A multi-analytical approach to the characterization of natural organic dyestuffs and inorganic substrates present in the 19th-century artistic oil paints manufactured by a French art materials supplier Richard Ainès†
Olga
Otłowska
a,
Marek
Ślebioda
b,
Mirosław
Wachowiak
c and
Magdalena
Śliwka-Kaszyńska
*a
aDepartment of Organic Chemistry, Gdansk University of Technology, Narutowicza 11/12, 80-233 Gdansk, Poland. E-mail: magkaszy@pg.gda.pl
bPerlan Technologies, Sp. z.o.o., Pulawska 303, 02-785 Warszawa, Poland
cDepartment of Conservation and Restoration of Modern Art, Nicolas Copernicus University, Sienkiewicza 30/32, 87-100 Torun, Poland
First published on 14th November 2016
Abstract
This paper presents a comprehensive analysis of artistic paints produced in the 19th century by a French art materials supplier Richard Ainès. Improved mild extraction with hydrofluoric acid enabled the observation of intact organic dyes. Reversed-phase liquid chromatography with diode-array and mass spectrometry detection was utilised for the identification of 35 dyes present in yellow and red paint samples, and in selected plant extracts. The developed analytical method allowed more efficient separation of several isomeric flavonoid and anthraquinone dye components of the paint samples. Persian berries and weld were identified as the dye sources in the yellow paint samples. The red oil paint had been coloured with the madder lake-type plant. Studies of dye extracts of the historical samples show the presence of uncommon dye components (quercetin-O-rhamnoside-glucuronide and rhamnasin-O-rhamnoside-glucoside) that could enhance this identification. The paint samples were additionally analyzed by X-ray fluorescence (XRF) and scanning electron microscopy with energy dispersive X-ray analysis (SEM-EDX). Aluminum, Sn, Zn, Ca, Cu, S, Si, and K were detected confirming the presence of aluminium hydrate and tin salts as carriers, as well as chalk, and other components used during the production of these paints. The SEM with the BSE detector images revealed the homogenous texture of finely ground lake pigments.
1 Introduction
Analysis of natural dyes in historical objects is a fundamental source of information for a wide range of specialists dealing with documentation and authentication of artwork and for developing appropriate conservation strategies. Artistic paints usually contain a large variety of inorganic and organic colorants, fillers or substrates and binding media (varnishes). Usually only tiny amounts of examined paints can be sampled in contrast to other objects, for instance textiles, for which sampling procedures are usually more flexible.Hyphenated methods, e.g. high performance separation techniques coupled to sensitive and selective spectrometric detectors, seem to be the most efficient tools for the analysis of such materials in complex matrices, as it was reviewed in detail by Rosenberg1 and by Pauk et al.2 Among them, liquid chromatography with mass spectrometric detection offers solutions to problems encountered in the analysis of real samples of unknown composition, especially those that lack proper standards. Inorganic pigments and substrates are characterised with vibrational spectroscopic techniques (IR and FTIR), X-ray fluorescence (XRF), and Raman or UV spectroscopy. Additionally, optical microscopy and scanning electron microscopy (SEM) with energy-dispersive X-ray detectors (EDX) and high resolution transmission electron techniques are applied.3–6 Natural organic dyestuffs exist in paints in the form of so-called lakes, which are complexes of colouring substances with metal ions, bonded on inorganic supports.7 The isolation of dyestuffs from such matrices is a difficult task without changing their original structure. Usually extraction of colorants from paints and lakes is carried out at elevated temperatures with methanol mixed with aqueous solutions of strong inorganic acids, such as sulphuric or hydrochloric acid.8,9 Unfortunately, strong mineral acids hydrolyse not only the metal–dye complex, but also decompose the glycosidic components into their parent aglycons. The strong acids cause also decarboxylation (e.g. pseudopurpurin to purpurin) or esterification of the dyes.8,10,11 Solutions of weak acids, e.g. formic, oxalic, ethylenediaminetetraacetic (EDTA), citric, trifluoroacetic acid, or boron trifluoride dissolved in methanol, facilitate the release of colorants and preserve glycoside linkages.12–14 However, the extraction efficiency with these reagents is very low. Latest studies on the use of hydrofluoric acid demonstrate high efficiency of dyestuff isolation from lakes without breaking the glycosidic bonds and causing other undesired reactions.11,15,16 As a result, the determination of real composition of the analysed paint has become possible.17
In this paper we describe the identification of natural organic colouring substances and inorganic substrates present in artistic paints produced in the 19th century by a French art materials supplier Richard Ainès (originally named Edouard, later taken over by Mulard and finally by Richard*). The opportunity of analysing historical paints with known commercial indication of the product is valuable and is important not only from the pure chemical point of view but also for historians trying to research on painting techniques and materials used among others by the French impressionists.
Four artistic oil paints with partially preserved paper labels attached to the tubes were fully characterized: red one with trade name Laque de Garance, and three yellows: Still de Grain, Laque de Robert no. 5 (lighter and warmer in hue) and Laque de Robert no. 6 (darker). Our preliminary study suggests the presence of a number of flavonoids and anthraquinones in the 19th century paints.18 Liquid chromatography-mass spectrometry with atmospheric pressure negative electrospray ionization LC-ESI(−)-MS was successfully applied for the identification of the main components in these artistic paints. Thirty-five dyestuffs were detected and identified or tentatively characterised, of which two compounds were not described before. LC-MS was also employed to analyse reference dyestuff samples in extracts from weld – Reseda luteola, Persian berries – Rhamni maturi and madder – Rubia tinctorum L. in order to provide indications about the structures of dyestuff components which are detected in the historical samples but are not available in pure form. The carriers of the dyes and fillers of the paints were investigated by means of XRF, SEM-EDX and FT-IR techniques. The results reported in the present paper enabled full dye fingerprints in paint samples.
2 Experimental
2.1 Chemicals and reference materials
Acetonitrile and methanol used as mobile phase components were of HPLC grade and were purchased from Merck (Darmstadt, Germany). Hydrofluoric acid (48% in water) was purchased from Sigma-Aldrich (Steinheim, Germany). Dimethyl sulfoxide (DMSO, ACS grade) was obtained from Merck KGaA (Darmstadt, Germany). Dichloromethane was purchased from POCh (Gliwice, Poland). Synthetic dyestuffs, alizarin, purpurin, rhamnetin, quercetin, kaempferol and apigenin, were available in pure form (Sigma-Aldrich). Raw dyestuff materials, weld (Reseda luteola), Persian berries (Rhamni maturi) and madder roots (Rubia tinctorum L.), were obtained from Kremer Pigmente (Aichstetten, Germany) in dried form. The plants were homogenized prior to analysis. All aqueous solutions were prepared using deionized Milli Q water.2.2 Origin of analysed historical paint samples
The historical samples of naturally aged paints manufactured by Richard Ainès (Paris) dated back to the 19th century were kindly offered by the National Museum in Krakow, Poland. The oil paint tubes formerly belonged to a famous Polish painter, Jan Matejko, and were being used between 1880 and 1893. The tubes had the name Richard Ainès imprinted on the metal screw tops. Yet, there is name of Edouard on the paper labels attached to tubes and brand of Mulard is pressed into the upper part of the tube. Maison Edouard was one of the most appreciated colourmen in Paris, famous for oil colours and pastels used by C. Monet, B. Morisot, A. Sisley, P.-A. Renoir, G. Caillebotte, M. Casatt, and E. Manet.19 Partially preserved labels on the paint tubes and the shopping list from Biasion's shop of painting materials acquired by Matejko allow us to match individual paints with their French brand name (Laque de Robert no. 5, Laque de Robert no. 6, Laque de Garance, and Still de Grain). Unfortunately, the paint composition suggested by the manufacturer was not always up to its specification.20,212.3 Extraction methods
The microsample (∼0.2 mg) placed in a 3 mL conical polypropylene vial was suspended in 300 μL of methylene chloride and kept in an ultrasonic bath for 15 min in order to swell the organic binding media. After evaporation of the solvent under the stream of nitrogen, the sample was re-suspended in 500 μL of a ACN/MeOH/DMSO/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixture. Fifty microliters of 4 M aqueous HF solution were added and the mixture was kept in an ultrasonic bath for 1 hour at temperature of 40 °C, then it was left in the darkness for 12 h. After this time the sample was centrifuged at 9000 rpm for 5 min. The supernatant was evaporated almost to dryness under a stream of nitrogen. The residue was taken up in 300 μL ACN/MeOH/DMSO, (1
1 v/v) mixture. Fifty microliters of 4 M aqueous HF solution were added and the mixture was kept in an ultrasonic bath for 1 hour at temperature of 40 °C, then it was left in the darkness for 12 h. After this time the sample was centrifuged at 9000 rpm for 5 min. The supernatant was evaporated almost to dryness under a stream of nitrogen. The residue was taken up in 300 μL ACN/MeOH/DMSO, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), out of which 2 μL were injected into the HPLC column. The HF extraction procedure was applied also to reference raw dyestuff materials (weld, Persian berries and madder roots) for comparison with the results obtained from the historical samples.
1, v/v), out of which 2 μL were injected into the HPLC column. The HF extraction procedure was applied also to reference raw dyestuff materials (weld, Persian berries and madder roots) for comparison with the results obtained from the historical samples.
2.4 Equipment
The samples (2 μL) were injected onto a Poroshell EC-C18 2.7 μm (3.0 × 150 mm) column thermostated at 40 °C. The mobile phase flow rate was 0.4 mL min−1, and elution was performed using 0.1% (v/v) formic acid in water (solvent A) and ACN/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) (solvent B) using composition gradient 10% B to 100% B in 20 min. The analysis was stopped after 30 minutes and the column was equilibrated for 10 minutes at 10% B. The UV signal was registered at 254 and 350 nm. All mass-spectrometric data were recorded in negative ionisation scan mode (m/z 50–1000). The nebulizer pressure, nitrogen flow rate, drying gas temperature, drying gas flow rate, and sheath gas temperature were 45 psi, 5 L min−1, 300 °C, 11 L min−1, and 250 °C, respectively. The capillary voltage was 3.5 kV and the fragmentation voltages were 100 and 250 V.
1; v/v) (solvent B) using composition gradient 10% B to 100% B in 20 min. The analysis was stopped after 30 minutes and the column was equilibrated for 10 minutes at 10% B. The UV signal was registered at 254 and 350 nm. All mass-spectrometric data were recorded in negative ionisation scan mode (m/z 50–1000). The nebulizer pressure, nitrogen flow rate, drying gas temperature, drying gas flow rate, and sheath gas temperature were 45 psi, 5 L min−1, 300 °C, 11 L min−1, and 250 °C, respectively. The capillary voltage was 3.5 kV and the fragmentation voltages were 100 and 250 V.
The structures of identified dyes were confirmed by LC-ESI(−)-QTOF analysis using an Agilent 1290 LC system coupled to an Agilent quadrupole time-of-flight (QTOF) mass spectrometer G6540 operated in negative ionisation scan mode under the same chromatographic conditions.
3 Results and discussion
3.1 HPLC analysis
| Peak no. | t R (min) | [M − H]−, m/z | Fragment ions (m/z) | Elemental composition | Diff. (ppm) | Proposed identification | λ max (nm) | |
|---|---|---|---|---|---|---|---|---|
| Nominal | Highly resolved | |||||||
| 1 | 9.4 | 609 | 609.1458 | 447, 285 | C27H30O16 | 0.49 | Kaempferol-O-dihexoside | 268, 325 |
| 2 | 10.6 | 609 | 609.1479 | 447, 285 | C27H30O16 | −2.95 | Luteolin 3′,7-O-diglucoside | 267, 342 |
| 3 | 11.0 | 755 | 755.2050 | 609, 463, 301 | C33H40O20 | −1.32 | Quercetin-O-dirhamnoside-glucoside | 256, 356 |
| 4 | 11.4 | 447 | 447.0941 | 327, 285, 151 | C21H20O11 | −1.79 | Luteolin-7-O-glucoside | 255, 340 |
| 5 | 11.6 | 739 | 739.2096 | 593, 447, 285 | C33H40O19 | −0.68 | Kaempferol-O-dirhamnoside-glucoside | 266, 348 |
| 6 | 11.9 | 609 | 609.1462 | 447, 301 | C27H30O16 | −0.16 | Quercetin-O-rhamnoside-glucoside | 256, 350 |
| 7 | 12.2 | 447 | 447.0935 | 301, 211, 151 | C21H20O11 | −0.45 | Quercetin-O-rhamnoside | 257, 349 |
| 8 | 13.4 | 769 | 769.2191 | 623, 447, 315 | C34H42O20 | 0.78 | Rhamnetin-O-dirhamnoside-glucoside | 257, 357 |
| 9 | 13.7 | 623 | 623.1253 | 447, 301 | C27H28O17 | 0.16 | Quercetin-O-rhamnoside-glucuronide | 260, 357 |
| 10 | 14.0 | 301 | 301.0360 | 232, 151, 121 | C15H10O7 | −1.99 | Quercetin | 255, 360 |
| 11 | 14.1 | 285 | 285.0400 | 197, 151, 133 | C15H10O6 | 1.75 | Luteolin | 254, 348 |
| 12 | 14.2 | 783 | 783.2357 | 637, 491, 329, 314, 299 | C35H44O20 | −0.5 | Rhamnazin-3-O-dirhamnoside-glucoside | 256, 356 |
| 13 | 14.5 | 637 | 637.1794 | 329, 314, 299 | C29H34O16 | −3.14 | Rhamnazin-O-rhamnoside-glucoside | 250, 355 |
| 14 | 14.8 | 623 | 623.1612 | 461, 315 | C28H32O16 | 0.80 | Rhamnetin-3-O-rutinoside | 257, 347 |
| 15 | 15.1 | 269 | 269.0457 | 227, 225, 151 | C15H10O5 | −0.74 | Apigenin | 267, 337 |
| 16 | 15.2 | 285 | 285.0414 | 257, 151 | C15H10O6 | −3.16 | Kaempferol | 266, 354 |
| 17 | 16.7 | 315 | 315.0509 | 300, 272, 244, 165, 121 | C16H12O7 | 0.32 | Rhamnetin | 256, 371 |
| 18 | 18.0 | 299 | 299.0563 | 284, 271, 256, 243, 228 | C16H12O6 | −0.67 | Rhamnocitrin | 266, 367 |
| 19 | 18.2 | 329 | 329.0667 | 314, 301, 299, 286, 271, 258 | C17H14O7 | 0.00 | Rhamnazin | 256, 371 |
| 20 | 20.1 | 269 | — | 241, 225, 197 | C15H10O5 | — | Emodin | 252, 286 |
| 21 | 12.9 | 447 | 447.0928 | 285, 225, 151 | C21H20O11 | 0.89 | Luteolin-O-glucoside | 268, 342 |
| 22 | 15.4 | 299 | 299.0568 | 284, 256, 243, 227, 199, 151 | C16H12O6 | −2.34 | Chrysoeriol | 267, 348 |
| 23 | 15.2 | 239 | 239.0352 | 211, 195, 183, 167 | C14H8O4 | −0.91 | Hystazarin | 282, 413 |
| 24 | 15.9 | 255 | 255.0295 | 227, 183 | C14H8O5 | 1.57 | Anthragallol | 279, 405 |
| 25 | 16.8 | 239 | 239.0348 | 211, 167 | C14H8O4 | −0.84 | Alizarin | 280, 427 |
| 26 | 18.1 | 239 | 239.0342 | 211, 195 | C14H8O4 | −3.35 | Xanthopurpurin | 251, 422 |
| 27 | 18.3 | 255 | 255.0296 | 227, 183 | C14H8O5 | −1.18 | Purpurin | 255, 480 |
| 28 | 19.8 | 253 | 253.0503 | 225, 197 | C15H10O4 | −1.18 | Rubiadin | 244, 409 |
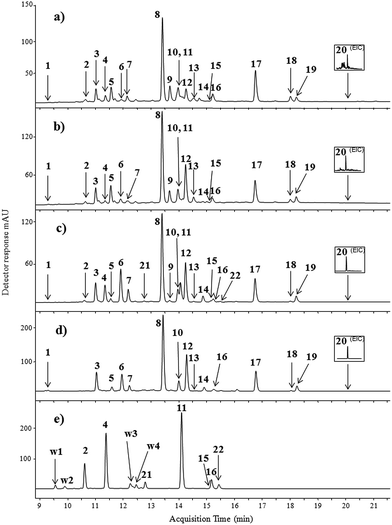
The identification of colouring compounds was performed by comparison of retention times, UV and mass spectra in the negative ionisation mode (ESI(−)-MS) to those obtained for the compounds found in Persian berries (Rhamni maturi) (Fig. 1d), and weld (Reseda luteola) (Fig. 1e) extracts to those of standard flavonoids under the same chromatographic conditions (see the Experimental section). Identification of standardless and unknown colouring substances was supported by high-resolution QTOF spectra.
The LC-ESI(−)-MS analyses of Laque de Robert no. 5 and Laque de Robert no. 6 showed the presence of 12 glycosides and 8 aglycons extracted with the use of organic solvents and hydrofluoric acid mixture. The chromatograms of both paint samples are very similar. Peak 1, appearing at a retention time of 9.4 min (hardly seen in UV spectrochromatogram), showed pseudo-molecular ion [M − H]− at m/z 609 corresponding to kaempferol-O-dihexoside, with fragment ions at m/z 447 and m/z 285 due to the loss of one and two glucose units, respectively. This compound has never been detected in weld and Persian berry extracts before; however it has been found in the Tamus communis family plant.22 The peak no. 2 detected in the both paint extracts was identified as luteolin 3′,7-O-diglucoside according to its mass spectrum with base peak [M − H]− at m/z 609 and fragment peaks at m/z 447 and 285 (Table 1). The identification of this compound was confirmed by mass spectrum data available in the literature for Reseda luteola L. extracts.23
Peak 3 showed pseudo-molecular ion [M − H]− at m/z 755 and fragment ions at m/z 609, 463, and 301. It was tentatively identified as quercetin-O-dirhamnoside-glucoside. The hypothesis was confirmed by the ESI(−)-QTOF product ion mass spectrum in which the peak of [M − H]− was observed at m/z 755.2050 (corresponding to the elemental composition of C33H40O20, mass diff. −1.32 ppm). Fragment ions at m/z 609 [M − H − 146]−, m/z 463 [M − H − 146 − 146]−, and m/z 301 [M − H − 146 − 146 − 162]− correspond to the loss of one rhamnose moiety, two rhamnose moieties and glucose moiety, respectively. The peak 4, appearing at a retention time of 11.4 min, with mass base peak [M − H]− at m/z 447 and the product ion at m/z 285 was identified as luteolin-7-O-glucoside based on the fragmentation pathway and data available in the literature.23 The compound 5, appearing in the chromatogram at a RT of 11.6 min, showed λmax of 350 and 266 nm, and pseudo-molecular ion [M − H]− at m/z 739. The fragment ions at m/z 593, 447 and 285 correspond to the elimination of one rhamnose unit, elimination of two rhamnose units, and finally the loss of glucose moiety, respectively. Identification of this compound as kaempferol-O-dirhamnoside-glucoside was supported by comparison of UV spectra of kaempferol and luteolin standards. Both aglycons have the same molecular mass but differ in UV maxima (see Table 1).
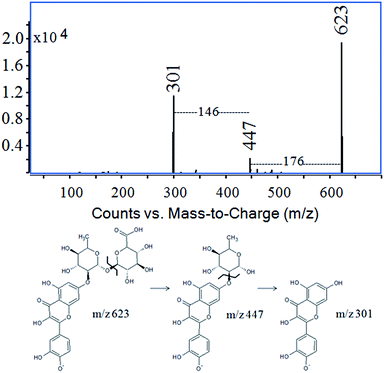
Peak 6 showed a pseudo-molecular ion [M − H]− at m/z 609 and fragment ions [M − H − 162]− at m/z 447 and [M − H − 146]− at m/z 301 corresponding to the successive losses of glucose and rhamnose moieties from quercetin glycoside. The fragmentation pattern of this compound was identical to peak 6 appearing at a retention time of 11.9 min in the chromatogram of the Persian berry extract (Fig. 1e). The data allowed us to identify compound 6 as quercetin-O-rhamnoside-glucoside. The ESI-MS spectrum of compound 7 showed an ion peak [M − H]− at m/z 447 and its fragment ion at m/z 301 formed by the loss of 146 mass units, indicating the elimination of the rhamnose unit from quercetin-O-rhamnoside. The main compound (peak 8, the pseudo-molecular ion at m/z 769) present in the samples Laque de Robert no. 5 and no. 6, as well as in the Persian berry extract, corresponds to O-triglycosylated xanthoramnin (also known as rhamnetin-O-dirhamnoside-glucoside).24 The pseudo-molecular ion (m/z 769) yielded three fragment ions: one intense at m/z 623 ([M − H − 146]−) by the loss of the rhamnose moiety, and two minor at m/z 477 ([M − H − 146 − 146]−) and m/z 315 ([M − H − 146 − 146 − 162]−) characteristic of double elimination of rhamnose units and finally the loss of the glucose moiety. Peak number 9, appearing at a retention time of 13.7 min, showed a pseudo-molecular ion [M − H]− at m/z 623 that generated two intense fragment ions at m/z 447 [M − H − 176]− (elimination of glucuronic acid) and m/z 301 [M − H − 176 − 146]− (elimination of glucuronic acid and rhamnose units from quercetin glycoside). This compound was identified as quercetin-O-rhamnoside-glucuronide (Fig. 2) which was confirmed by the ESI(−)-QTOF product ion mass spectrum with the molecular mass peak of [M − H]− at m/z 623.1253 (corresponding to the elemental composition of C27H28O17, mass diff. 0.16 ppm), fragment ion of [M − H − 176]− at m/z 447.0969 (C21H20O11, mass diff. 8.27 ppm) and [M − H − 176 − 146]− at m/z 301.0349 (C15H10O7, mass diff. 1.66 ppm).
This compound is present only in the naturally aged paint samples but not in the Persian berry extract. It was probably formed from its precursor quercetin-O-rhamnoside-glucoside (compound no. 6) in the oxidation process. In Laque de Robert samples the intensity of peak no. 9 predominate the intensity of peak no. 6, whereas in Persian berry extract the intensity of compound 6 is one of the most intensive. To the best of our knowledge, this colouring compound has not been reported so far in the literature. Peak 10 was assigned to quercetin according to its UV spectrum; the retention time corresponds to the reference standard material, pseudo-molecular ion [M − H]− at m/z 301, and the main fragment ion at m/z 151 which is characteristic of 1,3B cleavage of the C ring in the quercetin molecule.25,26 Quercetin and its glycosides, widely distributed in the plant kingdom, were also detected in the Persian berry extract.27 Peak no. 11 (RT 14.1 min) was identified as luteolin due to λmax at 350 nm, molecular ion [M − H]− at m/z 285, and the unique fragment ion at m/z 133 that could be attributed to the 1,3B flavonoid fragmentation pathway. Compound 12 had a pseudo-molecular ion at m/z 783 [M − H]− and daughter ions at m/z 637 [M − H − 146]− (elimination of rhamnose), m/z 491 [M − H − 146 − 146]− (elimination of two rhamnose units), m/z 329 [M − H − 146 − 146 − 162]− (elimination of all sugar units), and at m/z 299 [M − H − 454 − 2CH3]−. These data, together with the results of ESI(−)-QTOF analysis (see Table 1), lead to the identification of the compound as rhamnazin-3-O-dirhamnoside-glucoside, previously cited in the literature.27 The small peak at a RT of 14.5 min (no. 13) with ion [M − H]− at m/z 637, one main fragment ion at m/z 329 [M − H − 308]− formed after cleavage of the rhamnose-glucoside moiety, and two minor fragment ions at m/z 314 [M − H − 308 − CH3]− and m/z 299 [M − H − 308 − 2CH3]− was identified as rhamnasin-O-rhamnoside-glucoside with elemental composition C29H34O16 (ESI(−)-QTOF m/z 637.1794, mass diff. −3.14 ppm). The accurate mass of the ions formed by the loss of one and two methylene radicals was found from the QTOF spectrum at m/z 314.0436 (mass diff. −1.27 ppm) and m/z 299.0181 (mass diff. 5.35 ppm), respectively. To the best of our knowledge, this compound was not detected in the Rhamni species plant before. Compound labelled as no. 14 had pseudo-molecular ion [M − H]− at m/z 623 and two main fragment ions at m/z 461 [M − H − 162]− and m/z 315 [M − H − 162 − 146]−, characteristic of elimination at the first glucose unit and subsequently the rhamnose moiety. It was identified as rhamnetin-3-O-rutinoside based on data available in the literature.22 Compound labelled as no. 15 was apigenin, frequently identified in Reseda luteola L. extracts,23 with pseudo-molecular ion [M − H]− at m/z 269 and fragment ions registered at m/z 227 and m/z 151. The ion at m/z 227 [M − H − 42]− corresponds to the loss of the carbene molecule (C2H2O). The ion at m/z 151 was assigned to fragmentation 1,3A of the C-ring in the flavonoid structure. Peak 16 with pseudo-molecular ion [M − H]− at m/z 285, and fragment ions at m/z 257 [M − H − 28]− and at m/z 151 (1,3A cleavage of flavonoid structure) was identified as kaempferol. The next peak (no. 17) detected in yellow paint samples and in Persian berry extract had ion [M − H]− at m/z 315, and fragment ions at m/z 300 [M − H − 15]− (loss of CH3 radical), at m/z 272 [M − H − CH3 − CO]− and at m/z 244 [M − H − CH3 − 2CO]−. This compound was identified as rhamnetin. The elimination of CH3˙ from the negative ion is characteristic of flavonoids containing methoxy substituents at the aromatic ring.28,29 The peak eluted at a retention time of 18.0 min (no. 18) showed a pseudo-molecular ion [M − H]− at m/z 299 and fragment ions at m/z 284, 271, 256, 243, and 228. Careful interpretation of this spectrum allows us to propose two fragmentation pathways for this compound. The first pathway is a series of ion reactions involving double decarbonylation ([M − H − CO]− and [M − H − CO − CO]−) followed by the loss of a methyl radical [M − H − CO − CO − CH3]˙−. Ions at m/z 284, 256, and 228 reflect a second fragmentation path involving firstly the loss of a methyl radical [M − H − CH3]˙−, and subsequently the double decarbonylation ([M − H − CH3 − CO]˙−, [M − H − CH3 − CO − CO]˙−). These results allow us to identify compound no. 18 as rhamnocitrin, widely distributed in the Rhamni family plant.27 Compound no. 19, with major ion [M − H]− at m/z 329, showed the fragment ions at m/z 314 [M − H − CH3]˙−, 301 [M − H − CO]−, 299 [M − H − 2CH3]−, 286 [M − H − CO − CH3]˙−, 271 [M − H − CO − 2CH3]−, and 258 (low intensity) [M − H − CO − CH3 − CO]˙−. This compound was identified as rhamnazin. The substance no. 20 found in the extracts of Laque de Robert paint was almost invisible in the UV chromatogram. The compound was found as emodin in the extracted ion chromatogram at m/z 269. The ion [M − H]− undergoes decarbonylation and decarboxylation, leading to ions at m/z 241 [M − H − CO]−, m/z 225 [M − H − CO2]−, and m/z 197 [M − H − CO2 − CO]−. The identification of aglycons (apigenin, kaempferol, rhamnetin and emodin) was straightforward as these compounds were available in pure form (standard).
Paint sample labelled as Still de Grain was compared to weld and Persian berry extracts (Fig. 1c–e). Besides the twenty compounds detected in the Laque de Robert no. 5 and Laque de Robert no. 6, two additional colouring substances have been found in Still de Grain paint. The compound appearing at a retention time of 12.9 (peak no. 21) had the same mass base peak [M − H]− as compound no. 4 at m/z 447 and the same product ions at m/z 285 and 151. This corresponds to luteolin-O-glucoside yielding a luteolin aglycone anion formed upon the detachment of the whole glucose moiety. Such fragmentation is characteristic of O-glycosides, although it is not possible to conclude the exact position of the sugar unit. The enlargement of the UV chromatographic trace shows small peak just after the peak of kaempferol at a retention time of 15.4 min (peak no. 22) with mass base peak [M − H]− at m/z 299 and daughter ions at m/z 284 [M − H − CH3]˙−, m/z 271 [M − H − CO]−, and m/z 256 [M − H − CO − CH3]−. Based on MS spectra and by comparison with data available in the literature, it was identified as chrysoeriol.30 This compound was also detected in the weld extract (Fig. 1e).
Additional compounds not present in the paint samples were identified in the weld extract (Table 2).
| Compound | RT (min) | [M − H]− | Fragment ions (m/z) | Proposed identification | λ max (nm) |
|---|---|---|---|---|---|
| W1 | 9.6 | 593 | 447, 431, 285 | Kaempferol-O-glucoside-O-rhamnoside | 272, 346 |
| W2 | 9.8 | 609 | 447, 285 | Luteolin-O-diglucoside | 267, 342 |
| W3 | 12.3 | 431 | 269 | Apigenin-O-glucoside | 268, 338 |
| W4 | 12.5 | 461 | 446, 299, 284 | Chrysoeriol-O-glucoside | 268, 350 |
| M1 | 12.9 | 563 | 269 | Lucidin primeveroside | 255, 410 |
| M2 | 12.9 | 533 | 239 | Alizarin primeveroside | 255, 410 |
| M3 | 20.5 | 239 | 211, 195 | Quinizarin | 256, 468 |
The first of them, labelled as W1, eluted at a retention time of 9.6 min and had main ion [M − H]− at m/z 593 that yielded fragment ions at m/z 447 (loss of rhamnose moiety), m/z 431 (loss of glucose unit), and m/z 285 (loss of rhamnose and glucose moieties). Formation of fragment ions derived by losses of separate sugar unit suggests that they were located at different positions at the aglycone. Based on MS spectra, the compound W1 was tentatively identified as kaempferol-O-glucoside-O-rhamnoside, although it was not possible to conclude the exact position of each sugar unit. Previous studies confirm the presence of kaempferol-3-O-glucoside-7-O-rhamnoside in several plants.26,31 Peak W2 with a pseudo-molecular ion at m/z 609 has similar fragment ions to luteolin 3′,7-O-diglucoside (peak no. 2). Both these compounds had the same UV spectra with maxima absorbance at 267 and 342 nm, and fragment ions [M − H]− at m/z 447 and m/z 285, corresponding to the successive losses of two glucose moieties, characteristic of luteolin-O-diglucoside (ESI(−)-QTOF m/z 609.1452, mass diff. 1.48 ppm). Different retention times of compounds no. 2 and W2 imply the presence of glucosyl residues attached to different positions at the aglycone. The position of the sugar moieties could not be definitely concluded. The pseudo-molecular ion in the MS spectrum of compound W3 is registered at m/z 431 [M − H]− (ESI(−)-QTOF m/z 431.0989 mass diff. −1.16 ppm), and main fragment ion at m/z 269 [M − H − Glc]− (ESI(−)-QTOF m/z 269.0442, mass diff. 4.83 ppm). The compound was tentatively identified as apigenin-O-glucoside. Peak labelled as W4 showed a main ion at m/z 461 [M − H]− and product ions at m/z 446 [M − H − CH3]˙−, m/z 299 [M − H − Glc]−, m/z 284 [M − H − Glc − CH3]− and m/z 255 [M − H − Glc − CO2]−. The accurate mass of the pseudo-molecular ion of W4 (m/z 461.1092, mass diff. 0.65 ppm) and the accurate mass of the main fragment ion (m/z 299.0555, mass diff. 2.01 ppm) allow us to identify the examined compound as the chrysoeriol-O-glucoside losing glucose unit.
Although most of the plants used to produce the yellow dyestuff have unique patterns of flavonoid components, it is hard to reliably identify the plant source without the appropriate plant reference material because many plants contain other classes of colorants. For example, luteolin and apigenin were identified in all yellow paints. Luteolin is a major dyestuff in a few natural sources, such as Reseda luteola L., Serratula tinctoria L., Genista tinctoria L., etc.32,33 Unfortunately, the presence of major components of natural dye is not sufficient to determine the origin of plant sources used to prepare paints and the minor colouring substances in the sample are fingerprints, characteristic to a specific plant. In the case of yellow paint investigated in our study, only those samples where chrysoeriol was identified together with apigenin and luteolin are likely to have been dyed using Reseda luteola L. In the other cases the use of another flavone-containing plant may not be excluded.30,34 The results may suggest that Persian berries and weld were the sources of dyes of yellow lake in Still de Grain paint. The absence of chrysoeriol in the samples of Laque de Robert no. 5 and Laque de Robert no. 6 indicates that other plants containing luteolin and apigenin were used beside Persian berries for paint preparation.
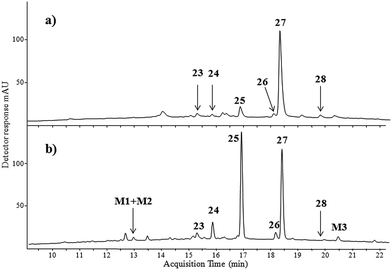 | ||
| Fig. 3 HPLC-DAD chromatograms (λ = 350 nm) of red extracts taken from: (a) Laque de Garance and (b) madder roots raw source. For chromatographic conditions see the Experimental section. | ||
Two main components eluting at retention times of 16.8 min (peak no. 25) and 18.3 min (peak no. 27) were observed together with only a small chromatographic peak labelled as: 23, 24, 26, and 28 at retention times of 15.2, 15.9, 18.1 and 19.8 min, respectively. Peak 25 had mass base peak [M − H]− at m/z 239 accompanied by product ions at m/z 211 and m/z 167. The ion m/z 239 corresponds to the alizarin anion [M − H]−, whereas the ion at m/z 211 differs by 28 atomic mass units from the alizarin anion by the loss of CO molecule. The second signal at m/z 167 is due to the subsequent loss of carbon dioxide. The next compound appearing at a retention time of 18.3 min (peak no. 27) was identified as purpurin based on the pseudo-molecular ion at m/z 255 and the product ions at m/z 227 [M − H − CO]− and m/z 183 [M − H − CO − CO2]−. Retention times, the MS and UV spectra of compounds no. 25 and 27 were identical to those of alizarin and purpurin reference standards. Compound labelled as no. 24 (RT 15.9 min) had base ion [M − H]− at m/z 255 and a similar fragmentation pathway to purpurin. The difference in retention time compared to that of purpurin is probably due to different locations of the three hydroxyl groups in the aromatic ring (see ESI†). This suggests the presence of anthragallol in the Laque de Garance extract, which was also found in the Rubia tinctorum origin plant.35 Peak no. 26 eluting at a retention time of 18.1 min had the same mass base peak [M − H]− at m/z 239 as alizarin and two fragment ions at m/z 211 and m/z 195 formed by the loss of CO and CO2 molecules, respectively. This fragmentation pathway is specific for anthraquinoids.36 Compound no. 26 eluted at higher retention time than alizarin. The difference in the retention time is related to hydrophobicity which led to tentative identification of compound 26 as xanthopurpurin as the meta position of hydroxyl groups in xanthopurpurin increases its hydrophobicity and increases its retention time in comparison to alizarin with hydroxyl groups in the ortho position.37 Compound no. 23 also had a mass spectrum similar to alizarin and xanthopurpurin with pseudo-molecular ion [M − H]− at m/z 239 and daughter ions at m/z 211 [M − H − CO]−, m/z 195 [M − H − CO2]−, m/z 183 [M − H − 2CO]−, and m/z 167 [M − H − CO2 − CO]−. This indicates the presence of the third positional anthraquinone isomer which differs in location of hydroxyl groups in the aromatic moiety. Based on data available in the literature,35,36 we suppose that compound no. 23 could be hystazarin. This anthraquinone to our knowledge has never been detected in the Rubia family plant. Compound 28 eluting at 19.8 min was classified as rubiadin on the basis of the pseudo-molecular ion (m/z 253) and its fragmentation path (m/z 225 [M − H − CO]− and m/z 197 [M − H − CO − CO]−).
The HPLC profile of the Laque de Garance paint extract was compared to that of the madder lake extract. A significant amount of alizarin and purpurin is present in madder lake (Fig. 3b). Identification of these compounds was straightforward as alizarin and purpurin standards are available. The four other compounds were found in the madder lake extract with the same molecular mass (240 Da). Three of them (no. 23, 25 and 26) were also present in the Laque de Garance paint sample, while the fourth, designated as M3, was present only in the plant extract. The last compound was identified as quinizarin by comparison to a standard. The high retention time of M3 (20.5 min) is due to the para arrangement of the hydroxyl groups, increasing its hydrophobicity. The small chromatographic peak seen in the UV spectrochromatogram of the madder lake extract at 12.9 min (M1 + M2) had a rather complicated mass spectrum. The chromatogram deconvolution algorithm of Agilent MassHunter software showed two co-eluting components. The reconstructed mass spectrum of the first component showed a pseudo-molecular peak at m/z 563 and the main product ion at m/z 269, while a pseudo-molecular peak at m/z 533 with a product ion at m/z 239 can be seen in the reconstructed mass spectrum of the second component (Table 1). The structure of the first component may correspond to a lucidin primeveroside with fragment ion m/z 269 [M − H − 294]− formed by the detachment of the whole rhamnosyl-pentoside moiety from lucidin glycoside. The alizarin primeveroside structure was assigned to the second component with a product ion at m/z 239, also formed by the elimination of the rhamnosyl-pentoside unit.38,39 It must be noted that these glycosides are not present in the Laque de Garance paint sample because the procedure of making the lake pigment may lead to the hydrolysis of the glycosides to parent aglycons.
Singh et al.40 stated that most species of Rubia contain many anthraquinones, though relative amounts are not given. We detected a significant amount of purpurin, and lower contents of alizarin, hystazarin, xanthopurpurin, and rubiadin in the red paint extract. This composition is typical to a number of species of Rubia-type plants that grow in Europe, e.g. Rubia tinctorum L., Rubia peregrina L. or Rubia cordifolia L.7 It is difficult to judge which plant from the Rubia family was the source for production of Laque de Garance paint, however.
3.2 Microscopic and spectroscopic study
The oil paint samples were also investigated by means of optical microscopy, XRF as well as SEM-EDX and FT-IR techniques. All samples were very homogenous and substrate particles, when visible, were relatively small. Samples of Laque de Robert no. 5 and no. 6 were close both in optical character and substrates and fillers.Calcium, Al, S, K, Si, Mg, Na, Fe, and Zn were identified in Laque de Robert no. 5 using SEM-EDX. All these elements, apart from Na, were confirmed by XRF analyses, and moreover Sn was detected as well. Detection of tin suggests possible addition of its salts in the form of tin chloride or oxide as another carrier for the dyes. It was a common practice in the 19th century to use both aluminium and tin containing substrates for production of red dyes. The presence of potassium and sulphur probably resulted from the alkali (K2CO3) and acids (H2SO4) added during the preparation of the lakes. Depending on the lake production method known in the second half of the 19th-century, it could happen that the resulting substrate was aluminium sulphate.41 The use of silica sand as a filler is indicated by silica crystallites clearly visible in the BSE images (Fig. S35 and S36 in the ESI†) obtained with a scanning microscope. Zinc probably originates from the zinc white additions lightening the hue of the paint. Tiny amounts of magnesium ions were also found that suggest addition of minerals like dolomites as fillers.
The sample of Laque de Robert no. 6 (Fig. S37 and S38†) was very close to the inorganic composition of the previous one. Calcium, S, Al, K, Si, and Mg were identified using SEM-EDX. Zinc was not present. All these elements were confirmed by the XRF analyses, and moreover Sn and Cu were detected as well. A tiny difference between these paints was the presence of copper which could be used as sulphate during dyestuff preparation or alternatively as siccative added because copper salt improves the drying properties of the paint. There were no grains of colouring pigments containing copper. The main reason of different hues is due to the addition of the zinc white to the lighter version.
Samples of Still de Grain paint were a little bit more heterogeneous, with tiny grains of the lake precipitated in amorphous aluminium hydrate and additionally of chalk as well as some silica sand grains. Aluminium, Ca, Si, K, Cl, S, P, Mg, and Na were identified using SEM-EDX. All these elements, apart from Na, were confirmed with the XRF analysis.
The mixture of grains of different ingredients was clearly visible in the UV lights. Although it is not obvious whether it is caused by the medium as well, it seems that the grains of the precipitated lake are bigger and have a tendency to darken in the UV light. The mixture of dyes is precipitated in the aluminium salts. The SEM-EDX maps of elemental distribution (Fig. S39 and S40 in ESI†) imply the presence of sulphur along with aluminium, and it cannot be excluded that the substrate is not only aluminium hydrate but its sulphate as well. The presence of some amount of tin suggests that the carrier of this kind was used in parallel to the aluminium based one. The detection of sodium and potassium can be related to the plant source or alkalis used during production (K2CO3 or Na2CO3). The presence of chlorine and sulphur can be attributed to the acids used in the preparation of the plant extract. The presence of arsenic is possibly related to the use of its salt during precipitation in the form of potassium arsenate. Copper and manganese salts were probably used to improve the drying properties of the paint.
The Laque de Garance sample (Fig. S41 and S42 in ESI†) contained Al, P, Sn, Si, Ca, S and Sb. Tin and Pb were additionally identified by XRF. The paint possibly contains both aluminium and tin based carriers as well as lead white and silica sand grains. The presence of other characteristic elements, phosphorus and potassium, originates probably from the plant source itself or from some stage in the production of this paint.
FT-IR analysis of the paint samples has been performed (Fig. S43 in the ESI†). Due to the fact that oil paint materials have complex composition, elucidation of infrared spectra is very complicated. FT-IR spectra of the paint samples were quite similar and revealed the presence of a broad O–H stretching band in the 3000 to 3600 cm−1 range. The strong bands centered at 2920 cm−1 and 2850 cm−1 could be assigned to C–H (aromatic) stretching vibration. The strong and broad absorption band centered at 1460 cm−1 probably resulted from the carboxylate ion, while skeletal vibrations involving carbon–carbon stretching within the aromatic ring absorbed in the 1560–1600 cm−1 region. The strong and broad band centered at 1100 cm−1 with a small shoulder at 990 cm−1 could be assigned to sulphate absorptions.
4 Conclusions
The combination of LC-ESI-MS analysis with SEM, EDX, FT-IR and XRF methods enabled the determination of composition of commercially available 19th-century artistic paints. Mass spectrometry facilitated the identification of 35 colour organic compounds present in one red and three yellow oil paints as well as three plant materials. Characteristic fragmentation paths of the compounds provided additional information on the structures of the analytes. An improved mild extraction method with hydrofluoric acid enabled the intact dye fingerprints in paint samples. Persian berries and weld were identified as the dye sources in yellow paint samples. Red oil paint had been coloured with madder lake-type plant although it is difficult to indicate which plant from the Rubia family was the dyestuff source for the production of this paint. The characteristic fragmentation pathways of the colouring substances were proposed. Studies of the extracts of the historical samples show the presence of additional and unusual dye components (quercetin-O-rhamnoside-glucuronide and rhamnasin-O-rhamnoside-glucoside). The paint samples were additionally analysed by means of XRF and SEM-EDX to identify carriers of the dyes based on both aluminium and tin salt as well as fillers like chalk, zinc white lightening the hue of the Laque de Robert no. 5 and lead white added to the Laque de Garance. In all samples tiny amounts of finely grinded silica sand were found. The presence of potassium and sulphur indicates alkali and/or acid solutions used during the extraction process. To the best of our knowledge this is the first attempt to investigate the manufactured 19th-century artistic paints with the known commercial indication using the combination of analytical techniques proposed here.Acknowledgements
The HPLC-MS part of research work has been financially supported by the National Science Centre of Poland Preludium project No. 2015/17/N/HS2/03310. The XRF and SEM-EDX part of research work has been financially supported by the National Science Centre of Poland Sonata project No. 2012/05/D/HS2/03385.Notes and references
- E. Rosenberg, Anal. Bioanal. Chem., 2008, 391, 33–57 Search PubMed.
- V. Pauk, P. Bartak and K. Lemr, J. Sep. Sci., 2014, 37, 3393–3410 Search PubMed.
- P. Buzzinia and E. Suzukib, J. Raman Spectrosc., 2016, 47, 16–27 Search PubMed.
- C. Miliani, F. Rosi, B. G. Brunetti and A. Sgamellotti, Acc. Chem. Res., 2010, 43, 728–738 Search PubMed.
- M. Elias, N. Mas and P. Cotte, J. Cult. Herit., 2011, 12, 335–345 Search PubMed.
- A. Domenech-Carbo, J. Solid State Electrochem., 2010, 14, 363–379 Search PubMed.
- J. H. Hofenk de Graaff, The Colorful Past: Origins, Chemistry and Identification of Natural Dyestuffs, Archetype Publications, 2004 Search PubMed.
- J. Wouters, Stud. Conserv., 1985, 30, 119–127 Search PubMed.
- S. Halpine, Stud. Conserv., 1996, 41, 76–94 Search PubMed.
- E. S. Ferreira, A. Quye, H. McNab and A. N. Hulme, Dyes Hist. Archaeol., 2002, 18, 63–72 Search PubMed.
- O. Otłowska, M. Ślebioda, M. Wachowiak and M. Śliwka-Kaszyńska, RSC Adv., 2015, 5, 48786–48792 Search PubMed.
- V. Pauk, P. Bartak and K. Lemr, J. Sep. Sci., 2014, 37, 3393–3410 Search PubMed.
- L. Valianou, I. Karapanagiotis and Y. Chryssoulakis, Anal. Bioanal. Chem., 2009, 395, 2175–2189 Search PubMed.
- J. Kirby and R. White, Natl. Gallery Tech. Bull., 1996, 17, 56–80 Search PubMed.
- J. Sanyova, Microchim. Acta, 2008, 162, 361–370 Search PubMed.
- J. Sanyova and J. Reisse, J. Cult. Herit., 2006, 7, 229–235 Search PubMed.
- X. Zhang and R. A. Laursen, Anal. Chem., 2005, 77, 2022–2025 Search PubMed.
- O. Otłowska, M. Ślebioda, M. Wachowiak, M. Śliwka-Kaszyńska and G. Trykowski, Zabytkoznawstwo i Konserwatorstwo XLVI, Acta Universitatis Nicolai Copernici, Toruń, 2015, pp. 287–314, DOI:10.12775/AUNC_ZiK.2015.011.
- I. Schaefer, C. von Saint-George and K. Lewerentz, Painting light, the hidden techniques of the impressionists, Wallraf-Richartz-Museum & Foundation Corboud, Koln, 2008 Search PubMed.
- J. H. Townsend, L. Carlyle, N. Khandekar and S. Woodcoock, Conservator, 1995, 19, 65–78 Search PubMed.
- L. Carlyle, Conservator, 1993, 17, 56–60 Search PubMed.
- L. Barros, M. Duenas, I. Ferreira, A. M. Carvalho and C. Santos Buelga, Food Chem., 2011, 127, 169–173 Search PubMed.
- C. Moiteiro, H. Gaspar, A. I. Rodrigues, J. F. Lopes and V. Carnide, J. Sep. Sci., 2008, 31, 3683–3687 Search PubMed.
- K. Bourhis, S. Blanc, C. Mathe, J. C. Dupin and C. Vieillescazes, Appl. Clay Sci., 2011, 53, 598–607 Search PubMed.
- Y. L. Ma, Q. M. Li, H. Van den Heuvel and M. Claeys, Rapid Commun. Mass Spectrom., 1997, 11, 1357–1364 Search PubMed.
- B. D. Davis, Structural Characterization of Isomeric Flavonoid Glycosides and Metabolites by Metal Complexation and Electrospray Ionization Tandem Mass Spectrometry, PhD thesis, The University of Texas, 2007.
- G. Cuoco, C. Mathe and C. Vieillescazes, Microchem. J., 2014, 115, 130–137 Search PubMed.
- K. Lech, K. Witkoś and M. Jarosz, Anal. Bioanal. Chem., 2014, 406, 3703–3708 Search PubMed.
- K. Lech and M. Jarosz, Anal. Bioanal. Chem., 2011, 399, 3241–3251 Search PubMed.
- D. Peggie, A. Hulme, H. McNab and A. Quye, Microchim. Acta, 2008, 162, 371–380 Search PubMed.
- N. Wyplosz, Laser desorption mass spectrometric studies of artists' organic pigments, PhD thesis, University of Amsterdam, 2003.
- E. S. Ferreira, A. N. Humle, H. McNab and A. Quye, Chem. Soc. Rev., 2004, 33, 329–336 Search PubMed.
- I. Karapanagiotis, J. Theologou, A. Lakka, A. Ozoline and C. Panayiotou, Archaeometry, 2011, 53, 587–599 CrossRef CAS.
- I. Petroviciu, I. Vanden Berghe, I. Cretu, F. Albu and A. Medvedovici, J. Cult. Herit., 2010, 13, 89–97 Search PubMed.
- L. Raffaelly, S. Heron, W. Nowik and A. Tchapla, Dyes Pigm., 2008, 77, 191–203 Search PubMed.
- I. Surowiec, B. Szostek and M. Trojanowicz, J. Sep. Sci., 2007, 30, 2070–2079 Search PubMed.
- K. Lech, K. Witkoś, B. Wileńska and M. Jarosz, Anal. Bioanal. Chem., 2015, 407, 855–867 Search PubMed.
- G. C. Derksen, M. Naayer, T. A. van Beek, A. Capelle, I. K. Haaksman, H. A. van Doren and A. de Groot, Phytochem. Anal., 2003, 14, 137–144 Search PubMed.
- C. Mouri and R. Laursen, Microchim. Acta, 2012, 179, 105–113 Search PubMed.
- R. Singh, Geetanjali and S. M. Chauhan, Chem. Biodiversity, 2004, 9, 1241–1264 Search PubMed.
- J. Kirby, Art of the past, sources and reconstructions, 2005, pp. 69–78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: MS spectra. See DOI: 10.1039/c6ay02959k |
| This journal is © The Royal Society of Chemistry 2017 |