophilic and non-fouling materials.50–53 They have both cationic and anionic moieties on the same chain while the overall charge remains neutral. Zwitterions usually include phosphobetaine, sulfobetaine, and carboxylbetaine. They are named according to the difference in the negatively charged groups, as described in Fig. 6. Zwitterionic betaines generate a tightly bound, structured water layer around the head groups via electrostatic and hydrogen bond-induced hydration in water, thus reducing protein adsorption and platelet adhesion. Because of that, it will effectively control the coagulation cascade and immune inflammation. The surface of modified biomaterials will be improved significantly.51,54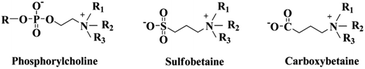 | ||
| Fig. 6 Chemical structures of phosphorylcholine, sulfobetaine, and carboxybetaine. Reproduced from ref. 10 with permission from The Royal Society of Chemistry. | ||
A zwitterionic sulfobetaine monomer was grafted onto a PU vascular catheter surface by ozone-induced polymerization by Yuan et al.55 The schematic of the process is described in Fig. 7.
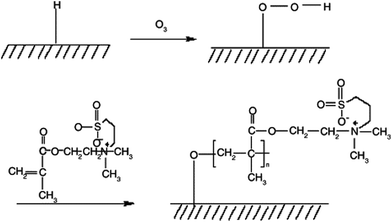 | ||
| Fig. 7 Schematic illustration to describe ozone-induced polymerization for grafting sulfobetaine onto a PU surface.55 Reprinted with permission from Elsevier © 2004. | ||
Sulfobetaine was also grafted onto a PU surface through two chemical reaction steps by Jiang et al.56 In the first step, PU was coupled with vinyl groups which were obtained through the reaction of the NH-urethane group and acrylic acid (AA), using dicyclohexylcarbodiimide (DCC) as a condensation agent and 4-[N,N-dimethylamino]pyridine (DMAP) as a catalyst. The reaction formed an amidic bond between the carboxyl group of AA and NH-urethane group of PU. In the second step, sulfobetaine was grafted using 2,2′-azobisisobutyronitrile (AIBN) as an initiator.
Elastic PU with sulfobetaine incorporated into the polymer backbone was synthesized by Ye et al., where the sulfobetaine content could be readily tuned by altering the molar ratios of diols employed in the synthesis, as shown in Fig. 8 and 9.36 This approach is straightforward and does not require a subsequent step to graft on the biofunctional moieties.
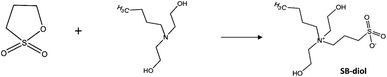 | ||
| Fig. 8 Schematic of the synthesis of sulfobetaine-diol (SB-diol).36 Reprinted with permission from American Chemical Society Publications © 2014. | ||
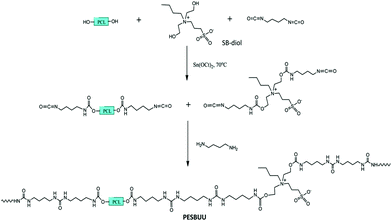 | ||
| Fig. 9 Schematic of the synthesis of biodegradable polyester sulfobetaine urethane ureas (PESBUUs).36 Reprinted with permission from American Chemical Society Publications © 2014. | ||
In several attempts, phospholipids were used for grafting through a zwitterion-mediated approach. Tan et al. grafted the phospholipids to carbon nanotubes through a zwitterion-mediated cycloaddition reaction, which avoided cutting the CNTs and kept their unique structures.57 These combined phospholipid–CNTs were used as additives in a PU composite forming phospholipid-biomimetic surfaces to improve hemocompatibility.
2-Methacryloyloxyethyl phosphorylcholine (MPC) is a custom methacrylate with a zwitterionic phosphorylcholine moiety on the side chain. It has been used as a building block over the years. MPC has been synthesized many times for biomaterial applications. It can be used alone or in combination with other materials.52 Morimoto et al. incorporated MPC in order to surface-modify a segmented PU film. The segmented PU film was immersed in a monomer solution and polymerization occurred at the mica interface to condense MPC to the surface.58 Tan et al. grafted phosphorylcholine to the surface of PU-PEI (polyethyleneimine) films in order to improve biocompatibility and anticoagulant activity of PU.59
Zwitterionic polynorbornene (poly(NSulfoZI)), which has many double bonds as well as zwitterions, can be used to modify PCU via the thiol–ene click-reaction, which has been done by Khan et al. and is described in Fig. 10.60 In another study regarding the thiol–ene click reaction, Khan et al. treated the PCU surface with thiol agents (L-cysteine and β-mercaptoethanol) to immobilize sulfhydryl groups, then polynorbornene was grafted onto these surfaces.53
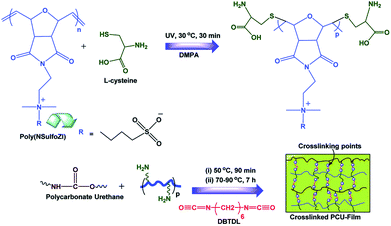 | ||
| Fig. 10 Schematic illustration of the preparation of crosslinked PCU materials using photo-initiated thiol–ene click-reaction and crosslinking.60 Reproduced from ref. 60 with permission from The Royal Society of Chemistry. | ||
Polysaccharides
Gradually, scientists have tended to adopt the design of bio-active surface modifications. The typical example is the well-known heparin-coated vascular graft. The strategy is to immobilize the pharmacologically active agents that regulate coagulation with molecules such as polysaccharides, anti-coagulant polypeptides, anti-proliferative agents, functional proteins and nitric oxide releasing compounds.14 Each of these active agents will be further discussed in the next section.Polysaccharides are actually biopolymers derived from renewable resources such as plants (starch, cellulose), animals (chitin/chitosan, heparin), algae (alginate, galactans), microorganisms (dextran, xanthan gum, pullulan), etc. and thus are abundant in nature.61 The most apparent properties compared with synthetic polymers are their biodegradability, abundance in nature, non-toxicity, low processing cost, and biocompatibility. The presence of free amino, carboxyl and hydroxyl groups made it possible to alter their properties, specifically their surface properties.62 Polysaccharides have found wide applications in clinical medicine on a large scale, including cellulose, heparin, starch, pullulan, chitin, chitosan, dextran, hyaluronan and alginate.62,63
Heparin is one of the most intensively studied glycosaminoglycans (GAGs) as a result of its anticoagulant properties.64 It has been used in both vascular grafts and stents and is clinically the most successfully applied polysaccharide. It is a relatively large polysaccharide, having a polydispersity of 1.2–1.4 and a molecular weight range of 5000 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. It is hydrophilic, holding 2–10% water even after extensive drying. It has a number of chemically reactive functional groups. Each disaccharide repeating unit contains a carboxyl group. All repeating units contain one or more hydroxyl groups and an average of 2–2.5 sulfo groups.
000. It is hydrophilic, holding 2–10% water even after extensive drying. It has a number of chemically reactive functional groups. Each disaccharide repeating unit contains a carboxyl group. All repeating units contain one or more hydroxyl groups and an average of 2–2.5 sulfo groups.
Aksoy et al. reported that a PU surface was successfully activated by oxygen plasma and modified by covalent immobilization of heparin to investigate the effect on cell adhesion.65 Oxygen plasma creates peroxide groups at the surface, which can react with acrylic acid and then be linked to heparin with the help of N′-3-(dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC).
By mixing a polyurethane and heparin solution, and then immersing a metal wire for creating tubes, Yan et al. successfully created PU/heparin vascular grafts.66 The metal wire was dipped in the solution, then drawn out and dried while being rotated in the air. After evaporation of the solvent, the wire was dipped again in the solution and the solvent evaporated again. This process was repeated until a thickness of 0.1 mm polymer was deposited on the wire surface.
Bae et al. used dopamine to form thin, surface-adherent films on inorganic surfaces.67 Further, they conjugated heparin to dopamine using EDC and NHS. The carboxyl groups of heparin were converted to reactive NHS-esters using EDC and NHS, and thereafter heparin was conjugated by reaction of NHS-activated Hep-COOH with residual primary amino groups in the EDC/NHS crosslinked dopamine matrix.
Another approach is by using an anti-thrombin–heparin (ATH) covalent complex, which has been developed and does not have the drawbacks of heparin, such as susceptibility to leaching, non-uniform distribution, and variable anticoagulant activity.68 Du et al. immobilized ATH onto a PU surface. It was shown that ATH distributed uniformly on the surface and was able to significantly attract antithrombin plasma protein as an important requirement for a heparinized surface to display anticoagulant properties.
Choi et al. immobilized heparin on the PU surface in a two-step process, first by introducing amino groups to the surface of PU. Heparin was then chemically immobilized onto the aminated PU-PEG surface. The carboxyl groups of heparin were activated by EDC and NHS. Later, the modified surface was rinsed with distilled water.69 Choi et al. do not only use heparin, but also several peptides to modify the surface.
Chitosan is a linear polyelectrolyte carrying positive charges. It has both amino and hydroxyl groups and been identified as a non-toxic, biodegradable, cell compatible and biocompatible material. It has been widely used as a wound healing accelerator, a health food to reduce blood cholesterol level, and an immune system stimulant.70 Lin et al. prepared water-soluble chitosan and used it to improve the hemocompatibility of a thermoplastic polyurethane (TPU) substrate via ozone-graft polymerization.71 Aside from chitosan, they also incorporated dextran to modify the PU surface. On the other hand, Kara et al. modified the PU surface with chitosan using oxygen plasma and incorporating acrylamide and glutaraldehyde to graft chitosan (Fig. 11).72
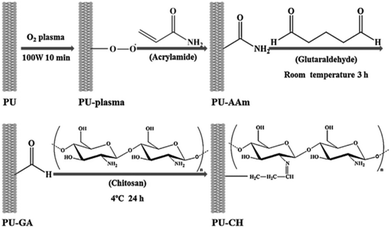 | ||
| Fig. 11 Schematic description of the surface modification of PU via oxygen plasma, involving two chemical processes to modify the PU surface with chitosan.72 Reprinted with permission from Elsevier © 2014. | ||
Hyaluronic acid (HA) is also being used to modify PUs. HA is a linear nonsulfated glycosaminoglycan made of glucuronic acid and N-acetyl glucosamine. A hyaluronan-based material as a graft, when implanted, could completely degrade with the formation of neotissue with complete endothelium, collagen, and elastin.5 PU-HA grafts outperformed those PUs modified with either PEG or heparin. Such grafts can clearly reduce protein adsorption and platelet and bacterial adhesion, as well as fibroblast and macrophage proliferation.73
A tubular PU sandwich-like adipose-derived stem cell (ADSC)/gelatin/alginate/fibrin construct was reported by He et al.74 They combined the advantages of the cell-assembly, low-temperature deposition, and cell cryopreservation techniques to fabricate a tubular PU sandwich-like cell/hydrogel construct. A cryoprotectant was basically incorporated in the ASDC/gelatin/alginate/fibrinogen hydrogel system and the PU was dissolved in a cell-compatible organic solvent, and then they were frozen using a double nozzle, low-temperature deposition method. This construct was stored at −80 °C for 1 week before being thawed and cultured.
The ADSCs survived during the fabrication, freezing, and thawing stages, and proliferated during the 2-week in vitro culture. In another report, Wang et al. successfully attained a high 80% cell survival rate with a formation time of within 20 min and with the ratio of gelatin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alginate
alginate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fibrinogen set as 2
fibrinogen set as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75
1.75
Surface endothelialization
When patients were implanted with a graft, the results was poor: endothelial cells do not adhere and proliferate on the implanted graft. On the other hand, animals will endothelialize any graft even if they are not seeded. This explains the development of numerous in vitro perfusion systems for testing the endothelialization of graft surfaces. One way to solve this problem is to incorporate a large amount of cells, which requires 2–4 weeks of culture time. However, this method has become unsuitable for use.76A complete alternative approach has been devised using a fully engineered graft made from a scaffold together with a mixture of smooth muscle cells and endothelial cells.76 This approach takes weeks to months of incubation in bioreactors before the vessels are ready for implantation. The term coined for this process is endothelialization. This section will review the surface endothelialization process, and how to enhance it.
Currently, endothelialization is the most promising strategy to fabricate non-thrombogenic materials. Endothelial cells can be applied onto the synthetic implant before the procedure (in vitro endothelialization) or can be recruited from the bloodstream and surrounding areas (in situ endothelialization).
In vitro endothelialization
Most synthetic vascular scaffolds often suffer from poor recognition by cells due to the lack of specific cell interactions, whereas endothelialization is essential for improving the blood compatibility of artificial vascular grafts.77 The endothelial cells are thus applied onto the surface of artificial vascular grafts and this is named in vitro endothelialization.Attempts to modify PU and other polymer surfaces have included surface modification by plasma treatment,78 chemical modification by grafting of hydrophilic components,45–47,50,55 immobilization of bioactive agents38,59,73,79–81 and direct surface introduction of proteins or endothelial cells (ECs) by either physical or chemical coupling.82–84
Chong et al. devised an approach to investigate the synergistic role of surface topography and chemistry in promoting endothelialization within nanocomposite vascular graft materials.17 Polyhedral oligomeric silsesquioxane polycarbonate urethane (POSS-PCU) patterned polymer films were fabricated using photolithography, electron beam lithography and then plasma treatment with various parameters. The oxygen plasma treatment successfully both increased the hydrophilicity of POSS-PCU and promoted endothelialization. Another POSS-PCU modification approach has also been reported.16 Several reports also showed the impact of surface topography in promoting endothelialization for vascular graft applications.84,85
RGD peptides and their derivatives
The survival, proliferation, migration and adhesion of ECs are largely dependent on their interactions with surrounding extracellular matrix (ECM) molecules, such as fibronectin (Fn), laminin (Ln), collagens, vitronectin (Vn), and HSPG.86 Some of these molecules have been studied and applied for surface endothelialization modification. Fn coatings may improve EC adhesion, spreading, proliferation, and migration.87 Integrins on the EC surface bind to specific amino acid sequences within ECM molecules and trigger signaling pathways. Therefore, biomaterial surfaces coated with adhesive proteins may improve EC adhesion, migration, and proliferation in vitro and thereby reduce the culture period.One of the most popular approaches is to immobilize the best Fn-derived peptide sequence, RGD (Arg-Gly-Asp). RGD peptides are the major functional amino acid sequences found in all major ECM proteins and have been known to be related to the attachment of ECs, specifically binding to αIIbβ3 or glycoprotein IIb/IIIa.88
There are numerous attempts with various methods that have been reported for conjugating RGD peptides and their derivatives to PU or another biomedical polymer.88–91 According to Wang et al., the molecular analysis of the peptides showed that hydrogen bonding is the primary driving force, and such peptide-treated PU materials showed much-improved hydrophilicity and enhanced anti-fouling properties. The binding affinity observed in their study is largely achieved through hydrogen bond interactions via the abundant carbonyl groups of PU.90
Oh et al. developed a tyrosinase-catalyzed oxidative reaction to immobilize bioactive peptides which can be applied to any type of surface.77 Tyrosinase (Ty) is an enzyme that catalyzes the oxidation of phenol molecules into o-quinones, immediately passing the intermediate state (o-dihydrocyphenol) in the presence of oxygen.77,92
RGD peptides with tyrosine and GGGGG (PRGD-Y) were employed as the Ty-reactive and spacer residues and using this approach RGD peptides can be immobilized onto PU microfibers by dipping the microfiber into an aqueous solution of the enzyme. The illustration and experimental scheme is depicted in Fig. 12 and 13.
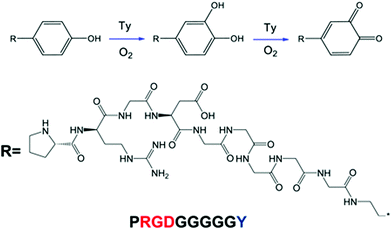 | ||
| Fig. 12 Schematic illustration of the immobilization of PRGD-Y peptides on PU meshes.77 Reprinted with permission from Springer © 2012. | ||
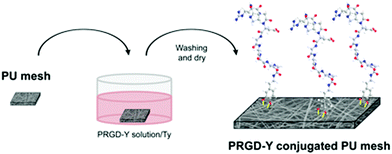 | ||
| Fig. 13 Schematic illustration of the experimental method used in Oh et al. study.77 Reprinted with permission from Springer © 2012. | ||
Numerous studies have proved that linear RGD and cyclic RGD peptides can enhance EC adhesion, growth, and proliferation.93,94 RGD-functionalized vascular grafts also lead to platelet deposition and adhesion because they have the ability to recognize αIIIb3β3 integrin and mediate platelet adhesion. In addition, another important goal in the modification of artificial vascular grafts is the balanced control of the adhesion and proliferation of ECs and SMCs.10 The competitive growth of SMCs or other cells can interfere with the formation of an endothelial monolayer, which leads to low patency.
REDV peptides
Arg-Glu-Asp-Val (REDV) is a fibronectin-derived peptide that can specifically bind to α4β1 integrin, which is abundant on ECs, whereas it is scarce on SMCs.10 Because of its ability to selectively adsorb and proliferate ECs rather than SMCs, REDV has gained much attention in the surface modification of biomaterials, especially for the surface endothelialisation.91,95,96Wei et al. immobilized the REDV peptide onto the surface via an active p-nitrophenyloxycarbonyl group.95 The surface coatings exhibit good hemocompatibility. This report concludes that the introduction of REDV peptides onto the surface could resist SMC adhesion and promote EC attachment, proliferation, and growth.
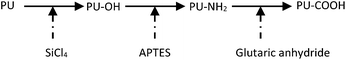
Butruk et al. reported a method to modify the PU surface with peptides containing the REDV sequence that can promote EC adhesion and proliferation.91 As shown in Fig. 14, the PU surface was silanized and then aminated and carboxylated before being coupled with the peptide. It was concluded from this work that the incorporation of REDV-containing peptides increased the number of surface-adhered ECs.
YIGSR peptides
Tyr-Lle-Gly-Ser-Arg (YIGSR) is a segment of the basement membrane matrix glycoprotein laminin.10 It is crucial to binding α4β1 on the cell membrane. This peptide mediates the attachment and migration of cells, such as ECs, fibroblasts, and SMCs.97 Also, the YIGSR peptide can interact with the 67 kDa laminin binding protein, which is highly expressed on the surface of ECs.98Ren et al. successfully fabricated a complementary gradient surface of PHEMA brushes and YIGSR peptides using a dynamically controlled reaction process.97 Due to the synergetic effects of repulsion by PHEMA and ligand attraction, ECs shall be guided in the direction of the ligand gradient. YIGSR is an active sequence derived from the beta1 chain of laminin and can interact with the 67 kDa laminin binding protein, which is expressed on the EC membrane surface. The surface-tethered YIGSR can therefore improve the attachment, spreading, and migration of ECs rather than SMCs.
Thus, ECs exhibited a significant preferential orientation and enhanced directional migration behavior on the gradient surface toward the region of lower PHEMA density and higher YIGSR density, while SMCs did not show either preferred directional migration or enhanced mobility on the gradient surfaces. The schematic of this complementary density gradient of PHEMA and YIGSR can be observed in Fig. 15.
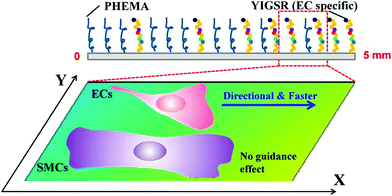 | ||
| Fig. 15 Schematic illustration showing the structure of a complementary density gradient of PHEMA and YIGSR and its influence on the mobility of ECs and SMCs.97 “The direction of increased YIGSR density and decreased PHEMA density is defined as the ‘+X’ direction.” Reprinted with permission from American Chemical Society Publications © 2014. | ||
As mentioned before, one of the strategies to achieve biomimicry is by constructing an endothelial cell-friendly microenvironment. One of the methods to prepare such a microenvironment is by using the construction of a NO releasing material. Kushwaha et al. developed a native endothelial ECM-mimicking self-assembled nanofibrous matrix formed by the self-assembly of peptide amphiphiles (Pas), which contain nitric oxide (NO) donating residues, endothelial cell-adhesive ligands composed of the YIGSR peptide sequence, and enzyme-mediated degradable sites.99 This endothelial ECM-mimicking nanofibrous matrix showed an increase in initial adhesion of endothelial cells due to the presence of endothelial cell-specific ligands, while the proliferation of SMCs was limited.
Other biomolecules
A healthy endothelium usually provides an anticoagulant and anti-proliferative surface, acting as natural protection against thrombosis, lipid uptake and inflammation by releasing signal molecules like nitric oxide, thrombomodulin, prostacyclin, heparin-like molecules, growth factors, tissue plasminogen activator and tissue factor pathway inhibitor.100 Most methods to prepare anti-coagulation coatings, for example the immobilization of heparin and construction of NO releasing materials, are in the scope of this strategy of mimicking endothelial function.Apart from that, the ECM, consisting of multiple functional proteins and signal molecules, plays an important role in supporting the survival of ECs. The various components of the ECM have been immobilized onto various surfaces of implants, including various amounts and types of collagens, adhesion molecules, proteoglycans, peptides, growth factors, cytokines or chemokines. The remodelling of the EC-friendly microenvironment is achievable by plenty of different methods. The results depend on the strategy to effectively construct the EC-friendly microenvironment for endothelialization of cardiovascular implants.14
However, unfortunately many previous attempts to develop bioactive coatings for artificial vascular grafts have been limited by overly simplistic approaches, often focusing on only one facet of biocompatibility at the expense of the others.101 Peptides mimicking cell-binding motifs such as RGD and YIGSR suffer the same problem, failing to achieve a multi-faceted effect.99
Wise et al. summarized biomolecule candidates that fit the goals, achieving a multi-faceted effect: reductions in neointimal hyperplasia, enhanced endothelialization, and low thrombogenicity. A subset of candidates is summarized in Table 2.
| Candidate | Effect on ECs | Effect on SMCs | Blood compatibility | Translation to date |
|---|---|---|---|---|
| Fibrillin-1 | Proliferation enhanced | Proliferation inhibited | Not yet tested | Enhanced fibroblast attachment to PU scaffold |
| Fibulin-5 | Attachment enhanced; apoptosis reduced | Proliferation inhibited | Not yet tested | No translation |
| Tropoelastin | Attachment and proliferation enhanced | Proliferation inhibited | Hemocompatible; minimal activation of platelets | Improves EC binding, growth on steel; reduces thrombogenicity of catheters and ePTFE grafts |
| Perlecan | Proliferation enhanced | Proliferation and hyperplasia (rat model) inhibited | Direct inhibition of thrombosis | Less thrombus present on ePTFE grafts, more ECs; stents show less neointima |
In vivo endothelialization
Another approach to overcome the shortcomings of in vitro EC seeded endothelialization is by in vivo induced rapid self-endothelialization or in situ endothelialization. The major challenge in in situ endothelialization is how to overcome the contradiction between the reality of low EC proliferation activity and the requirement of rapid endothelium regeneration. The emergence of endothelial progenitor cells (EPCs) may provide a promising approach to solving this particular problem.41,86Endothelial progenitor cells (EPC)
To support rapid self-endothelialization on artificial grafts, one study showed the incorporation of antibodies as a marker and its prospects. The strategy focused on promoting the attachment and proliferation of ECs and EPCs on the inner lumen of grafts after implantation.EPCs, which have the potential to differentiate into mature functional ECs, are initially characterized by the expression of CD34 and vascular endothelial growth factor receptor-2 (VEGFR-2) markers.86 EPCs are bone marrow-derived cells and circulate at low concentrations in the peripheral blood of adults. A report showed that EPCs that are seeded on a biomaterial surface can prevent thrombogenic complications and improve long-term patency.102 They also reported accelerated re-endothelialization and reduced neointimal formation.103
Liu et al. described that there are several properties needed in order to design EPC-based in vivo-induced endothelialization:86
1. Induction of the mobilization of EPCs and directed homing to the implanted site.
2. Favorable bioactivity to promote in situ EPC and EC adhesion and proliferation and inhibit SMCs overproliferation.
3. The ability to enhance the migration of EPCs on a material surface and to improve EC migration from native vasculature.
4. The ability to induce the oriented differentiation of EPCs into ECs.
It is also important to take the effect of biomaterial design on thrombosis formation into consideration. The endothelialization rate and stability are dependent on the in situ EPC/EC adhesion and migration on the material surface, which is mainly because of the presence of adsorbed proteins from blood serum, plasma or cellular secretions. The physical and chemical properties of the material surface indirectly regulate cell behavior.104 Differences in hydrophilicity may trigger quantitative and qualitative variations in the adsorbed proteins. Therefore, surface modification becomes important in this case as well, as described in the previous section.
Biofunctional molecules
These EPCs may secrete angiogenic cytokines to support other EPCs and ECs. Although the exact role of EPCs is still under debate, some studies show positive effects on vascular artificial grafts in vivo.105–107 EPCs are generally identified by the expression of specific surface markers such as CD34, CD133, and VEGFR-2.A variety of specific and nonspecific molecules have been investigated to induce cell capture and attachment. One such biofunctional molecule is an antibody against CD34 (anti-CD34Ab) which has been used to induce endothelialization of permanent vascular stents.108–111
Melchior et al. proposed two coating strategies that utilized heparin-crosslinked surfaces to either load VEGF or immobilize anti-CD34Ab.107 Heparin crosslinking was done using the EDC and NHS reaction, then the scaffolds were incubated in VEGF solution and lastly coated with anti-CD34Ab.
Chiono et al. proposed a cytocompatible high molecular weight PU for the preparation of scaffolds for myocardial tissue engineering by melt-extrusion additive manufacturing (AM).112 It is found that CD117-positive cardiac progenitor cells (CPCs) reportedly adhere on the scaffolds, showing a spreading morphology and retaining viability.
Aside from monoclonal antibodies, nucleic acid aptamers are also considered for use. Nucleic acid aptamers are a group of oligonucleic acid molecules that bind to a wide range of target molecules such as peptides, proteins, small molecules, nucleic acids and even whole cells, with high specificity and affinity.86
There is one report regarding the usage of these aptamers. Hoffman et al. first selected single-stranded DNA aptamers that were specific for porcine CD31+ EPCs using the SELEX technique and proved that EPCs can rapidly and specifically adhere to aptamer-coated implants and differentiate into endothelial-like cells.113
As previously described, the behavior and function of EPCs are temporally controlled by the microenvironment, where cytokines play an important role. After cytokine binding, a series of intracellular signaling cascades are activated and regulate the cells’ behavior. Among these cytokines, VEGF is the most widely used in microenvironment engineering. However, studies reported an uncontrolled initial burst in the release kinetics of VEGF and this high concentration of VEGF in the local site may lead to malformed vessels.114,115 Some researchers suggest that soluble VEGF may be internalized and subsequently degraded after binding to its receptors on cells, which reduces its activity and efficiency.107,116 A similar method was used to covalently immobilize VEGF on different materials to provide microenvironments for cell adhesion, proliferation and differentiation.107,115
Cytokines have a low half-life in the circulation; for example, that of VEGF is less than 6 min, resulting in a serious loss in its efficacy. It is reported that gene therapy may improve continuous expression of proteins. After the therapeutic gene is successfully transfected into the target cells, the desired cytokines are expressed continuously and efficiently, thereby regulating the behavior of the cells. The success rate depends on the delivery system.
In the past few years, gene therapy has provided a promising option to promote endothelialization from the transfected ECs.117 Such genetic modifiers can be effectively trapped and carried using nanoparticle (NP) systems.118 The most widely studied genetic modifier for EPC-based cardiovascular therapy is the VEGF gene.119
The most recent study on the effect of VEGF transfected ECs on endothelialization has been done by Wu et al.120 VEGF overexpression promoted cell proliferation, migration, and endothelial capillary-like tube formation, while on the other hand, downregulation of VEGF expression inhibited these activities.
Ke et al. demonstrated that an adenovirus sero-type 5 (Ad5) vector expressing β2AR expression used for gene delivery into EPCs is involved in the restoration of damaged endothelium.121 This gene transfer treatment enhanced β2AR expression in EPCs and increased the capacity of EPCs to migrate, adhere, proliferate, and secrete NO in vitro, as well as the re-endothelialization capacity of EPCs in vivo.
Besides the commonly used VEGF gene, the ZNF580 gene is also a good choice, since its expression will enhance the expression of VEGF protein, and the migration and proliferation of the cells.122 In their study, Yang et al. used the core–shell structure of REDV peptide-functionalized NPs as gene carriers for the ZNF580 gene and their results demonstrated that HUVECs were efficiently transfected by REDV peptide-functionalized NP/pZNF580 complexes.123
Aside from using genes, one study by Daniel et al. shows that the inhibition of microRNA (miR)-92a accelerates re-endothelialization and reduces neointimal lesion formation.124 This may serve as an attractive new strategy to improve surface modification for vascular grafts applications.
Conclusion
Synthetic polymers such as PET, ePTFE, and PU in vascular graft applications have become a popular treatment for vascular diseases. However, thrombosis, restenosis, and low long-term patency always hinder and limit their usage, specifically for small-diameter artificial vascular grafts. Many strategies have been developed to overcome these problems.This review is mainly focused on polyurethane (PU), which has gained substantial interest because of its tunable biological, biochemical and biomechanical properties and various favorable properties, including high wear resistance, no toxicity, excellent physical/mechanical properties and relative hemocompatibility with human blood and cells. The major problem with PU is its thrombogenicity. There are several relevant and recent techniques concerning vascular tissue engineering for small diameter blood vessel applications, as discussed in this review, including surface modification and endothelialization.
The area of vascular graft tissue engineering is in an exciting period today. Significant progress has been made in the development of recent technologies in scaffold engineering and cell engineering, including gene delivery and improvement of endothelial progenitor cells. Over the years, the strategy to fabricate and modify the vascular tissue grafts has shifted towards biomimicry and in vivo endothelialization. The development of these studies in clinical settings will determine the future of the vascular implants. There are still many scientific questions that need to be addressed and there is still enough space for creativity and innovation in this field of research. There are many improvements that have been made and that need to be achieved.
These strategies have successfully improved the hemocompatibility and thrombogenicity of PU grafts without changing their bulk properties significantly. One option to further improve hemocompatibility and thrombogenicity is to functionalize their inner surface. Hydrophilic surfaces can be created to fulfill this purpose. Examples of improving the hydrophilicity of artificial vascular grafts include surface grafting with PEGMA, PEG, MPC, and monomers having sulfobetaine, as well as immobilization of heparin, gelatin, and other bioactive compounds.
The ideal vascular graft surface does not only need hemocompatibility, but also needs a non-thrombogenic surface. Ideally, they should have superior hemocompatibility and regulate blood–graft responses spatiotemporally, enhance endothelialization, and accelerate the formation of an endothelial monolayer. All the physical, biological and chemical properties of the surfaces should be integrated and readily tuned for vascular graft applications.
Covering the inner surface of an artificial vascular graft with a biofunctional and confluent layer of ECs could mimic a healthy blood vessel and potentially enable the long-term application of implants. Since cell adhesion onto an artificial scaffold plays an important role in the generation of an EC layer, one strategy has been developed to enhance the surface with the ability to selectively adhere ECs. Most of them involve immobilizing or fixing cell-adhesive proteins and active peptides on the surface of artificial vascular grafts. This approach, namely in vitro endothelialization, is promising for enhancing EC adhesion and promoting self-endothelialization.
However, rapid endothelialization on artificial vascular grafts is a complex process that involves EC adhesion, migration, proliferation, and differentiation, which are regulated by numerous signals. ECM proteins act to modulate cellular fate through cell signaling cascades. Growth factors, especially VEGFs, are implicated in the regulation of EC activities and new blood vessel formation. Thus, another approach to overcome the shortcomings of in vitro endothelialization is by in vivo endothelialization. An example to improve in vivo endothelialization is to immobilize endothelial progenitor cells (EPC) onto the surface of artificial vascular grafts or to use gene carriers to improve endothelialization.
With this recent development, specifically in surface modification and endothelialization, there is still space to be filled up. A lot of mechanisms are still yet unknown; for example the exact role of EPCs is still debated. For PU alone, a lot of creativity and innovation are still needed to improve its performance, either for its hemocompatibility or for endothelialization.
Acknowledgements
The authors would like to express our gratitude to the financial support of the Ministry of Science and Technology, Taiwan, under the grant MOST-104-2923-E-011-004-MY3 and the National Centre for Research and Development, Poland, under the Taiwan–Poland Cooperation.References
- A. S. Go, D. Mozaffarian, V. L. Roger, E. J. Benjamin, J. D. Berry, M. J. Blaha, S. Dai, E. S. Ford, C. S. Fox, S. Franco, H. J. Fullerton, C. Gillespie, S. M. Hailpern, J. A. Heit, V. J. Howard, M. D. Huffman, S. E. Judd, B. M. Kissela, S. J. Kittner, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, R. H. Mackey, D. J. Magid, G. M. Marcus, A. Marelli, D. B. Matchar, D. K. McGuire, E. R. Mohler, C. S. Moy, M. E. Mussolino, R. W. Neumar, G. Nichol, D. K. Pandey, N. P. Paynter, M. J. Reeves, P. D. Sorlie, J. Stein, A. Towfighi, T. N. Turan, S. S. Virani, N. D. Wong, D. Woo and M. B. Turner, Heart Disease and Stroke Statistics – 2014 Update: A report from the American Heart Association, 2014, p. 129 Search PubMed.
- K. Zhang, T. Liu, J. A. Li, J. Y. Chen, J. Wang and N. Huang, J. Biomed. Mater. Res.,Part A, 2014, 102, 588–609 CrossRef PubMed.
- J. Andersson, P. Libby and G. K. Hansson, Clin. Immunol., 2010, 134(1), 33–46 CrossRef CAS PubMed.
- J. Chlupác, E. Filová and L. Bačáková, Physiol. Res., 2009, 58, 119–140 Search PubMed.
- N. Thottappillil and P. D. Nair, Vasc. Health Risk Manage., 2015, 11, 79–91 CAS.
- J. M. Rhodes and M. Simons, J. Cell. Mol. Med., 2007, 11, 176–205 CrossRef CAS PubMed.
- M. Boffito, S. Sartori and G. Ciardelli, Polym. Int., 2014, 63, 2–11 CrossRef CAS.
- B. P. Chan and K. W. Leong, Eur. Spine J., 2008, 17, 467–479 CrossRef PubMed.
- M. S. Shoichet, Macromolecules, 2010, 43, 581–591 CrossRef CAS.
- X. Ren, Y. Feng, J. Guo, H. Wang, Q. Li, J. Yang, X. Hao, J. Lv, N. Ma and W. Li, Chem. Soc. Rev., 2015, 44, 5680–5742 RSC.
- N. Burke and A. Hasirci, Biomaterials: From Molecules to Engineered Tissues, 2004 Search PubMed.
- K. A. Rocco, M. W. Maxfield, C. A. Best, E. W. Dean and C. K. Breuer, Tissue Eng., Part B, 2014, 20, 628–640 CrossRef CAS PubMed.
- K. Singha and M. Singha, Int. J. Biol. Eng., 2012, 2, 1–8 CrossRef CAS.
- P. Qi, M. F. Maitz and N. Huang, Surf. Coat. Technol., 2013, 233, 80–90 CrossRef CAS.
- S. Li, D. Sengupta and S. Chien, Wiley Interdiscip. Rev.: Syst. Biol. Med., 2014, 6, 61–76 CrossRef CAS PubMed.
- A. Solouk, B. G. Cousins, F. Mirahmadi, H. Mirzadeh, M. R. J. Nadoushan, M. A. Shokrgozar and A. M. Seifalian, Mater. Sci. Eng., C, 2015, 46, 400–408 CrossRef CAS PubMed.
- D. S. T. Chong, L.-A. Turner, N. Gadegaard, A. M. Seifalian, M. J. Dalby and G. Hamilton, Eur. J. Vasc. Endovasc. Surg., 2015, 49, 335–343 CrossRef CAS PubMed.
- B. Seal, Mater. Sci. Eng., R, 2001, 34, 147–230 CrossRef.
- S. Ravi and E. Chaikof, Regener. Med., 2010, 5, 1–21 CrossRef PubMed.
- A. S. Hoffman, Blood—Biomaterial Interactions: An Overview, in Biomaterials: Interfacial Phenomena and Applications, ed. S. Cooper, no. 3, Advances in Chemistry, American Chemical Society, 1982, pp. 3–8 Search PubMed.
- S. Sarkar, K. M. Sales, G. Hamilton and A. M. Seifalian, J. Biomed. Mater. Res., Part B, 2006, 82, 100–108 Search PubMed.
- P. Ferreira, P. Alves, P. Coimbra and M. H. Gil, J. Coat. Technol. Res., 2015, 12, 463–475 CrossRef CAS.
- A. de Mel, B. G. Cousins and A. M. Seifalian, Int. J. Biomater., 2012, 2012, 1–8 CrossRef PubMed.
- S. Koch, T. C. Flanagan, J. S. Sachweh, F. Tanios, H. Schnoering, T. Deichmann, V. Ellä, M. Kellomäki, N. Gronloh, T. Gries, R. Tolba, T. Schmitz-Rode and S. Jockenhoevel, Biomaterials, 2010, 31, 4731–4739 CrossRef CAS PubMed.
- K. A. McKenna, M. T. Hinds, R. C. Sarao, P. C. Wu, C. L. Maslen, R. W. Glanville, D. Babcock and K. W. Gregory, Acta Biomater., 2012, 8, 225–233 CrossRef CAS PubMed.
- V. Catto, S. Farè, G. Freddi and M. C. Tanzi, ISRN Vasc. Med., 2014, 2014, 1–27 CrossRef.
- H. Cao, Y. Feng, H. Wang, L. Zhang, M. Khan and J. Guo, Front. Chem. Sci. Eng., 2011, 5, 409–415 CrossRef CAS.
- F. Couet, N. Rajan and D. Mantovani, Macromol. Biosci., 2007, 7, 701–718 CrossRef CAS PubMed.
- K. Kim, M. Yu, X. Zong, J. Chiu, D. Fang, Y. S. Seo, B. S. Hsiao, B. Chu and M. Hadjiargyrou, Biomaterials, 2003, 24, 4977–4985 CrossRef CAS PubMed.
- Y. Feng, H. Zhao, L. Zhang and J. Guo, Front. Chem. Eng. China, 2010, 4, 372–381 CrossRef CAS.
- H. Bergmeister, N. Seyidova, C. Schreiber, M. Strobl, C. Grasl, I. Walter, B. Messner, S. Baudis, S. Fröhlich, M. Marchetti-Deschmann, M. Griesser, M. di Franco, M. Krssak, R. Liska and H. Schima, Acta Biomater., 2015, 11, 104–113 CrossRef CAS PubMed.
- J. W. Boretos and W. S. Pierce, J. Biomed. Mater. Res., 1968, 2, 121–130 CrossRef CAS PubMed.
- X. Zhang, K. G. Battiston, J. E. McBane, L. A. Matheson, R. S. Labow and J. P. Santerre, Advances in Polyurethane Biomaterials, 2016, pp. 75–114 Search PubMed.
- R. Hashizume, Y. Hong, K. Takanari, K. L. Fujimoto, K. Tobita and W. R. Wagner, Biomaterials, 2013, 34, 7353–7363 CrossRef CAS PubMed.
- S. Sharifpoor, C. A. Simmons, R. S. Labow and J. Paul Santerre, Biomaterials, 2011, 32, 4816–4829 CrossRef CAS PubMed.
- S. H. Ye, Y. Hong, H. Sakaguchi, V. Shankarraman, S. K. Luketich, A. D'Amore and W. R. Wagner, ACS Appl. Mater. Interfaces, 2014, 6, 22796–22806 CAS.
- W. Ou, H. Qiu, Z. Chen and K. Xu, Biomaterials, 2011, 32, 3178–3188 CrossRef CAS PubMed.
- R. Y. Kannan, H. J. Salacinski, P. E. Butler, G. Hamilton and A. M. Seifalian, J. Biomed. Mater. Res., Part B, 2005, 74, 570–581 CrossRef PubMed.
- R. Y. Kannan, H. J. Salacinski, M. Odlyha, P. E. Butler and A. M. Seifalian, Biomaterials, 2006, 27, 1971–1979 CrossRef CAS PubMed.
- H. Yahyaei, M. Mohseni and H. Ghanbari, J. Inorg. Organomet. Polym. Mater., 2015, 25, 1305–1312 CrossRef CAS.
- M. Avci-Adali, G. Ziemer and H. P. Wendel, Biotechnol. Adv., 2010, 28, 119–129 CrossRef CAS PubMed.
- S. R. Meyers and M. W. Grinstaff, Chem. Rev., 2012, 112, 1615–1632 CrossRef CAS PubMed.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- B. D. Ratner, J. Dent. Educ., 2001, 65, 1340–1347 CAS.
- I. K. Jung, J. W. Bae, W. S. Choi, J. H. Choi and K. D. Park, J. Biomater. Sci., Polym. Ed., 2009, 20, 1473–1482 CrossRef CAS PubMed.
- Y. X. Qiu, D. Klee, W. Pluster, B. Severich and H. Hockery, J. Appl. Polym. Sci., 1996, 61, 2373–2382 CrossRef CAS.
- H. Wang, Y. Feng, B. An, W. Zhang, M. Sun, Z. Fang, W. Yuan and M. Khan, J. Mater. Sci. Mater. Med., 2012, 23, 1499–1510 CrossRef CAS PubMed.
- W. Yuan, Y. Feng, H. Wang, D. Yang, B. An, W. Zhang, M. Khan and J. Guo, Mater. Sci. Eng., C, 2013, 33, 3644–3651 CrossRef CAS PubMed.
- J. Kucinska-Lipka, I. Gubanska, H. Janik and M. Sienkiewicz, Mater. Sci. Eng., C, 2015, 46, 166–176 CrossRef CAS PubMed.
- C. Leng, H.-C. Hung, S. Sun, D. Wang, Y. Li, S. Jiang and Z. Chen, ACS Appl. Mater. Interfaces, 2015, 7(30), 16881–16888 CAS.
- Q. Shao and S. Jiang, Adv. Mater., 2015, 27, 15–26 CrossRef CAS PubMed.
- T. Goda, K. Ishihara and Y. Miyahara, J. Appl. Polym. Sci., 2015, 132, 1–10 CrossRef.
- M. Khan, J. Yang, C. Shi, Y. Feng, W. Zhang, K. Gibney and G. N. Tew, Macromol. Mater. Eng., 2015, 300, 802–809 CrossRef CAS.
- X. Cai, J. Yuan, S. Chen, P. Li, L. Li and J. Shen, Mater. Sci. Eng., C, 2014, 36, 42–48 CrossRef CAS PubMed.
- Y. Yuan, F. Ai, X. Zang, W. Zhuang, J. Shen and S. Lin, Colloids Surf., B, 2004, 35, 1–5 CrossRef CAS PubMed.
- Y. Jiang, B. Rongbing, T. Ling, S. Jian and L. Sicong, Colloids Surf., B, 2004, 36, 27–33 CrossRef CAS PubMed.
- D. Tan, L. Liu, Z. Li and Q. Fu, J. Biomed. Mater. Res., Part A, 2015, 103, 2711–2719 CrossRef CAS PubMed.
- N. Morimoto, A. Watanabe, Y. Iwasaki, K. Akiyoshi and K. Ishihara, Biomaterials, 2004, 25, 5353–5361 CrossRef CAS PubMed.
- M. Tan, Y. Feng, H. Wang, L. Zhang, M. Khan, J. Guo, Q. Chen and J. Liu, Macromol. Res., 2013, 21, 541–549 CrossRef CAS.
- M. Khan, J. Yang, C. Shi, Y. Feng, W. Zhang, K. Gibney and G. N. Tew, RSC Adv., 2015, 5, 11284–11292 RSC.
- J. F. Mano, G. a Silva, H. S. Azevedo, P. B. Malafaya, R. A. Sousa, S. S. Silva, L. F. Boesel, J. M. Oliveira, T. C. Santos, a P. Marques, N. M. Neves and R. L. Reis, J. R. Soc., Interface, 2007, 4, 999–1030 CrossRef CAS PubMed.
- J. Guo, D. Y. Nguyen, R. T. Tran, Z. Xie, X. Bai and J. Yang, Natural and Synthetic Biomedical Polymers, 2014, pp. 259–285 Search PubMed.
- F. Zia, K. M. Zia, M. Zuber, S. Tabasum and S. Rehman, Int. J. Biol. Macromol., 2016, 84, 101–111 CrossRef CAS PubMed.
- S. Murugesan, J. Xie and R. J. Linhardt, Curr. Top. Med. Chem., 2008, 8, 80–100 CrossRef CAS PubMed.
- A. E. Aksoy, V. Hasirci and N. Hasirci, Macromol. Symp., 2008, 269, 145–153 CrossRef CAS.
- Y. Yan, X. Hong Wang, D. Yin and R. Zhang, J. Bioact. Compat. Polym., 2007, 22, 323–341 CrossRef CAS.
- I.-H. Bae, I.-K. Park, D. S. Park, H. Lee and M. H. Jeong, J. Mater. Sci. Mater. Med., 2012, 23, 1259–1269 CrossRef CAS PubMed.
- Y. J. Du, J. L. Brash, G. Mcclung, L. R. Berry, P. Klement and A. K. C. Chan, J. Biomed. Mater. Res., A, 2006, 80, 216–225 Search PubMed.
- W. S. Choi, Y. K. Joung, Y. Lee, J. W. Bae, H. K. Park, Y. H. Park, J.-C. Park and K. D. Park, ACS Appl. Mater. Interfaces, 2016, 8, 4336–4346 CAS.
- C. Chatelet, O. Damour and A. Domard, Biomaterials, 2001, 22, 261–268 CrossRef CAS PubMed.
- W.-C. Lin, D.-G. Yu and M.-C. Yang, Colloids Surf., B, 2005, 44, 82–92 CrossRef CAS PubMed.
- F. Kara, E. A. Aksoy, Z. Yuksekdag, N. Hasirci and S. Aksoy, Carbohydr. Polym., 2014, 112, 39–47 CrossRef CAS PubMed.
- T. W. Chuang and K. S. Masters, Biomaterials, 2009, 30, 5341–5351 CrossRef CAS PubMed.
- K. He and X. Wang, J. Bioact. Compat. Polym., 2011, 26, 363–374 CrossRef CAS.
- X. Wang, K. He and W. Zhang, J. Bioact. Compat. Polym., 2013, 28, 303–319 CrossRef.
- A. M. Seifalian, A. Tiwari, G. Hamilton and H. J. Salacinski, Artif. Organs, 2002, 26, 307–320 CrossRef PubMed.
- J. H. Oh, J. S. Lee, K. M. Park, H. T. Moon and K. D. Park, Macromol. Res., 2012, 20, 1150–1155 CrossRef CAS.
- P. Alves, R. Cardoso, T. R. Correia, B. P. Antunes, I. J. Correia and P. Ferreira, Colloids Surf., B, 2014, 113, 25–32 CrossRef CAS PubMed.
- I. You, S. M. Kang, Y. Byun and H. Lee, Bioconjugate. Chem., 2011, 22, 1264–1269 CrossRef CAS PubMed.
- C. K. Hashi, N. Derugin, R. R. R. Janairo, R. Lee, D. Schultz, J. Lotz and S. Li, Arterioscler., Thromb., Vasc. Biol., 2010, 30, 1621–1627 CrossRef CAS PubMed.
- S. Yamamoto, H. Okamoto, M. Haga, K. Shigematsu, T. Miyata, T. Watanabe, Y. Ogawa, Y. Takagi and T. Asakura, J. Mater. Chem. B, 2016, 4, 938–946 RSC.
- L. Jia, M. P. Prabhakaran, X. Qin, D. Kai and S. Ramakrishna, J. Mater. Sci., 2013, 48, 5113–5124 CrossRef CAS.
- D.-A. Wang, L.-X. Feng, J. Ji, Y.-H. Sun, X.-X. Zheng and J. H. Elisseeff, J. Biomed. Mater. Res., Part A, 2003, 65, 498–510 CrossRef PubMed.
- E. Potthoff, D. Franco and V. D'Alessandro, Nanoletters, 2014, 14, 1069–1079 CrossRef CAS PubMed.
- R. Liu, Y. Qin, H. Wang, Y. Zhao, Z. Hu and S. Wang, BMC Cardiovasc. Disord., 2013, 13, 1–7 Search PubMed.
- T. Liu, S. Liu, K. Zhang, J. Chen and N. Huang, J. Biomed. Mater. Res., Part A, 2013, 102, 3754–3772 CrossRef PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. García, J. Biomed. Mater. Res., Part A, 2003, 66, 247–259 CrossRef PubMed.
- N. Q. Tran, Y. K. Joung, E. Lih, K. M. Park and K. D. Park, Macromol. Res., 2011, 19, 300–306 CrossRef CAS.
- M. F. A. Cutiongco, D. E. J. Anderson, M. T. Hinds and E. K. F. Yim, Acta Biomater., 2015, 25, 97–108 CrossRef CAS PubMed.
- Y. Wang, Y. Yu, L. Zhang, P. Qin and P. Wang, J. Biomater. Sci., Polym. Ed., 2015, 26, 459–467 CrossRef CAS PubMed.
- B. Butruk, P. Bąbik, B. Marczak and T. Ciach, Procedia Eng., 2013, 59, 126–132 CrossRef CAS.
- A. Wieckowska, A. B. Braunschweig and I. Willner, Chem. Commun., 2007, 3918–3920 RSC.
- C. Tao, J. Huang, Y. Lu, H. Zou, X. He, Y. Chen and Y. Zhong, Colloids Surf., B, 2014, 122, 439–446 CrossRef CAS PubMed.
- J. S. Lee, K. Lee, S. H. Moon, H. M. Chung, J. H. Lee, S. H. Um, D. I. Kim and S. W. Cho, Macromol. Biosci., 2014, 14, 1181–1189 CrossRef CAS PubMed.
- Y. Wei, Y. Ji, L. Xiao, Q. Lin and J. Ji, Colloids Surf., B, 2011, 84, 369–378 CrossRef CAS PubMed.
- B. D. Plouffe, M. Radisic and S. K. Murthy, Lab Chip, 2008, 8, 462–472 RSC.
- T. Ren, S. Yu, Z. Mao, S. E. Moya, L. Han and C. Gao, Biomacromolecules, 2014, 15, 2256–2264 CrossRef CAS PubMed.
- C. Hundt, J. M. Peyrin, S. Haik, S. Gauczynski, C. Leucht, R. Rieger, M. L. Riley, J. P. Deslys, D. Dormont, C. I. Lasmezas and S. Weiss, EMBO J., 2001, 20, 5876–5886 CrossRef CAS PubMed.
- M. Kushwaha, J. M. Anderson, C. A. Bosworth, A. Andukuri, W. P. Minor, J. R. Lancaster, P. G. Anderson, B. C. Brott and H. W. Jun, Biomaterials, 2010, 31, 1502–1508 CrossRef CAS PubMed.
- A. McGuigan and M. Sefton, Biomaterials, 2007, 28, 2547–2571 CrossRef CAS PubMed.
- S. G. Wise, A. Waterhouse, P. Michael and M. K. C. Ng, J. Funct. Biomater., 2012, 3, 569–587 CrossRef CAS PubMed.
- D. P. Griese, A. Ehsan, L. G. Melo, D. Kong, L. Zhang, M. J. Mann, R. E. Pratt, R. C. Mulligan and V. J. Dzau, Circulation, 2003, 108, 2710–2715 CrossRef PubMed.
- N. Kipshidze, G. Dangas, M. Tsapenko, J. Moses, M. B. Leon, M. Kutryk and P. Serruys, J. Am. Coll. Cardiol., 2004, 44, 733–739 CAS.
- V. Llopis-Hernandez, P. Rico, J. Ballester-Beltran, D. Moratal and M. Salmeran-Sanchez, PLoS One, 2011, 6, e19610 CAS.
- M. K. Hagensen, P. M. Vanhoutte and J. F. Bentzon, Cardiovasc. Res., 2012, 95, 281–289 CrossRef CAS PubMed.
- M. C. Yoder, Cold Spring Harbor Perspect. Med., 2012, 1–14 Search PubMed.
- A. J. Melchiorri, N. Hibino, T. Yi, Y. U. Lee, T. Sugiura, S. Tara, T. Shinoka, C. Breuer and J. P. Fisher, Biomacromolecules, 2015, 16, 437–446 CrossRef CAS PubMed.
- M. Yin, Y. Yuan, C. Liu and J. Wang, J. Mater. Sci. Mater. Med., 2009, 20, 1513–1523 CrossRef CAS PubMed.
- Q. Lin, X. Ding, F. Qiu, X. Song, G. Fu and J. Ji, Biomaterials, 2010, 31, 4017–4025 CrossRef CAS PubMed.
- K. Larsen, C. Cheng, D. Tempel, S. Parker, S. Yazdani, W. K. Den Dekker, J. H. Houtgraaf, R. De Jong, S. Swager-Ten Hoor, E. Ligtenberg, S. R. Hanson, S. Rowland, F. Kolodgie, P. W. Serruys, R. Virmani and H. J. Duckers, Eur. Heart J., 2012, 33, 120–128 CrossRef CAS PubMed.
- G. Nakazawa, J. F. Granada, C. L. Alviar, A. Tellez, G. L. Kaluza, M. Y. Guilhermier, S. Parker, S. M. Rowland, F. D. Kolodgie, M. B. Leon and R. Virmani, JACC Cardiovasc. Interv., 2010, 3, 68–75 CrossRef PubMed.
- V. Chiono, P. Mozetic, M. Boffito, S. Sartori, E. Gioffredi, A. Silvestri, A. Rainer, S. M. Giannitelli, M. Trombetta, D. Nurzynska, F. Di Meglio, C. Castaldo, R. Miraglia, S. Montagnani and G. Ciardelli, Interfaces Focus, 2014, 4, 1–11 Search PubMed.
- J. Hoffmann, A. Paul, M. Harwardt, J. Groll, T. Reeswinkel, D. Klee, M. Moeller, H. Fischer, T. Walker, T. Greiner, G. Ziemer and H. P. Wendel, J. Biomed. Mater. Res., Part A, 2008, 84A, 614–621 CrossRef CAS PubMed.
- A. Lode, C. Wolf-Brandstetter, A. Reinstorf, A. Bernhardt, U. König, W. Pompe and M. Gelinsky, J. Biomed. Mater. Res., Part A, 2007, 81A, 474–483 CrossRef CAS PubMed.
- C. K. Poh, Z. Shi, T. Y. Lim, K. G. Neoh and W. Wang, Biomaterials, 2010, 31(7), 1578–1585 CrossRef CAS PubMed.
- A. H. Zisch, U. Schenk, J. C. Schense, S. E. Sakiyama-Elbert and J. A. Hubbell, J. Controlled Release, 2001, 72, 101–113 CrossRef CAS PubMed.
- W. Chen, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2014, 190, 398–414 CrossRef CAS PubMed.
- K. Nakano, K. Egashira, S. Masuda, K. Funakoshi, G. Zhao, S. Kimura, T. Matoba, K. Sueishi, Y. Endo, Y. Kawashima, K. Hara, H. Tsujimoto, R. Tominaga and K. Sunagawa, JACC Cardiovasc. Interv., 2009, 2, 277–283 CrossRef PubMed.
- H. Iwaguro, Circulation, 2002, 105, 732–738 CrossRef CAS PubMed.
- X. Wu, Y. Zhao, C. Tang, T. Yin, R. Du, J. Tian, J. Huang, H. Gregersen and G. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 7578–7589 CAS.
- X. Ke, X. R. Shu, F. Wu, Q. S. Hu, B. Q. Deng, J. F. Wang and R. Q. Nie, Stem Cell Res. Ther., 2016, 7, 73–85 CrossRef PubMed.
- H. Y. Sun, S. P. Wei, R. C. Xu, P. X. Xu and W. C. Zhang, Biochem. Biophys. Res. Commun., 2010, 395, 361–366 CrossRef CAS PubMed.
- J. Yang, W. Liu, Y. Feng, X. Ren and W. Zhang, J. Mater. Chem. B, 2016, 4, 3365–3376 RSC.
- J. M. Daniel, D. Penzkofer, R. Teske, J. Dutzman, A. Koch, W. Bielenburg, A. Bonauer, R. A. Boon, A. Fischer, J. Bauersachs, E. v. Rooij, S. Dimmeler and D. G. Sedding, Cardiovasc. Res., 2014, 564–572 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |