Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure and luminescence properties of Eu2+ doped LuxSr2−xSiNxO4−x phosphors evolved from chemical unit cosubstitution
Zhiguo
Xia
*a,
Shihai
Miao
b,
Maxim S.
Molokeev
 cd,
Mingyue
Chen
a and
Quanlin
Liu
a
cd,
Mingyue
Chen
a and
Quanlin
Liu
a
aThe Beijing Municipal Key Laboratory of New Energy Materials and Technologies, School of Materials Sciences and Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: xiazg@ustb.edu.cn; Fax: +86-10-8237-7955; Tel: +86-10-8237-7955
bSchool of Materials Sciences and Technology, China University of Geosciences, Beijing 100083, China
cLaboratory of Crystal Physics, Kirensky Institute of Physics, SB RAS, Krasnoyarsk 660036, Russia
dDepartment of Physics, Far Eastern State Transport University, Khabarovsk, 680021, Russia
First published on 12th January 2016
Abstract
The design scheme of the chemical unit cosubstitution of [Lu3+–N3−] for [Sr2+–O2−] in Sr2SiO4:Eu2+ has been put into practice to discover the new phosphor systems with tunable luminescence properties, and the structures and photoluminescence tuning of yellow-emitting LuxSr2−xSiNxO4−x:Eu2+ phosphors have been investigated. Crystal structures of LuxSr2−x−ySiNxO4−x:yEu2+ samples were resolved using the Rietveld method, suggesting that the as-prepared Sr2SiO4 belonged to monoclinic symmetry (P21/n) of β-phase Sr2SiO4, while Sr1.97Eu0.03SiO4 and Sr1.965Eu0.03Lu0.005SiO3.995N0.005 belonged to orthorhombic symmetry (Pnma) of α-Sr2SiO4. The emission peaks of LuxSr1.97−xSiNxO4−x:0.03Eu2+ phosphors were red-shifted from 563 to 583 nm upon increasing the [Lu3+–N3−] substitution content from x = 0 to x = 0.005, furthermore, the PL emission peaks of Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ also showed a red-shift from 583 nm to 595 nm with increasing Eu2+ concentration (y = 0.03, 0.07, 0.10 and 0.15), and their corresponding red-shift mechanism has been discussed. The temperature dependent luminescence results further verified that the introduction of [Lu3+–N3−] for [Sr2+–O2−] in Sr2SiO4:Eu2+ can improve the thermal stability of the photoluminescence, which indicated that the LuxSr2−x−ySiNxO4−x:yEu2+ phosphors have potential applications in white light-emitting diodes (wLEDs).
1. Introduction
The discovery of new inorganic materials is always a hot issue in solid-state materials chemistry and is an important objective highlighted by the “Materials Genome Initiative”.1,2 To establish suitable strategies discovering new host materials for advanced optical materials, many conventional or modified approaches have been developed, such as cation/anion replacement in the single site of the inorganic phases,3,4 prototype substitution from an original “old” phase to find out a “new” one,5,6 combinatorial chemistry screening via the phase diagram,7,8 the single-particle-diagnosis approach along with the advanced measurement devices,9 and our recently reported chemical unit cosubstitution strategy for photoluminescence tuning,10 and so on. These strategies used for the discovery of the new phosphors demonstrated great success, and many important phosphors have been reported and used finally. Nevertheless, increasingly demanded by newly developed optical materials and devices, phosphors with tunable optical properties are continuously pursued by chemists or material scientists.Recently, the exploration of new materials for white light-emitting diodes (wLEDs) has become a hot issue.11,12 Among them, silicate phosphors represented by Eu2+ or Ce3+ doped orthosilicates A2SiO4 (A = Ca, Sr, Ba) have drawn much attention owing to their versatile polymorphs and chemical compositions, broad excitation/emission bands and tunable optical properties.13,14 As we know, crystal site engineering can be used to tune the luminescence properties by changing the coordination environment for phosphors employing Ce3+ or Eu2+ ions characterized by d–f transitions. Therefore, the nitridation of the orthosilicate phosphors has demonstrated great potential.15–23 For example, Sohn reported the Sr2SiO4−xN2x/3:Eu2+ phosphors.15 Gu reported the N doped Sr2SiO4:Eu2+ phosphors.16 Lee reported the (Sr,M)2Si(O1−xNx)4:Eu2+ (M: Mg2+, Ca2+, Ba2+) phosphors.17 Zhao found the red-emitting oxonitridosilicate phosphors Sr2SiNzO4−1.5z:Eu2+.18 Park reported the effects of N3−, Eu2+, and Ca2+ substitutions on the structural and luminescence properties of Sr2SiO4:Eu2+.19 Ju reported the modification of the coordination environment of Eu2+ in Sr2SiO4:Eu2+ phosphors to achieve full color emission.20 Li reported the luminescence properties and the crystal structure of α-Sr2Si3x/4O2Nx:Eu2+ phosphors with different concentrations of N3− ions.21 Tian reported the optical spectrum adjustment of yellow-green Sr1.99SiO4−3x/2Nx:0.01Eu2+ phosphor.22
Recently, Black found the new LaSrSiO3N and LaBaSiO3N phases, and these compounds activated with Eu2+ showed orange-red emission.23 Such an example is in accord with our previously reported chemical unit cosubstitution strategy,10 so that the [La3+–N3−] chemical unit can be used to cosubstitute for the [Sr2+/Ba2+–O2−] couple in the (Sr,Ba)2SiO4:Eu2+ phosphors. This is also the main topic highlighted in the present paper, and it is believed that such a strategy can be successfully used to discover the new phosphor materials and tune the luminescence properties. Therefore, the usage and verification of the design scheme of chemical unit cosubstitution will be significant in discovering other new solid state materials. Herein, the chemical unit cosubstitution of [Lu3+–N3−] for [Sr2+–O2−] in Sr2SiO4:Eu2+ has been proposed in this paper, and the yellow-emitting LuxSr2−xSiNxO4−x:Eu2+ phosphors with tunable photoluminescence have been studied. As also mentioned above, the nitridation of the orthosilicate phosphors brings us some opportunities to search for new phosphors. In this work, we studied the phase structure evolution and luminescence properties of yellow-emitting LuxSr2−x−ySiNxO4−x:yEu2+ phosphors, and the observed spectral red-shift with increasing [Lu3+–N3−] and Eu2+ content has been discussed in detail.
2. Experimental
The designed LuxSr1.97−x−ySiNxO4−x:yEu2+ phosphors were synthesized by a conventional high temperature solid-state reaction. The starting materials were as follows: SrCO3 (A.R.), SiO2 (A.R.), Lu2O3 (A.R.), Si3N4 (99.99%), and Eu2O3 (99.99%). After mixing and grinding in an agate mortar for 20 min, the mixture was placed in a crucible and then sintered at 1500 °C for 4 h in a H2(10%)/N2(90%) reducing atmosphere to produce the final samples. Finally, the prepared phosphors were cooled to room temperature and reground for further measurements.The powder X-ray diffraction (XRD) measurements were performed on a D8 Advance diffractometer (Bruker Corporation, Germany) operating at 40 kV and 40 mA with Cu Kα radiation (λ = 1.5406 Å). The scanning rate for phase identification was fixed at 4° min−1 with 2θ ranges from 10° to 70° and the data for Rietveld analysis were collected in a step-scanning mode with the step size of 0.02° and 10 s counting time per step over the 2θ range from 10° to 120°. The morphology and chemical compositions have been detected by using the scanning electron microscopy (SEM, JEOL JSM-6510A) and the attached energy disperse spectroscopy (EDS) techniques. The PL and photoluminescence excitation (PLE) spectra were recorded using a Hitachi F-4600 spectrophotometer (HITACHI, Tokyo, Japan) equipped with a 150 W xenon lamp as the excitation source. The temperature-dependent luminescence properties were also measured on the same spectrophotometer, and it was combined with a self-made heating attachment and a computer-controlled electric furnace (Tianjin Orient KOJI Co., Ltd, TAP-02). The decay curves were recorded on an instrument (Edinburgh Instruments Ltd, UK) (FLSP920) with an F900 flash lamp as the excitation source.
3. Results and discussion
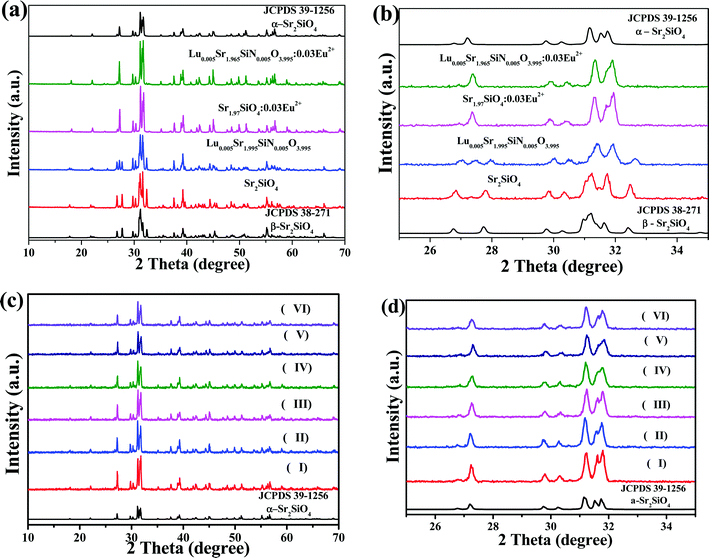
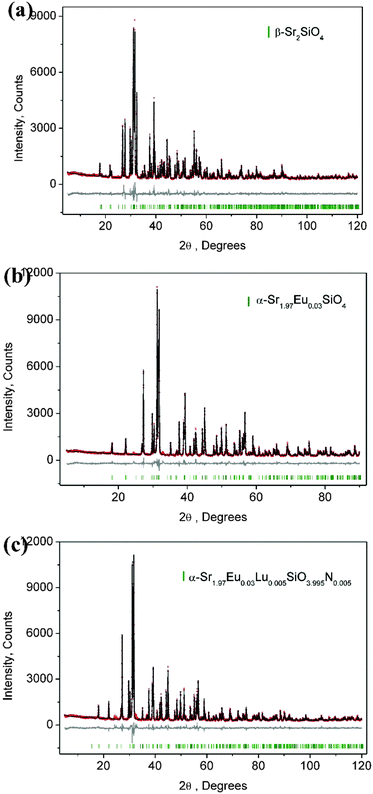
The full range XRD patterns and the magnified range between 25° and 35° of as-prepared Sr2SiO4, Lu0.005Sr1.995SiN0.005O3.995, Sr1.97SiO4:0.03Eu2+ and Lu0.005Sr1.965Si N0.005O3.995:0.03Eu2+ samples are given in Fig. 1a and b, respectively. The standard data for α-Sr2SiO4 (JCPDS 39-1256) and β-Sr2SiO4 (JCPDS 38-271) are also shown as a reference. From Fig. 1a and b, we can see that the as-obtained Sr2SiO4 and Lu0.005Sr1.995SiN0.005O3.995 samples can be indexed to the β-Sr2SiO4 phase (JCPDS 38-271) suggesting that the introduction of the [Lu3+–N3−] chemical unit in a small amount (x = 0.005) will not affect the phase transition. Moreover, we can find that diffraction peaks of the as-prepared Lu0.005Sr1.995SiN0.005O3.995 sample shift to the high-angle direction compared to that of the as-obtained Sr2SiO4 phase, which verified that the Lu–N dopant can enter the structural framework of the β-Sr2SiO4 phase leading to the shrinkage of the unit cell. However, the impurities appear when the contents of the [Lu3+–N3−] chemical unit exceed x = 0.005 from our experimental results. So that we select the content of x = 0.005 for the following discussion. Furthermore, Fig. 1c and d displays the full range XRD patterns and the magnified range between 25° and 35° for the as-prepared Lu0.005Sr1.995−ySiN0.005O3.995:yEu2+ (y = 0.03, 0.05, 0.07, 0.10, 0.15 and 0.20) phosphors. The standard data for α-Sr2SiO4 (JCPDS 39-1256) are shown as a reference. The six samples exhibit the same diffraction peaks, which can be indexed to the α-Sr2SiO4 phase (JCPDS 39-1256), and no other polymorphs, or other impurities were detected. It is believed that the introduction of Eu2+ on the Sr2+ sites will induce the phase transition from the β-Sr2SiO4 phase to the α-Sr2SiO4 phase, so that Eu2+ will further stabilize the α-Sr2SiO4 phase, which agree with the previous report.24In order to further compare the difference of the phase structure evolution depending on the introduction of the Eu2+ and [Lu3+–N3−] chemical unit, the detailed Rietveld structure analysis of the selected Sr2SiO4, Sr1.97Eu0.03SiO4 and Sr1.965Eu0.03Lu0.005SiO3.995N0.005 samples has been performed. Fig. 2 displays the difference Rietveld plots of Sr2SiO4, Sr1.97Eu0.03SiO4 and Sr1.965Eu0.03Lu0.005SiO3.995N0.005. It is found that all the peaks of the as-prepared Sr2SiO4 compound were indexed by a monoclinic unit cell (P21/n) with parameters close to β-phase Sr2SiO4. In contrast, all the peaks of Sr1.97Eu0.03SiO4 and Sr1.965Eu0.03Lu0.005SiO3.995N0.005 are well fitted by orthorhombic cell (Pnma) corresponding to α-phase Sr2SiO4. During the Rietveld structural analysis, sites of Sr and O atoms were occupied by Eu/Lu and N atoms with fixed occupations, respectively, according to suggested chemical formula. Refinement was stable and gives low R-factors as shown in Table 1. Moreover, the coordinates of atoms are shown in Table 2, which verified the proposed chemical unit cosubstitution model.
| Compound | Sr2SiO4 | Sr1.97Eu0.03SiO4 | Sr1.965Eu0.03Lu0.005SiO3.995N0.005 |
|---|---|---|---|
| Sp. gr. | P21/n | Pnma | Pnma |
| a, Å | 5.66012(9) | 7.07515(9) | 7.07370(7) |
| b, Å | 7.0808(1) | 5.66437(8) | 5.66778(6) |
| c, Å | 9.7547(2) | 9.7314(1) | 9.7363(1) |
| β, ° | 92.594(8) | — | — |
| V, Å3 | 390.55(1) | 389.998(9) | 390.348(7) |
| Z | 4 | 4 | 4 |
| 2θ-interval, ° | 5–120 | 5–90 | 5–120 |
| No. of reflections | 584 | 183 | 326 |
| No. of refined parameters | 54 | 56 | 59 |
| R wp, % | 6.76 | 6.10 | 7.14 |
| R p, % | 4.94 | 4.59 | 5.32 |
| R exp, % | 4.38 | 4.27 | 4.44 |
| χ 2 | 1.54 | 1.43 | 1.61 |
| R B, % | 2.34 | 1.88 | 2.09 |
| x | y | z | B iso | Occ. | |
|---|---|---|---|---|---|
| Sr2SiO4 | |||||
| Sr1 | 0.2624 (3) | 0.3429 (2) | 0.5783 (2) | 1.1 (2) | 1 |
| Sr2 | 0.2699 (3) | 0.0009 (2) | 0.3021 (2) | 1.1 (2) | 1 |
| Si | 0.2459 (9) | 0.7783 (5) | 0.5819 (6) | 1.0 (2) | 1 |
| O1 | 0.273 (2) | 0.005 (1) | 0.5739 (8) | 1.5 (2) | 1 |
| O2 | 0.196 (2) | 0.679 (2) | 0.430 (1) | 1.5 (2) | 1 |
| O3 | 0.492 (2) | 0.682 (2) | 0.646 (1) | 1.5 (2) | 1 |
| O4 | 0.017 (2) | 0.729 (1) | 0.673 (1) | 1.5 (2) | 1 |
| Sr1.97Eu0.03SiO4 | |||||
| Sr1 | 0.3409 (2) | 0.269 (2) | 0.5786 (2) | 1.4 (3) | 0.4925 |
| Eu1 | 0.3409 (2) | 0.269 (2) | 0.5786 (2) | 1.4 (3) | 0.0075 |
| Sr2 | 0.9989 (2) | 0.271 (1) | 0.3036 (1) | 0.9 (3) | 0.4925 |
| Eu2 | 0.9989 (2) | 0.271 (1) | 0.3036 (1) | 0.9 (3) | 0.0075 |
| Si | 0.7780 (6) | 0.25 | 0.5831 (7) | 1.5 (3) | 1 |
| O1 | 1.005 (1) | 0.300 (3) | 0.5657 (9) | 1.5 (3) | 0.5 |
| O2 | 0.732 (3) | 0.025 (4) | 0.674 (2) | 1.5 (3) | 0.5 |
| O3 | 0.670 (2) | 0.208 (3) | 0.433 (1) | 1.5 (3) | 0.5 |
| O4 | 0.689 (3) | 0.492 (4) | 0.647 (2) | 1.5 (3) | 0.5 |
| Sr1.965Eu0.03Lu0.005SiO3.995N0.005 | |||||
| Sr1 | 0.3403 (2) | 0.268 (1) | 0.5794 (2) | 1.2 (2) | 0.49125 |
| Eu1 | 0.3403 (2) | 0.268 (1) | 0.5794 (2) | 1.2 (2) | 0.0075 |
| Lu1 | 0.3403 (2) | 0.268 (1) | 0.5794 (2) | 1.2 (2) | 0.00125 |
| Sr2 | 0.9993 (2) | 0.230 (1) | 0.3023 (2) | 0.8 (2) | 0.49125 |
| Eu2 | 0.9993 (2) | 0.230 (1) | 0.3023 (2) | 0.8 (2) | 0.0075 |
| Lu2 | 0.9993 (2) | 0.230 (1) | 0.3023 (2) | 0.8 (2) | 0.00125 |
| Si | 0.7775 (6) | 0.25 | 0.5828 (7) | 1.2 (2) | 1 |
| O1 | 1.005 (1) | 0.303 (2) | 0.569 (1) | 1.5 (2) | 0.5 |
| O2 | 0.736 (3) | 0.024 (3) | 0.677 (2) | 1.5 (2) | 0.5 |
| O3 | 0.670 (2) | 0.207 (3) | 0.430 (1) | 1.5 (2) | 0.5 |
| O4 | 0.685 (3) | 0.493 (3) | 0.647 (2) | 1.5 (2) | 0.5 |
Therefore, the above results verified that the [Lu3+–N3−] chemical unit and the Eu2+ ions entered into the crystal lattice of Sr2SiO4 can maintain the same phase structures. In the present study, the chemical unit cosubstitution takes place at the cation and coordination anion's sites of the polyhedra simultaneously, not the central tetrahedral sites, which are different from our previous study.10 The substitution on such a site will have the direct effect on the crystal field environment of doped Eu2+, so that we can possibly find the tunable photoluminescence even if the content of the substituted [Lu3+–N3−] chemical unit is relatively low. In order to clearly demonstrate the proposed chemical unit cosubstitution, the possible atoms' transfer between Sr1.97Eu0.3SiO4 and Sr1.965Eu0.3Lu0.005SiO3.995N0.005 can be clearly seen in Fig. 3. Two ions Sr2+ and O2−, which together have zero sum of charge, are substituted by two ions Lu3+ and N3−, which also have zero sum of charge. The scheme can be presented as: Sr2+ + O2− → Lu3+ + N3−. From Fig. 3 we can know that the component replacement affects the lattice environment of the luminescence center, and further impacts the spectrum emission of phosphors.25,26 In order to demonstrate the chemical composition information of the typical samples, SEM-EDS analysis of the typical Lu0.005Sr1.965SiN0.005O3.995:0.03Eu2+ phosphor for a semi-quantitative measurement has been performed and demonstrated in Fig. 4. As shown in Fig. 4a, the sample has irregular morphology, and when we focused on one minor aggregate for the EDS analysis, we can find that all the elements can be detected, and the contents in the same order of magnitude are in agreement with the designed ones in the chemical formula. Since the doped contents of the Lu/N are very minor, it is difficult to be measured by using the chemical method, so that the present result can also support the successful substitution of the [Lu3+–N3−] couple for [Sr2+–O2−] in the Sr2SiO4:Eu2+.
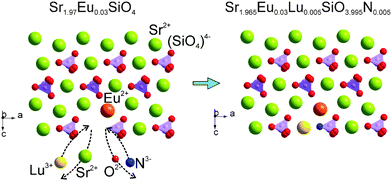 | ||
| Fig. 3 The proposed chemical unit cosubstitution strategy of [Lu3+–N3−] for [Sr2+–O2−] to highlight the possible atoms' transfer between Sr1.97Eu0.3SiO4 and Sr1.965Eu0.3Lu0.005SiO3.995N0.005. | ||
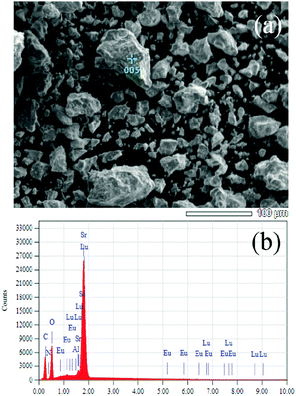 | ||
| Fig. 4 SEM image (a) and EDS (b) analysis of the selected sample of Sr1.965Eu0.3Lu0.005SiO3.995N0.005 to check the presence of existed elements. | ||
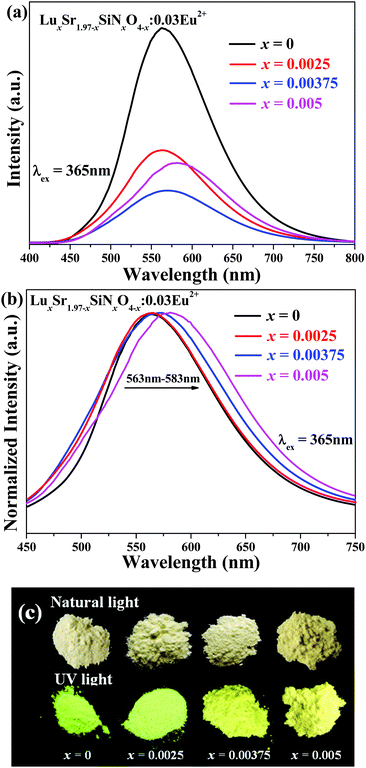
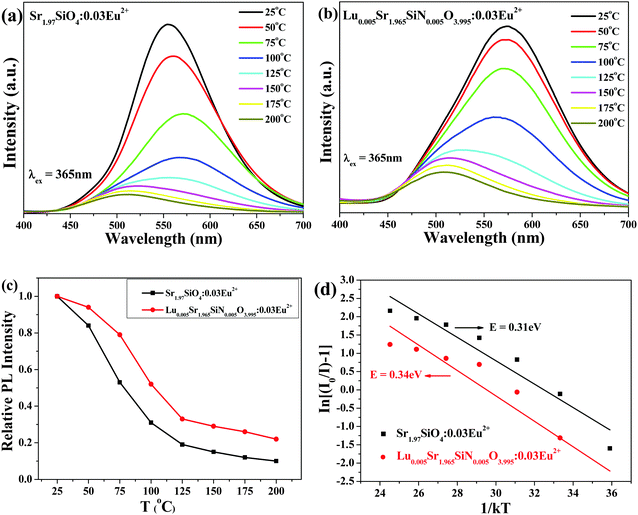
The as-measured and normalized PL spectra of LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.0025, 0.00375, 0.005) phosphors under 365 nm excitation are shown in Fig. 5a and b, respectively. The emission spectra consist of an asymmetric broad band centered at around 570 nm, which is ascribed to the electric dipole allowing the transition of the Eu2+ ions from the lowest level of the 5d excited state to the 4f ground state.27 As shown in Fig. 5b (with increasing) [Lu3+–N3−] content, the emission peaks give a red-shift from 563 nm to 583 nm. Fig. 5c comparatively gives the typical images of LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.0025, 0.00375 and 0.005) phosphors under a 365 nm UV lamp and natural light, respectively. We can clearly find the emission color evolution from yellow green to yellow with increasing [Lu3+–N3−] content for the cosubstitution of [Sr2+–O2−]. As we know, the N3− is less electronegative and more polarizable than O2− and its introduction in Sr1.97Eu0.03SiO4 increases the covalent character of the bonds with the Sr2+/Eu2+, and we can infer that some of the Eu2+ ions in the LuxSr1.97−xSiNxO4−x:0.03Eu2+ phosphor are coordinated with nitrogen, so that we can find the obvious red-shift behavior.
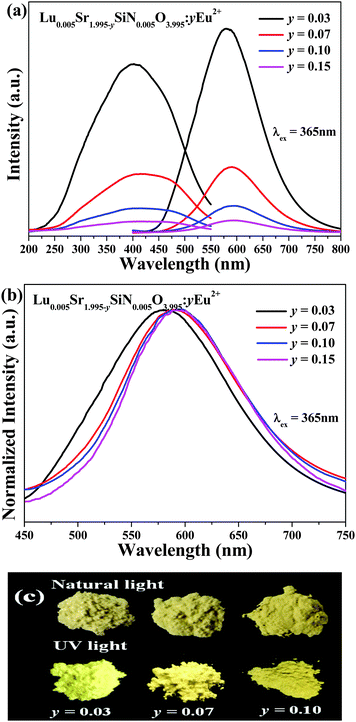
Fig. 6a shows the as-measured PL and PLE spectra of Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ (y = 0.03, 0.07, 0.10 and 0.15) phosphors, and the normalized PL spectra are shown in Fig. 6b. Broad emission bands were clearly observed in the 450–750 nm range for Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ phosphors. From Fig. 6b (we can clearly see that) (with increasing) Eu2+ concentration, the PL emission peaks show a red-shift from 583 nm to 595 nm. Fig. 6c also presents the images of Lu0.005Sr1.995−ySiN0.005O3.995:yEu2+ (y = 0.03, 0.07, 0.10 and 0.15) phosphors under a 365 nm UV lamp and natural light, respectively. We can see that the emission color evolves from light yellow to deep yellow with increasing Eu2+ doping content. The main reason for this red-shift is an increase of crystal field splitting, which in turn decreases the 5d–4f transition energy.28
The temperature dependence of the emission spectra and the relative PL intensity of Sr1.97SiO4:0.03Eu2+ and Lu0.005Sr1.965SiN0.005O3.995:0.03Eu2+ were comparatively investigated and are given in Fig. 7a and b. Furthermore, as shown in Fig. 7c, the emission intensity at 100 °C is about 30% of that measured at room temperature for the Sr1.97SiO4:0.03Eu2+ phosphor while this value is about 51% for Lu0.005Sr1.965SiN0.005O3.995:0.03Eu2+ phosphor. The improved thermal stability depending on the introduced [Lu3+–N3−] chemical unit can be clearly found, which should be ascribed to the enhanced chemical bond effect with a rigid structure originating from the incorporated N atoms and the charge balance originating from such a cosubstitution. In order to better understand the temperature dependence of the luminescence, the activation energy ΔE can be calculated using the following equation:29
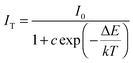 | (1) |
Decay curves of Eu2+ emission in LuxSr1.97−xSiNxO4−x:0.03Eu2+ and Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ phosphors under excitation at 365 nm, monitored at 563 nm, are shown in Fig. 8. As depicted in Fig. 8a and b, we can find that all the decay curves of Eu2+ emission in LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.00125, 0.0025, 0.00375 and 0.005) phosphors and Lu0.005Sr1.995−ySiN0.005O3.995:yEu2+ (x = 0.03, 0.05, 0.07, 0.10 and 0.15) phosphors demonstrate a second-order exponential decay and they can be fitted by this formula:30
I(t) = I0 + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2) | (2) |
| τ* = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (3) |
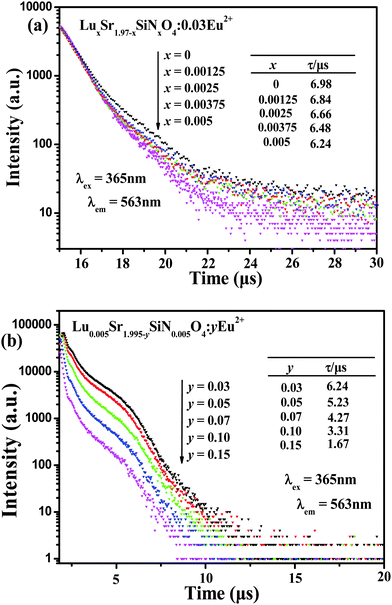 | ||
| Fig. 8 Decay curves of Eu2+ emission in LuxSr1.97−xSiNxO4−x:0.03Eu2+ (a), Lu0.005Sr1.995−ySiN0.005O3.995:yEu2+ (b) phosphors monitored at corresponding emission peaks under excitation at 365 nm. | ||
The calculated average decay lifetime values of Eu2+ ions in LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.00125, 0.0025, 0.00375, 0.005) are determined to be 6.98, 6.84, 6.66, 6.48 and 6.24 μs, respectively, depending on the different [Lu3+–N3−] contents. From Fig. 8b we can find that the lifetime values of Eu2+ ions are determined to be 6.24, 5.23, 4.27, 3.31 and 1.67 μs for the Lu0.005Sr1.965−ySiN0.005O3.995:yEu2+ phosphors with y = 0.03, 0.05, 0.07, 0.10 and 0.15, respectively. Obviously the decay time decreases gradually with increasing Eu2+ concentration. It is proposed that the nonradiative and self-absorption rate of the internal doped ions evidently increases when activators cross the critical separation between the acceptor (quenching site) and the donor (activator ion) with increasing Eu2+ concentration.31,32
Fig. 9 shows the CIE chromaticity diagram of the select LuxSr1.97−xSiNxO4−x:0.03Eu2+ and Lu0.005Sr1.895SiN0.005O3.995:0.10Eu2+ phosphors (λex = 365 nm). The CIE results are listed in Table 3. From Fig. 9, one can see that the emission colors of LuxSr2−x−ySiNxO4−x:yEu2+ phosphors can be shifted from yellowish green to deep yellow by the chemical unit cosubstitution of [Lu3+–N3−] and the increasing Eu2+ doping concentration compared to the initial phosphor Sr2SiO4:Eu2+. Accordingly, the corresponding CIE coordinates of LuxSr2−x−ySiNxO4−x:yEu2+ phosphors can be changed from (0.396, 0.537) to (0.482, 0.473). Also on the right side, we can clearly find the red shift trend. The internal quantum efficiency (QE) of the selected LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.005) phosphors were measured, and the internal QE values of Sr1.97SiO4:0.03Eu2+ and Lu0.005Sr1.965SiN0.005O3.995:0.03Eu2+ phosphors under 365 nm excitation are determined to be 81.8% and 24.7%, respectively. We can find that the QE values decreased sharply with the introduction of the [Lu3+–N3−] couple, however, it could be improved via controlling the particle size, size distribution, morphology and crystalline defects in the future work. Therefore, this series of LuxSr2−x−ySiNxO4−x:yEu2+ phosphors may be promising candidates as color tunable luminescence materials for application in wLEDs.
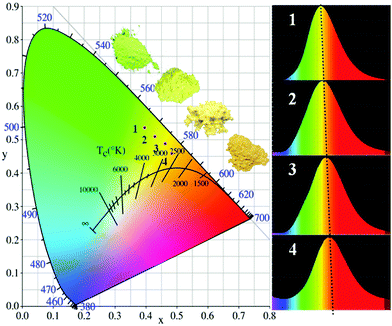 | ||
| Fig. 9 CIE chromaticity diagram of the select LuxSr1.97−xSiNxO4−x:0.03Eu2+ (x = 0, 0.0025, 0.005) and Lu0.005Sr1.895SiN0.005O3.995:0.10Eu2+ phosphors (λex = 365 nm). | ||
| Sample no. | CIE coordinates (x, y) |
|---|---|
| 1 | (0.396, 0.537) |
| 2 | (0.428, 0.501) |
| 3 | (0.462, 0.488) |
| 4 | (0.482, 0.473) |
4. Conclusions
The effects of [Lu3+–N3−] chemical unit cosubstitution on the phase structures and luminescence properties of LuxSr2−x−ySiNxO4−x:yEu2+ phosphors were investigated in this paper. The crystal chemistry analysis, also combining with the XRD patterns and PL spectral results, revealed that the [Lu3+–N3−] couple could be incorporated into the Sr2SiO4 host via cosubstitution with [Sr2+–O2−], which further affects the crystal field environment of doped Eu2+ and further tunes the photoluminescence of the as-obtained phosphor. The PL emission reveals a red-shift and the emission color evolution from yellow green to deep yellow with increasing [Lu3+–N3−] substitution content. Accordingly, the CIE coordinates of LuxSr2−x−ySiNxO4−x:yEu2+ phosphors can be changed from (0.396, 0.537) to (0.482, 0.473). The tunable luminescence properties and high thermal stability demonstrate that LuxSr2−x−ySiNxO4−x:yEu2+ phosphors can be applied as potential yellow phosphors for wLEDs. The demonstrated chemical unit cosubstitution strategy will also be significant in discovering new systems for advanced optical materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51572023 and 51272242), the Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-12-0950), Beijing Nova Program (Z131103000413047), the Funds of the State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, CAS (RERU2015022), and Fundamental Research Funds for the Central Universities (FRF-TP-15-003A2).References
- R. Potyrailo, K. Rajan, K. Stoewe, I. Takeuchi, B. Chisholm and H. Lam, ACS Comb. Sci., 2011, 13, 579–633 CrossRef CAS PubMed.
- J. J. Pablo, B. Jones, C. L. Kovacs, V. Ozolins and A. P. Ramirez, Curr. Opin. Solid State Mater. Sci., 2014, 18, 99–117 CrossRef.
- C. C. Wu, K. B. Chen, C. S. Lee, T. M. Chen and B. M. Cheng, Chem. Mater., 2007, 19, 3278–3285 CrossRef CAS.
- H. P. Ji, Z. H. Huang, Z. G. Xia, M. S. Molokeev, V. V. Atuchin, M. H. Fang and S. F. Huang, J. Phys. Chem. C, 2015, 119, 2038–2045 CAS.
- Z. G. Xia, Y. Y. Zhang, M. S. Molokeev, V. V. Atuchin and Y. Luo, Sci. Rep., 2013, 3, 3310–3317 Search PubMed.
- S. Schmiechen, P. Strobel, C. Hecht, T. Reith, M. Siegert, P. J. Schmidt, P. Huppertz, D. Wiechert and W. Schnick, Chem. Mater., 2015, 27, 1780–1785 CrossRef CAS.
- W. B. Park, N. Shin, K. P. Hong, M. Pyo and K. S. Sohn, Adv. Funct. Mater., 2012, 22, 2258–2266 CrossRef CAS.
- W. B. Park, S. P. Singh and K. S. Sohn, J. Am. Chem. Soc., 2014, 136, 2363–2373 CrossRef CAS PubMed.
- N. Hirosaki, T. Takeda, S. Funahashi and R. J. Xie, Chem. Mater., 2014, 26, 4280–4288 CrossRef CAS.
- Z. G. Xia, C. G. Ma, M. S. Molokeev, Q. L. Liu, K. Rickert and K. R. Poeppelmeier, J. Am. Chem. Soc., 2015, 137, 12494–12497 CrossRef CAS PubMed.
- M. M. Shang, C. X. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- X. J. Zhang, J. Wang, L. Huang, F. J. Pan, Y. Chen, B. F. Lei, M. Y. Peng and M. M. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 10044–10054 CAS.
- A. Kalaji, M. Mikami and A. K. Cheetham, Chem. Mater., 2014, 26, 3966–3975 CrossRef CAS.
- S. H. Miao, Z. G. Xia, M. S. Molokeev, M. Y. Chen, J. Zhang and Q. L. Liu, J. Mater. Chem. C, 2015, 3, 4616–4622 RSC.
- K. S. Sohn, J. H. Hwak, Y. S. Jung, H. X. Yan and M. J. Reece, J. Electrochem. Soc., 2008, 155, 58–61 CrossRef.
- Y. X. Gu, Q. H. Zhang, Y. G. Li and H. Z. Wang, J. Alloys Compd., 2011, 509, 109–112 CrossRef.
- S. J. Lee, S. H. Hong and Y. J. Kim, J. Electrochem. Soc., 2012, 159, 163–167 CrossRef.
- Z. Y. Zhao, Z. G. Yang, Y. R. Shi, C. Wang, B. T. Liu, G. Zhu and Y. H. Wang, J. Mater. Chem. C, 2013, 1, 1407–1412 RSC.
- J. Park, S. J. Lee and Y. J. Kim, Cryst. Growth Des., 2013, 13, 5204–5210 CAS.
- L. C. Ju, X. Xu, L. Y. Hao, Y. Lin and M. H. Lee, J. Mater. Chem. C, 2015, 3, 1567–1575 RSC.
- X. J. Li, Y. J. Hua, H. P. Ma, D. G. Deng, G. H. Jia and S. Q. Xu, RSC Adv., 2015, 5, 62659–62669 RSC.
- W. Y. Tian, K. X. Song, F. F. Zhang, P. Zhang, J. X. Deng, J. Jiang, J. M. Xu and H. B. Qin, J. Alloys Compd., 2015, 638, 249–253 CrossRef CAS.
- A. P. Black, K. A. Denault, J. Ora-Sole, A. R. Goni and A. Fuertes, Chem. Commun., 2015, 51, 2166–2169 RSC.
- Z. G. Xia, S. H. Miao, M. Y. Chen, M. S. Molokeev and Q. L. Liu, Inorg. Chem., 2015, 54, 7684–7691 CrossRef CAS PubMed.
- M. Catti, G. Gazzoni and G. Ivaldi, Acta Crystallogr., Sect. A: Found. Crystallogr., 1992, 38, 217–220 Search PubMed.
- M. Catti, G. Gazzoni, G. Ivaldi and G. Zanini, Acta Crystallogr., Sect. B: Struct. Sci., 1983, 39, 674–679 CrossRef.
- S. H. Miao, Z. G. Xia, J. Zhang and Q. L. Liu, Inorg. Chem., 2014, 53, 10386–10393 CrossRef CAS PubMed.
- S. S. Wang, W. T. Chen, Y. Li, J. Wang, H. S. Sheu and R. S. Liu, J. Am. Chem. Soc., 2013, 135, 12504–12507 CrossRef CAS PubMed.
- X. Chen, Z. G. Xia and Q. L. Liu, Dalton Trans., 2014, 43, 13370–13376 RSC.
- Z. G. Xia, X. M. Wang, Y. X. Wang, L. B. Liao and X. P. Jing, Inorg. Chem., 2011, 50, 10134–10142 CrossRef CAS PubMed.
- Y. F. Liu, X. Zhang, Z. D. Hao, Y. S. Luo, X. J. Wang and J. H. Zhang, J. Mater. Chem., 2011, 21, 16379–16384 RSC.
- M. M. Jiao, Y. C. Jia, W. Lv, W. Z. Lv, Q. Zhao, B. Q. Shao and H. P. You, J. Mater. Chem. C, 2014, 2, 90–97 RSC.
| This journal is © The Royal Society of Chemistry 2016 |