Sodium tanshinone IIA sulfonate suppresses pulmonary fibroblast proliferation and activation induced by silica: role of the Nrf2/Trx pathway
Zhonghui
Zhu
ab,
Yan
Wang
ab,
Di
Liang
ab,
Gengxia
Yang
c,
Li
Chen
ab,
Piye
Niu
ab and
Lin
Tian
*ab
aSchool of Public Health, Capital Medical University, Beijing 100069, China
bBeijing Key Laboratory of Environmental Toxicology, Capital Medical University, Beijing, 100069, P.R. China
cOncology Minimally Invasive Interventional Center, Beijing Youan Hospital, Capital Medical University, Beijing 100069, China. E-mail: tian_lin@163.com; Fax: +86 10 83911506; Tel: +86 10 83911506
First published on 2nd December 2015
Abstract
Alveolar macrophages are believed to induce oxidative stress via reactive oxygen species (ROS) when silica particles are inhaled. This process can contribute to the pathogenesis of silicosis, but the mechanism is unclear. A traditional Chinese herbal derivative, sodium tanshinone IIA sulfonate (STS), displays significant antioxidant effects. Here, we determine whether STS can attenuate the oxidative stress induced by silica. Traditionally, studies on the toxic effects of silica have focused on monocultures of macrophages or fibroblasts. A coculture model of macrophages (Raw 264.7) and pulmonary fibroblasts (MRC-5) was used in this study to mimic a more in vivo-like environment. We investigated the protective effects of STS on the abnormal proliferation of MRC-5 fibroblasts in an in vitro model. The results showed that fibroblast viability increased with the accumulation of intracellular ROS induced by cocultured Raw 264.7 cells after silica exposure. Treatment with STS markedly ameliorated the silica-induced cell proliferation and oxidative stress. Western blotting and immunofluorescence analysis of the Nrf2 and thioredoxin (Trx) system were conducted, and the results confirmed that treatment with STS enhanced nuclear Nrf2 accumulation and mediated antioxidant Trx system expression. These findings suggest that silica exposure might induce some level of oxidative stress in fibroblasts and that STS might augment antioxidant activities via up-regulation of the Nrf2 and Trx system pathways in MRC-5 cells in vitro.
1. Introduction
Silicosis, a disease caused by chronic inhalation of crystalline silica, is characterized by inflammation and scarring in the form of nodular lesions in the lungs. The presence of silicosis has been known for centuries, and industrial dust has led to the increase of silicosis worldwide, especially in developing countries. However, the pathological mechanism of silicosis is unclear. Recent studies have found that when a silica particle is inhaled, it is inevitably engulfed by macrophages that act as the primary barrier and release cytokines, such as TGF-β1.1 These stimulate fibroblast proliferation and the production of collagen around the silica particles, resulting in pulmonary fibrosis and the formation of silicotic nodules.2 However, enhancing anti-inflammatory therapy has shown limited therapeutic efficacy in silicosis.During the development of silicosis, contact between alveolar macrophages and silica drives the subsequent pathogenic process. The uptake of silica particles by macrophages triggers the production of reactive oxygen species (ROS) via the oxidative stress pathway, which in turn contributes to pulmonary damage and macrophage death by apoptosis.3 In addition, recent evidence has suggested that nonphagocytic cells, including fibroblasts, also produce ROS following stimulation by cytokines and growth factors.4,5 Sustained ROS generation perpetuates the continuum of phagocytosis, cell death, inflammatory cell recruitment and silica deposition, and it is responsible for progressive and irreversible lung injury.6–8 These data suggest that scavenging excessive ROS could be a possible therapeutic approach to modulate the fibrogenic response.
Over the past few decades, studies have found an emerging role for the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in resistance to oxidative stress. Under normal conditions, Nrf2 is predominantly sequestered in the cytoplasm with its chaperone. Oxidative insults cause dissociation of the chaperone complex, triggering the translocation of Nrf2 from the cytoplasm to the nucleus.9 Then, nuclear Nrf2 binds to the antioxidant response element (ARE) and up-regulates the transcription of genes, such as thioredoxin (Trx) and thioredoxin reductase (TrxR),10 which are critical to the cellular antioxidant process.11,12 Trx, TrxR and NADPH comprise the Trx system, which plays an important role in some biological functions such as the regulation of redox.13 To clear ROS, TrxR is involved in catalyzing oxidized Trx to regenerate its reduced form using NADPH.14 We have previously observed the relationship between Nrf2 and Trx in a rat silicosis model.15 In addition, knockdown of Nrf2 in mice can substantially increase susceptibility to a variety of exogenous toxicities and disease situations associated with oxidative pathology,16–18 whereas pharmacological boosting of Nrf2 activity with chemoprotective agents protects animals from oxidative damage.19 However, activating the Nrf2 and Trx pathways has seldom been studied in alleviating oxidative stress in silicosis.
Sodium tanshinone IIA sulfonate (STS) is a water-soluble derivative of tanshinone IIA, an important ingredient in the well-known traditional Chinese herb Danshen (Salvia miltiorrhiza). With superior bio-availability and various pharmacological actions, STS has been used for the treatment of antioxidative stress and reversing myocardial fibrosis, among other conditions.20 However, there are few studies available on the efficacy of STS in pulmonary fibrosis, and the exact mechanisms of the protective actions of STS in silicosis remain largely undefinable.
Monoculture studies of pulmonary fibroblasts are the predominant form of silica particle toxicological testing in vitro, but it is difficult to show the specific mechanism of silica particle pathogenesis both in vitro and in vivo. Cocultures of multiple cell types have been proposed to be superior to monoculture and to more adequately reflect a signaling environment in vivo.21,22 In this study, we cocultured macrophages (Raw 264.7) with lung fibroblasts (MRC-5) in a Transwell system to determine the potential toxic effects of silica particles and to explore the protective effects of STS. The effects were indirectly similar to those observed in lung tissue and further support the hypothesis that macrophages mediate the pulmonary response following silica exposure. Through the measurement of crystalline silica particles, ROS production, fibroblast viability, and transcription factor and downstream antioxidant protein expression in MRC-5, this study determined that silica exposure may induce oxidative stress to stimulate the proliferation of fibroblasts and that treatment with STS may augment antioxidant activities via the up-regulation of Nrf2/Trx to restore the oxidant/antioxidant equilibrium and to attenuate MRC-5 fibroblast activation in vitro.
2. Materials and methods
2.1 Silica particles and STS
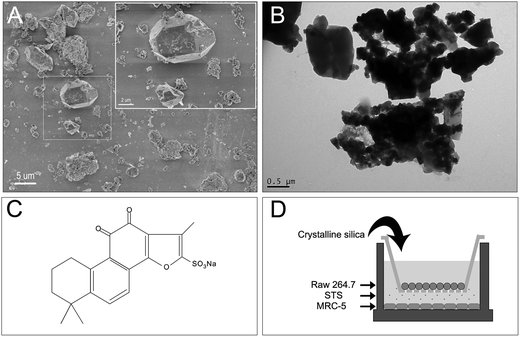
Silica particles (Sigma-Aldrich Co. LLC.) with a nominal size of 0.5–10 μm (approx. 80% between 1–5 μm) were used. The morphology of the crystalline silica particles was assessed by transmission electron microscopy (TEM, JEOL, JEM-2100) and scanning electron microscopy (SEM, HITACHI, S-4800) (Fig. 1A and B). For cell culture, silica was first prepared as stock solution in phosphate-buffered saline (PBS) at a final concentration of 2 mg mL−1 after sonication. The silica stock solution was kept at 4 °C and used within 1 week.STS was obtained from the National Institutes for Food and Drug Control (Beijing, China) and was prepared fresh as a 0.5 mg mL−1 stock solution in PBS. The molecule structure of STS is shown in Fig. 1C.
2.2 Cell culture
Human fetal lung fibroblasts (MRC-5) (Cell Culture Center of Peking Union Medical College, China) and murine macrophage cells (Raw 264.7) (Xiang Ya Central Experiment Laboratory, China) were used in this experiment. Briefly, MRC-5 cells were cultured in minimal essential medium (MEM) with 10% fetal bovine serum (FBS, Gibco) and 1% non-essential amino acids (NEAA, Gibco). Raw 264.7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) and supplemented with 10% FBS, 100 units per mL penicillin G (Sigma), and 100 mg mL−1 streptomycin (Sigma). All cells were maintained in an incubator at 37 °C and 5% CO2.To prepare the coculture, inserts with a 0.4 μm pore size were removed from a polyester Transwell (Corning, USA) and seeded with Raw 264.7 cells (6-well Transwell: 1.5 × 105 cells per well, 96-well Transwell: 3 × 103 cells per well, individually) in complete media in an empty companion dish for at least 12 h. MRC-5 cells (6-well Transwell: 2 × 105 cells per well, 96-well Transwell: 4 × 103 cells per well, individually) were plated at the bottom of each plate well and allowed to adhere for at least 12 h without apical chamber inserts. Then, the inserts were returned to the Transwell. MRC-5 cells were maintained in 2.5 ml or 235 μl of MEM without serum in the basolateral chamber while Raw 264.7 cells were maintained in 1.5 ml or 75 μl of DMEM without serum in the apical chamber. Cells were exposed to silica solution at a final concentration of 12.5, 25, 50, or 100 μg mL−1 for 24 h. As the control group, an MRC-5 monoculture was seeded on the Transwell, and as the negative control group, a coculture of Raw 264.7 and MRC-5 cells without silica was kept under the same culture conditions. Furthermore, to explore the role of STS, MRC-5 cells were supplemented with various concentrations of STS (0, 1, 5, 10, 25, or 50 μg mL−1) in the basolateral chamber with silica exposure (final concentration of 50 μg mL−1 only) and co-incubated for 24 h (Fig. 1D).
2.3 Cell viability
The measurement of cell viability was performed using an MTT ((4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. Briefly, Raw 264.7 cells were plated in the 96-well chamber inserts, and MRC-5 cells were added to the bottom; the cells were then incubated at 37 °C for 12 h. As a control group, MRC-5 cells were solo seeded at 4 × 103 cells per well before incubating for 12 h. Then cells were treated with various concentrations of STS and/or silica suspension and then incubated at 37 °C for an additional 24 h in silica/STS groups. Alternatively, the medium was replaced with DMEM containing a final concentration of 10−3, 10−4, 10−5, 10−6, or 10−7 M H2O2 and cultured for 24 h. After the incubation, the inserts and media in the bottom were removed, and then, 100 μL of MTT solution (0.5 mg mL−1 in PBS) and 100 μL of DMEM were added into each bottom well. MRC-5 cells were incubated under the same conditions for an additional 4 h. MTT was cleaved by living cells into visible formazan crystals during this incubation. The formazan crystals were dissolved in DMSO, and the resulting absorbance was measured at 490 nm using an enzyme-linked immunosorbent assay. The relative cell viability was calculated and compared with the absorbance of the negative control group. All MTT assays were performed more than five times.2.4 Intracellular ROS measurement
Intracellular ROS production was analyzed using a DCFH-DA probe according to the manufacturer's instructions (Jiancheng Biotech, Nanjing, China). DCFH-DA, a nonfluorescent cell-permeant compound, is cleaved by endogenous esterases within the cell, and the de-esterified product can be converted into the fluorescent compound DCF upon oxidation by intracellular ROS. To estimate intracellular ROS, after 24 h of incubation, MRC-5 cells were loaded with 100 nM DCFH-DA for 10 minutes at 37 °C, washed with PBS, and immediately analyzed using a fluorospectrophotometer (SHIMADZU, RF-5301PC) at an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The level of total ROS is expressed as fluorescence intensity per mg protein.2.5 Western blot analysis
Whole MRC-5 extracts were prepared using lysis buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1% sodium deoxycholate; 0.1% SDS) (KeyGEN Biotech, China) on ice after 24 h of incubation. The protein concentration was determined by Bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein extracts (50 μg) were separated by 10% or 15% SDS-Polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ, USA). The transblotted membranes were blocked with 5% nonfat milk in TBS containing 0.1% Tween 20 (TBST) for 1 h. The membranes were incubated overnight at 4 °C with specific antibodies against Nrf2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution, Abcam, UK), Trx (1
1000 dilution, Abcam, UK), Trx (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution, Cell Signaling Technology, USA), TrxR (1
1000 dilution, Cell Signaling Technology, USA), TrxR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution, Santa Cruz Biotechnology, USA) and β-actin (1
500 dilution, Santa Cruz Biotechnology, USA) and β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution, Cell Signaling Technology, USA). The blots were then incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (1
1000 dilution, Cell Signaling Technology, USA). The blots were then incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution, Cell Signaling, USA) for 2 h. The blots were developed using ECL plus western blot detection reagents (Thermo Scientific, USA) and detected with a Molecular Imager Bio-Rad Gel Doc™ XR+ with Image Lab™ Software (Bio-Rad, California, USA).
2000 dilution, Cell Signaling, USA) for 2 h. The blots were developed using ECL plus western blot detection reagents (Thermo Scientific, USA) and detected with a Molecular Imager Bio-Rad Gel Doc™ XR+ with Image Lab™ Software (Bio-Rad, California, USA).
2.6 Confocal laser scanning microscopy
Immunofluorescence labeling was performed to assess the subcellular localization of Nrf2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution, Abcam, UK) and Trx (1
200 dilution, Abcam, UK) and Trx (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution, Abcam, UK) in MRC-5 cultures. Nuclear immunofluorescence was quantified by co-localization with DAPI under observation with a LEICA TCS SP8X (LEICA, Germany).
200 dilution, Abcam, UK) in MRC-5 cultures. Nuclear immunofluorescence was quantified by co-localization with DAPI under observation with a LEICA TCS SP8X (LEICA, Germany).
2.7 Knockdown of Nrf2 by lentiviral transfection
Nrf2-shRNAs were designed to target the Nrf2 gene (NM_001145412); the specific shRNA sequences were cloned into GV248 (Shanghai GeneChem Co., Ltd, Shanghai, China). A green fluorescent protein (GFP) lentiviral vector was used as a negative control. The lentivirus was produced by transfecting lentiviral plasmids into HEK-293 T cells with Lipofectamine 2000 (Invitrogen). After transfection for 48 h, cell supernatant containing the lentivirus was harvested and concentrated, and the virus titer was calibrated. The lentivirus, with a final concentration of 4 × 108 TU ml−1, was stored at −80 °C.MRC-5 cells were plated at a density of 1 × 104 cells per well in six-well plates in MEM supplemented with 10% FBS and 1% NEAA in a humidified atmosphere of 5% CO2 at 37 °C for 12 h before transfection. The medium was then replaced with 1 ml of serum-free medium, and the cells were transfected with lentivirus at a multiplicity of infection (MOI) of 10 in the presence of 5 μg ml−1 polybrene (Sigma). The medium was changed to complete medium after 12 h of transfection. After 72 h of incubation, the rate of infection was observed using a fluorescence microscope, and the total cellular RNA was extracted for further validation or the transfected cells were used for cell function experiments.
2.8 RNA isolation and reverse transcription–polymerase chain reaction
At the indicated time points, total RNA was isolated with the SV Total RNA Isolation System (Promega) and spectrophotometrically quantified, and reverse transcription–polymerase chain reaction (RT-PCR) was performed with the OneTaq® RT-PCR Kit (New England Biolabs) according to the manufacturer's instructions. The reactions were started at first-strand cDNA synthesis, with incubation at 42 °C for one hour. The PCR cycling conditions were as follows: 95 °C for 30 seconds and then immediately cycled 30 times through a 30-second denaturing step at 94 °C, a 30-second annealing step at 61 °C, and a 1 min extension step at 68 °C. After the cycling procedure, a final 5 min elongation step at 68 °C was performed. All amplifications were performed in the linear range of the assay. The reaction products were separated on a 1.0% agarose gel and imaged using a Molecular Imager Bio-Rad Gel Doc™ XR+ with Image Lab™ Software (BIO-RAD, California, USA). The primers used are shown in Table 1.| β-Actin | (F) 5′-TGAGACCTTCAACACCCCAG-3′ |
| (R) 5′-GCCATCTCTTGCTCGAAGTC-3′; | |
| Nrf2 | (F) 5′-GAATTGCCTGTAAGTCCTGGTC-3′ |
| (R) 5′-GGTGAAGGCTTTTGTCATTTTC-3′; | |
| Trx-1 | (F) 5′-GAAGCAGATCGAGAGCAAGACT-3′ |
| (R) 5′-CACTCTGAAGCAACATCCTGAC-3′; | |
| TrxR | (F) 5′-TCTTTTCTCCTTGCCTTACTGC-3′ |
| (R) 5′-TCTTCACCCCTACGGTTTCTAA-3′. |
2.9 Statistical analysis
Individual dependent variable data were analyzed statistically by one-way analysis of variance (ANOVA), followed by Duncan's multiple range test when appropriate. Differences between groups were considered to be significant when p < 0.05. Data are expressed as the mean ± S.D.3. Results
3.1 Effect of STS on the suppression of both silica- and H2O2-induced MRC-5 proliferation
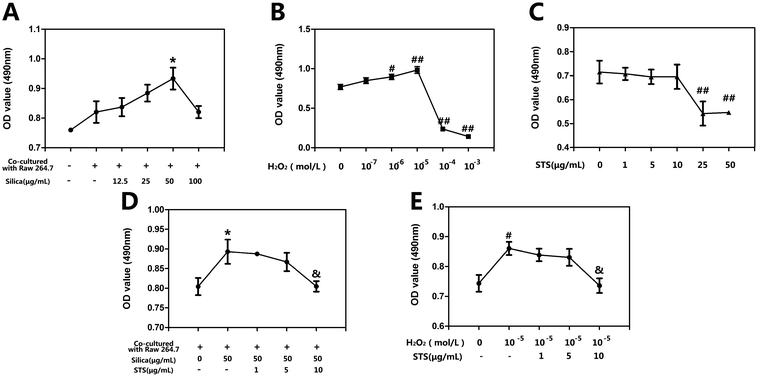
We first explored the viability of MRC-5 cells following treatment with either silica or H2O2 by MTT assay. As shown in Fig. 2A, the viability of MRC-5 cells cocultured with Raw 264.7 cells was increased compared with the quiescent monocultured MRC-5 cells, while at low concentrations (12.5 or 25 μg mL−1) silica administration had an impact on cocultured MRC-5 cells. When examining cells treated with higher amounts of silica, MRC-5 fibroblasts treated with 50 μg mL−1 silica showed peak and significant proliferation compared with the cocultured group without silica treatment. Based on this result, 50 μg mL−1 silica was used for all subsequent STS treatment experiments. As shown in Fig. 2B, a range of concentrations of H2O2 (10−3, 10−4, 10−5, 10−6, and 10−7 M) were used to determine their effects on MRC-5 cell proliferation. In the case of H2O2, an increase of 20–30% in cell proliferation compared with the control group was measured after treatment with 10−5 M H2O2, which was the concentration that was determined for the subsequent study. As the concentration increased further to 10−4 M, the toxic effect of H2O2 became apparent. Furthermore, the toxicity of STS on MRC-5 was not obvious upto 10 μg mL−1 in Fig. 2C. The effects of various STS concentrations below 10 μg mL−1 were measured following their addition after silica or H2O2 treatment as indication previously. As shown in Fig. 2D, STS treatment (10 μg mL−1) suppressed the MRC-5 proliferation induced by 50 μg mL−1 silica. STS administration also showed similar effects on the cell proliferation induced by 10−5 M H2O2 (Fig. 2E).3.2 Effect of STS on the inhibition of silica-induced MRC-5 activation
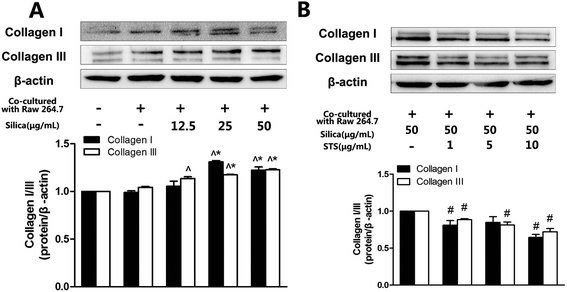
To evaluate whether fibroblast activation plays a key role in silicosis, the expression of extracellular matrix proteins (collagen I and III) was determined. As shown in Fig. 3A and B, corresponding with MRC-5 proliferation, both collagen I expression and collagen III expression were increased in a silica-concentration-dependent manner. The expression of collagen in the 50 μg mL−1 silica treatment groups was significantly increased compared to the negative control group. We also used different concentrations of STS to elucidate the possible effects of STS on extracellular matrix degradation. Compared to the silica treatment group, STS treatment decreased the expression of collagen I and collagen III in MRC-5 cells in a dose-dependent manner.3.3 STS suppressed the ROS production induced by silica in MRC-5 cells
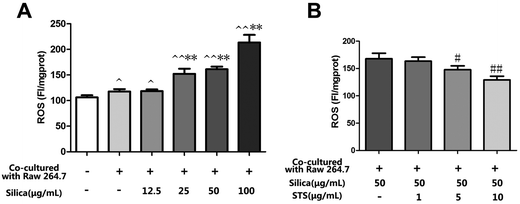
The effect of silica is likely to be mediated by ROS, which could play an important role in the progress of pulmonary fibrosis. To determine whether STS exerts its cytoprotective effects by inhibiting ROS production, intracellular ROS levels were measured in MRC-5 stained with DCFH-DA. As shown in Fig. 4A, silica exposure could markedly increase the generation of ROS in MRC-5 cells in a dose-dependent manner. Interestingly, the macrophage/fibroblast coculture system showed a mild increase in intracellular ROS generation compared with monocultured MRC-5 cells. This finding may to some extent be due to the coexistence of the cell types and could reflect their interaction under physical conditions. On this basis, we aimed to explore the toxic effect of silica in an in vivo-like system. The results showed that silica exposure could clearly aggravate intracellular oxidative stress. In consideration of this, treatment with STS markedly reduced the ROS generation induced by silica.3.4 Effect of STS on Nrf2 protein expression in MRC-5 cells
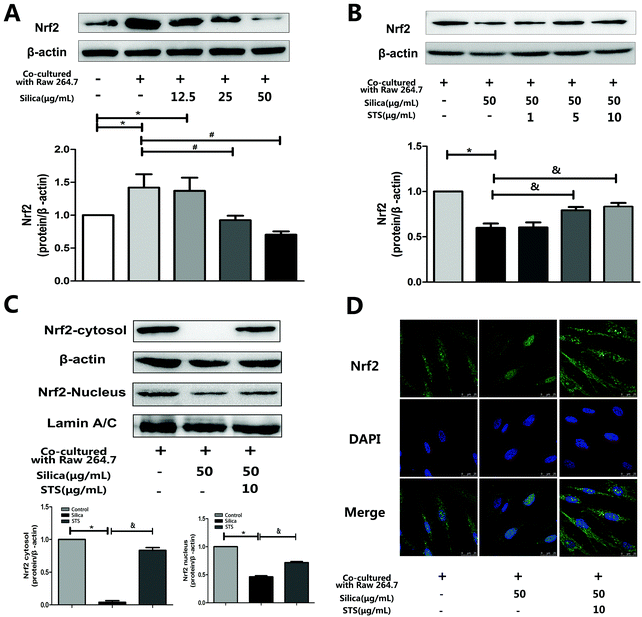
To investigate whether STS could affect Nrf2 expression in MRC-5 cells, the coculture system was treated with the indicated concentration of silica or STS. Following treatment for 24 h, the amount of Nrf2 protein was analyzed by Western blot and immunofluorescence in MRC-5 cells (Fig. 5). We first examined Nrf2 protein expression in whole MRC-5 cells, and the results showed that the Nrf2 expression was increased in the negative control group compared with that in the control group, whereas silica exposure caused a gradual decrease in Nrf2 expression (Fig. 5A). After STS treatment, the amount of Nrf2 protein in whole cells increased, especially at 10 μg mL−1 STS (p < 0.05) (Fig. 5B). Moreover, to further explore Nrf2 activation, the nuclear and cytoplasm levels of Nrf2 protein were determined (Fig. 5C). Silica exposure resulted in decreased levels of nuclear and cytoplasmic Nrf2 in MRC-5 cells. In addition, immunofluorescence showed that Nrf2 was barely expressed in the cytoplasm (Fig. 5D). However, the translocation of Nrf2 protein to the nucleus was significantly increased by STS treatment, as assessed using both Western blotting and immunofluorescence techniques, suggesting that STS treatment might contribute to nuclear Nrf2 protein up-regulation.3.5 Nrf2 knockdown inhibited TrxR and Trx gene expression in MRC-5 cells
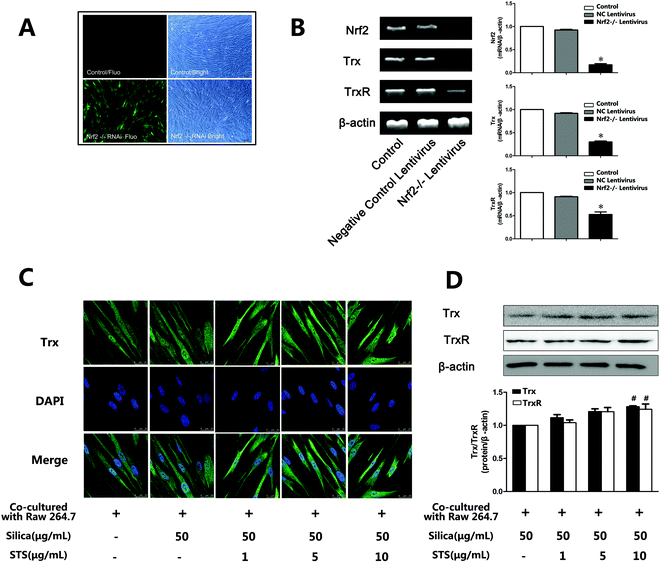
The Trx system plays a key role in antioxidative stress. To evaluate whether the transcription factor Nrf2 could regulate the expression of TrxR and Trx, Nrf2 was knocked down by lentivirus transfection. First, shRNA-Nrf2 or shRNA-control was transfected into MRC-5 cells. After 72 h of transfection, significantly increased expression of GFP was observed in the shRNA-Nrf2 cells using an inverted fluorescence microscope (Fig. 6A). Next, Nrf2 mRNA expression was detected by RT-PCR. Compared with the shRNA-control, transfection with shRNA-Nrf2 significantly ablated Nrf2 mRNA expression at 72 h post-transfection (Fig. 6B). These results indicate that transfection with shRNA-Nrf2 was effective in MRC-5 cells. To elucidate the downstream effectors of Nrf2, we examined Trx and TrxR mRNA expression. We found that Nrf2 knockdown by shRNA significantly decreased Trx mRNA and TrxR mRNA expression compared with the siRNA-control. This may indicate that Nrf2 was an important transcription factor that could regulate the expression of Trx and TrxR.3.6 Effect of STS on Trx and TrxR expression in MRC-5 cells
According to the effect of STS on the induction of Nrf2, we wondered whether the Trx system was involved in the STS mechanism of action. As shown in Fig. 6C and D, STS treatment increased Trx and TrxR protein expression, as assessed by western blotting and/or immunofluorescence analysis.4. Discussion
Most silica particles are engulfed by macrophages in the process of entering the lung in vivo, and interstitial macrophages are activated after particle uptake to produce ROS.23 Therefore, this study used a macrophage/fibroblast coculture system to determine if the interaction of macrophages with silica in vitro could affect the underlying fibroblasts, as their activation plays a key role in the pathophysiologic process of silicosis. Fibroblasts, activated and differentiated into myofibroblasts and acquiring a pro-fibrosing phenotype, are defined by the expression of extracellular matrix proteins (collagen I and III) and cell proliferation.24 Our current study demonstrated for the first time that exposure to silica could significantly promote the proliferation and activation of MRC-5 cells in a coculture system. The results shown in Fig. 2A demonstrated that the viability of MRC-5 cells coculture with Raw 264.7 cells was increased compared with the quiescent monocultured MRC-5 cells. By contrast, macrophage/fibroblast coculture with silica treatment showed significantly increased proliferation compared with the negative control group. Additionally, collagen I and III expression was significantly increased in the silica treatment group compared with the negative control group (Fig. 3A). These results indicated that the coculture model could partially simulate the complex in vivo environment, enabling the exploration of silica pathogenesis mediated by the stimulation of fibrogenesis.The abnormal proliferation and activation of MRC-5 cells by silica could therefore be partially attributed to the increased intracellular ROS levels. First, a growing body of evidence indicates that ROS may act as a fundamental signaling molecule in many cellular processes. Previous reports have indicated that low concentrations of H2O2 (∼10 nM–10 μM) are effective at stimulating the growth of mammalian fibroblasts in a cell culture system.25,26 It is now clear that H2O2 can stimulate growth responses in a variety of mammalian cell types when added exogenously to the medium. In the present study, 10−5 M H2O2 (Fig. 2B) was needed to promote maximal proliferation of MRC-5 cells. Additionally, mice deficient for ROS production through the NADPH-oxidase pathway do not develop pulmonary fibrosis after the intranasal administration of bleomycin, suggesting that NADPH-oxidase-derived ROS are essential for the development of pulmonary fibrosis.27 These data are in agreement with previous publications indicating that there is a significant decrease in erythrocyte GSH, GPx and SOD as well as a significant increase in the MDA level in silicotic patients, suggesting oxidative stress status.28 We have previously observed that both ROS and MDA levels are markedly increased during the early stage of silica exposure in rat lung.15 Second, a recent publication also confirmed that ROS increase active TGF-β1 release, which works as a fibrotic factor and directly induces the activation of human lung fibroblasts.29 Moreover, superoxide production is markedly enhanced in vascular smooth muscle cells after TGF-β1 stimulation,30 suggesting a positive feedback loop in TGF-β1 activation by ROS. We found that the concentration of TGF-β1 in Raw 264.7 supernatants gradually increased with the silica-concentration-dependent exposure (data not shown). In this study, the results show that indirect silica exposure of fibroblasts moderately increased the generation of ROS in a dose-dependent manner and that this was accompanied by fibroblast proliferation and activation. Collectively, these data suggest that blocking fibroblast activation by scavenging excessive ROS could be a possible therapeutic approach to modulate the fibrogenic response.
Numerous reports have shown that Tanshinone IIA has antioxidant properties and that it can alter the expression and/or activity of specific anti-oxidant enzymes to protect cells from oxidant damage.31 It has been reported that STS can markedly modulate the redox-sensitive steps to attenuate angiotensin II-induced collagen I expression in cardiac fibroblasts in vitro.20 In this study, we explored the potential protective mechanism of STS, which was used to treat MRC-5 cells. The results showed that both the proliferation and activation of MRC-5 fibroblasts induced by silica were reversed by STS treatment and that STS eliminated the excessive ROS production. These findings suggest that the effect elicited by STS might be due to its potent antioxidant property and its ability to mitigate fibrosis. Although the protective effects of STS against fibrosis have been widely investigated, the molecular mechanisms remain elusive, especially because the direct target of STS is unknown. The salient findings of this study are summarized as follows: (1) STS markedly decreased ROS generation via the Nrf2/Trx pathway to exert its antioxidant function; (2) STS may increase the myofibroblastic dedifferentiation associated with Nrf2 activation. These observations shed new light on the potential anti-fibrotic properties of STS in silicosis.
First, the oxidant/antioxidant imbalance in pulmonary fibroblasts was associated with decreased nuclear Nrf2 expression. Nrf2, a transcription factor, directly regulates pathways that control ROS homeostasis and antioxidant defense by modulating the transcription of critical genes to influence the onset and severity of pulmonary fibrosis. In a murine model of bleomycin-induced pulmonary fibrosis, researchers have shown increased sensitivity to bleomycin and more severe inflammatory lesions in Nrf2-knockout mice.32 In the present study, silica treatment gradually attenuated the induction of total Nrf2 in cocultured MRC-5 cells with increasing doses of silica (Fig. 5), accompanied by increasing ROS levels. Our results are in agreement with recent evidence showing decreased total Nrf2 expression in fibroblastic foci in idiopathic pulmonary fibrosis (IPF) lung biopsies.33 Moreover, the down-regulation of Nrf2 was confirmed in our previous results in a rat silicosis model, which showed that Nrf2 was absent in rat lungs on day 30 after silica inhalation.15 These results suggest that low Nrf2 expression could be the reason underlying redox balance disorders. Previous studies have provided evidence indicating that the Nrf2 transcription factor binds to the ARE in the Trx and TrxR promoters in response to oxidative stress stimuli, leading to the induction of Trx and TrxR expression.34–36 We further found that Nrf2 knockdown by shRNA significantly decreased Trx mRNA expression and TrxR mRNA expression compared with the siRNA-control (Fig. 6B). Additionally, we have previously reported that silica can induce ROS accumulation and some oxidative cellular damage by down-regulating both the mRNA and protein expression of Trx and TrxR in vitro.37 These data are in agreement with data showing that ROS are counterbalanced by a web of antioxidant systems in the body and that the Nrf2/Trx pathway might play a key role in ROS homeostasis. However, STS treatment could significantly up-regulate the intracellular expression of Nrf2 not only in the cytoplasm but also in the nucleus (Fig. 5C and D). Furthermore, it could induce the nuclear translocation of Nrf2 and subsequently increase the protein expression of Trx and TrxR (Fig. 6C and D), which consequently enhanced the resistance to oxidative stress induced by silica. Hence, the role of STS we observed was that of facilitating Nrf2 translocation to the nucleus, where Nrf2 played a pivotal role in the transcriptional activation of Trx and TrxR, which mitigated the oxidative stress induced by silica.
Except for a clear antioxidative role in respiratory diseases, Nrf2 has been found to be involved in cellular differentiation, including the myofibroblastic differentiation of fibroblasts during IPF.38 In that study, Nrf2 knockdown induced oxidative stress and myofibroblastic differentiation in control fibroblasts. Conversely, Nrf2 activation increased antioxidant defenses and myofibroblastic dedifferentiation in IPF fibroblasts. Furthermore, Nrf2 has been found to down-regulate the collagen I mRNA level in mice.39 In agreement with this, we found that the Nrf2 down-regulation induced by silica was associated with the up-regulation of collagen I/III expression. However, STS treatment down-regulated the collagen I/III expression accompanied by Nrf2 activation. Further research is needed to explore the exact mechanism underlying the effect on STS-induced Nrf2 activation.
5. Conclusions
Our results suggest that STS likely inhibited silica-induced MRC-5 proliferation and activation by inducing Nrf2 expression and up-regulation of the oxidative response genes Trx and TrxR. This finding may form the basis of a new strategy for treating silicosis.Conflicts of interest
The authors confirm that there are no conflicts of interest.Acknowledgements
This work was supported by grants from the Natural Science Foundation of Beijing (no. 7112015) and the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20111107110016).References
- L. A. Cox Jr., An exposure-response threshold for lung diseases and lung cancer caused by crystalline silica, Risk Anal., 2011, 31(10), 1543–1560 CrossRef PubMed.
- R. M. Gilberti, G. N. Joshi and D. A. Knecht, The phagocytosis of crystalline silica particles by macrophages, Am. J. Respir. Cell Mol. Biol., 2008, 39(5), 619–627 CrossRef CAS PubMed.
- H. M. Shen, Z. Zhang, Q. F. Zhang and C. N. Ong, Reactive oxygen species and caspase activation mediate silica-induced apoptosis in alveolar macrophages, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2001, 280(1), L10–LL7 CAS.
- M. Sundaresan, Z. X. Yu, V. J. Ferrans, D. J. Sulciner, J. S. Gutkind and K. Irani, et al., Regulation of reactive-oxygen-species generation in fibroblasts by Rac1, Biochem. J., 1996, 318(Pt 2), 379–382 CrossRef CAS PubMed.
- H. I. Krieger-Brauer and H. Kather, Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells, Biochem. J., 1995, 307(Pt 2), 549–556 CrossRef CAS PubMed.
- M. Lopes-Pacheco, T. G. Ventura, H. D. de Oliveira, L. C. Moncao-Ribeiro, B. Gutfilen and S. A. de Souza, et al., Infusion of bone marrow mononuclear cells reduces lung fibrosis but not inflammation in the late stages of murine silicosis, PLoS One, 2014, 9(10), e109982 Search PubMed.
- X. L. Shi, Y. Mao, U. Saffiotti, L. Y. Wang, Y. Rojanasakul and S. S. Leonard, et al., Antioxidant Activity Of Tetrandrine And Its Inhibition Of Quartz-Induced Lipid-Peroxidation, J. Toxicol. Environ. Health, 1995, 46(2), 233–248 CrossRef CAS PubMed.
- X. L. Shi, D. C. Flynn, D. W. Porter, S. S. Leonard, V. Valllyathan and V. Castranova, Efficacy of taurine based compounds as hydroxyl radical scavengers in silica induced peroxidation, Ann. Clin. Lab. Sci., 1997, 27(5), 365–374 CAS.
- H. Motohashi and M. Yamamoto, Nrf2-Keap1 defines a physiologically important stress response mechanism, Trends Mol. Med., 2004, 10(11), 549–557 CrossRef CAS PubMed.
- H. J. Hawkes, T. C. Karlenius and K. F. Tonissen, Regulation of the human thioredoxin gene promoter and its key substrates: a study of functional and putative regulatory elements, Biochim. Biophys. Acta, 2014, 1840(1), 303–314 CrossRef CAS PubMed.
- H. Y. Cho, A. E. Jedlicka, S. P. Reddy, T. W. Kensler, M. Yamamoto and L. Y. Zhang, et al., Role of NRF2 in protection against hyperoxic lung injury in mice, Am. J. Respir. Cell Mol. Biol., 2002, 26(2), 175–182 CrossRef CAS PubMed.
- S. Papaiahgari, Q. Zhang, S. R. Kleeberger, H. Y. Cho and S. P. Reddy, Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells, Antioxid. Redox Signaling, 2006, 8(1–2), 43–52 CrossRef CAS PubMed.
- N. Kondo, H. Nakamura, H. Masutani and J. Yodoi, Redox regulation of human thioredoxin network, Antioxid. Redox Signaling, 2006, 8(9–10), 1881–1890 CrossRef CAS PubMed.
- E. S. J. Arner and A. Holmgren, Physiological functions of thioredoxin and thioredoxin reductase, Eur. J. Biochem., 2000, 267(20), 6102–6109 CrossRef CAS PubMed.
- Z. Zhu, G. Yang, Y. Wang, J. Yang, A. Gao and P. Niu, et al., Suppression of thioredoxin system contributes to silica-induced oxidative stress and pulmonary fibrogenesis in rats, Toxicol. Lett., 2013, 222(3), 289–294 CrossRef CAS PubMed.
- D. M. Walters, H. Y. Cho and S. R. Kleeberger, Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2, Antioxid. Redox Signaling, 2008, 10(2), 321–332 CrossRef CAS PubMed.
- T. W. Kensler, N. Wakabayashi and S. Biswal, Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway, Annu. Rev. Pharmacol. Toxicol., 2007, 47, 89–116 CrossRef CAS PubMed.
- Q. Ma and X. He, Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2, Pharmacol. Rev., 2012, 64(4), 1055–1081 CrossRef CAS PubMed.
- P. Talalay, A. T. Dinkova-Kostova and W. D. Holtzclaw, Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis, Adv. Enzyme Regul., 2003, 43, 121–134 CrossRef CAS PubMed.
- L. Yang, X. J. Zou, X. Gao, H. Chen, J. L. Luo and Z. H. Wang, et al., Sodium tanshinone IIA sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro, Exp. Mol. Med., 2009, 41(7), 508–516 CrossRef CAS PubMed.
- J. Kasper, M. I. Hermanns, C. Bantz, M. Maskos, R. Stauber and C. Pohl, et al., Inflammatory and cytotoxic responses of an alveolar-capillary coculture model to silica nanoparticles: comparison with conventional monocultures, Part. Fibre Toxicol., 2011, 8(1), 6 CrossRef CAS PubMed.
- B. Rothen-Rutishauser, F. Blank, C. Muhlfeld and P. Gehr, In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter, Expert Opin. Drug Metab. Toxicol., 2008, 4(8), 1075–1089 CrossRef CAS PubMed.
- R. F. Hamilton Jr., S. A. Thakur and A. Holian, Silica binding and toxicity in alveolar macrophages, Free Radicals Biol. Med., 2008, 44(7), 1246–1258 CrossRef PubMed.
- S. H. Phan, Genesis of the myofibroblast in lung injury and fibrosis, Proc. Am. Thorac. Soc., 2012, 9(3), 148–152 CrossRef CAS PubMed.
- B. Y. Kim, M. J. Han and A. S. Chung, Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells, Free Radicals Biol. Med., 2001, 30(6), 686–698 CrossRef CAS PubMed.
- G. A. Murrell, M. J. Francis and L. Bromley, Modulation of fibroblast proliferation by oxygen free radicals, Biochem. J., 1990, 265(3), 659–665 CrossRef CAS PubMed.
- B. Manoury, S. Nenan, O. Leclerc, I. Guenon, E. Boichot and J. M. Planquois, et al., The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis, Respir. Res., 2005, 6, 11 CrossRef PubMed.
- A. Orman, A. Kahraman, H. Cakar, H. Ellidokuz and M. Serteser, Plasma malondialdehyde and erythrocyte glutathione levels in workers with cement dust-exposure [corrected], Toxicology, 2005, 207(1), 15–20 CrossRef CAS PubMed.
- S. Qi, G. J. den Hartog and A. Bast, Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels, Toxicol. Appl. Pharmacol., 2009, 237(1), 111–118 CrossRef CAS PubMed.
- A. T. Churchman, A. A. Anwar, F. Y. Li, H. Sato, T. Ishii and G. E. Mann, et al., Transforming growth factor-beta1 elicits Nrf2-mediated antioxidant responses in aortic smooth muscle cells, J. Cell. Mol. Med., 2009, 13(8B), 2282–2292 CrossRef PubMed.
- Y. I. Li, G. Elmer and R. C. Leboeuf, Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase, Life Sci., 2008, 83(15–16), 557–562 CrossRef CAS PubMed.
- H. Y. Cho, S. P. Reddy, M. Yamamoto and S. R. Kleeberger, The transcription factor NRF2 protects against pulmonary fibrosis, FASEB J., 2004, 18(11), 1258–1260 CAS.
- W. Mazur, P. Lindholm, K. Vuorinen, M. Myllarniemi, K. Salmenkivi and V. L. Kinnula, Cell-specific elevation of NRF2 and sulfiredoxin-1 as markers of oxidative stress in the lungs of idiopathic pulmonary fibrosis and non-specific interstitial pneumonia, Acta Pathologica, Microbiologica et Immunologica Scandinavica, 2010, 118(9), 703–712 CrossRef CAS PubMed.
- B. N. Chorley, M. R. Campbell, X. T. Wang, M. Karaca, D. Sambandan and F. Bangura, et al., Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha, Nucleic Acids Res., 2012, 40(15), 7416–7429 CrossRef CAS PubMed.
- Y. C. Kim, Y. Yamaguchi, N. Kondo, H. Masutani and J. Yodoi, Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response, Oncogene, 2003, 22(12), 1860–1865 CrossRef CAS PubMed.
- A. Sakurai, M. Nishimoto, S. Himeno, N. Imura, M. Tsujimoto and M. Kunimoto, et al., Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: Role of NF-E2-related factor-2, J. Cell. Physiol., 2005, 203(3), 529–537 CrossRef CAS PubMed.
- W. Guo, J. B. Cao, Z. H. Zhu and L. Tian, Effect of expression of TrxR and Trx on human embryo lung fibroblast cell induced by quartz, J. Pract. Med., 2012, 28, 2315–2317 CAS.
- E. Artaud-Macari, D. Goven, S. Brayer, A. Hamimi, V. Besnard and J. Marchal-Somme, et al., Nuclear Factor Erythroid 2-Related Factor 2 Nuclear Translocation Induces Myofibroblastic Dedifferentiation in Idiopathic Pulmonary Fibrosis, Antioxid. Redox Signaling, 2013, 18(1), 66–79 CrossRef CAS PubMed.
- D. Malhotra, E. Portales-Casamar, A. Singh, S. Srivastava, D. Arenillas and C. Happel, et al., Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis, Nucleic Acids Res., 2010, 38(17), 5718–5734 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |