Construction of hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell nanowire arrays for high-performance supercapacitors†
Wenbin
Fu
a,
Yaling
Wang
a,
Weihua
Han
*a,
Zemin
Zhang
a,
Heming
Zha
b and
Erqing
Xie
 *a
*a
aSchool of Physical Science and Technology, Lanzhou University, Lanzhou, 730000, China. E-mail: hanwh@lzu.edu.cn; xieeq@lzu.edu.cn; Tel: +86 931 8912616
bCuiying Honors College, Lanzhou University, Lanzhou, 730000, China
First published on 11th November 2015
Abstract
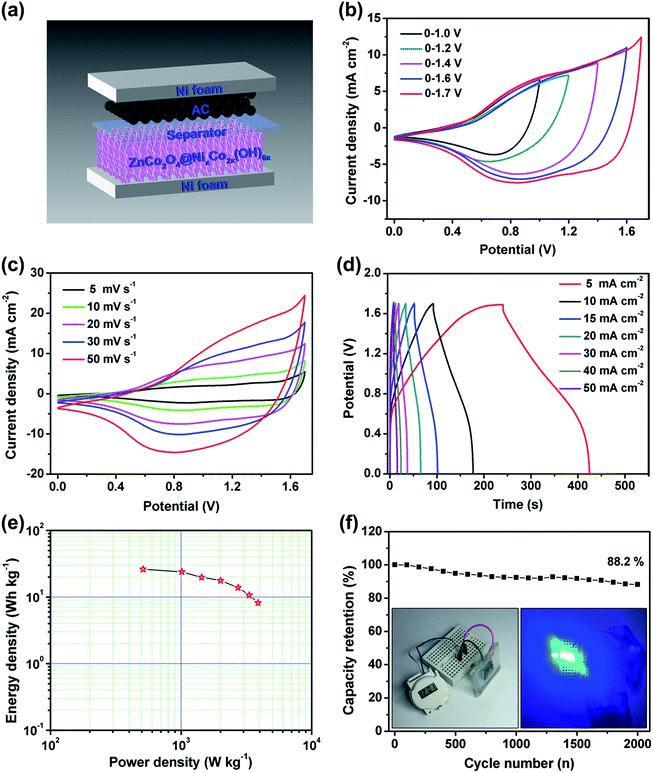
Rational design and synthesis of core/shell nanostructures as binder-free electrodes has been believed to be an effective strategy to improve the electrochemical performance of supercapacitors. In this work, hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell nanowire arrays (NWAs) have been successfully constructed by electrodepositing NixCo2x(OH)6x nanosheets onto hydrothermally grown ZnCo2O4 nanowires and investigated as a battery-type electrode for hybrid supercapacitors. Taking advantage of the hierarchical core/shell structures and the synergetic effect between ZnCo2O4 nanowires and NixCo2x(OH)6x nanosheets, the optimised core/shell electrode exhibits remarkable electrochemical performance with a high areal capacity (419.1 μA h cm−2), good rate capability and cycling stability. Moreover, the assembled ZnCo2O4@NixCo2x(OH)6x//activated carbon (AC) hybrid device can be reversibly cycled in a large potential range of 0–1.7 V and deliver a maximum energy density of 26.2 W h kg−1 at 511.8 W kg−1. Our findings indicate that the hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs have great potential for applications in energy storage devices.
1. Introduction
With the increasing desire for the utilization of renewable and clean energy sources, energy storage has become one of the biggest challenges in the 21st century.1–3 Supercapacitors have attracted significant attention as one type of efficient energy storage device due to their high power density, fast charge–discharge rate, long cycle life and safe operation.4 Based on the charge storage mechanism, supercapacitors are generally divided into electrical double layer capacitors (EDLCs) and pseudocapacitors.5 However, as for the two categories, their energy density is still too low, which impedes their practical applications.6,7 Hybrid supercapacitors consisting of an EDLC-type (or pseudocapacitive) electrode and a battery-type electrode can achieve higher energy density than conventional capacitors and higher power density than batteries.8–10 Thus, numerous efforts have been dedicated to develop battery-type materials that undergo faradic reactions, including metal oxides, hydroxides and sulfides.11–14 Among them, Ni(OH)2 and Co(OH)2 have been widely studied as electrode materials for hybrid supercapacitors due to their natural abundance and low cost.15,16 Ni(OH)2 has a high theoretical specific capacity but suffers from poor conductivity which restricts its rate capability,17,18 while Co(OH)2 can exhibit higher conductivity than Ni(OH)2. More interestingly, Ni–Co hydroxide is found to integrate the advantages of Ni(OH)2 and Co(OH)2 and exhibits higher rate capability.19–21 However, the rate capability and cycling performance are still unsatisfactory.Recently, ZnCo2O4 has been particularly concerned for energy storage applications owing to its superior electrochemical properties.22–24 By partially replacing Co2+ by Zn2+, ZnCo2O4 can exhibit higher conductivity and better electrochemical activity than pure Co3O4.25–27 Thus far, various types of ZnCo2O4 nanostructures have been prepared as battery-type electrode materials for hybrid supercapacitors.27–31 Although some progress has been made on ZnCo2O4, its areal (specific) capacity is still limited and needs to be further improved to meet the demand for high-performance devices.
To improve the electrochemical performance, constructing core/shell hybrid nanostructures on conductive substrates as binder-free electrodes has been considered as one of the effective strategies.32–34 Generally, the core materials are one-dimensional (1D) nanowires (or nanorods/nanotubes), which would create stable and efficient pathways for electron transport. The shell materials are promising as two-dimensional (2D) nanosheets (or nanoflakes), which can significantly increase the surface area and provide more electrochemical active sites.33,35,36 As a result, such core/shell structures can shorten the distance for electrolyte ion diffusion and open up more efficient pathways for electron transport.37,38 Given these merits, considerable efforts have been devoted to developing various core/shell configurations (e.g., H-CoOx@Ni(OH)2 NWAs,39 NiCo2O4@MnO2 NWAs,40 and NiCo2S4/CoxNi1−x(OH)2 nanotube arrays41) for supercapacitors and achieved improved electrochemical performance.
Herein, we demonstrate a facile strategy for the construction of hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs, including hydrothermal synthesis of ZnCo2O4 NWAs and electrodeposition of NixCo2x(OH)6x nanosheets (a mixture of Ni(OH)2 and Co(OH)2 with a molar ratio of 1/2). Benefiting from the rational construction, the core/shell nanostructures can show good conductivity, large surface area and sufficient active sites, which are favorable for the electrolyte ion diffusion and provide more efficient pathways for electron transport. This hybrid electrode based on the core/shell nanostructures exhibits a high areal capacity of 419.1 μA h cm−2 at a current density of 5 mA cm−2, good rate capability (253.4 μA h cm−2 at 50 mA cm−2) and cycling stability (81.4% of capacity retention after 2000 cycles at 20 mA cm−2). A hybrid supercapacitor has been fabricated by using the ZnCo2O4@NixCo2x(OH)6x NWAs as the positive electrode and activated carbon (AC) as the negative electrode. The hybrid device can present a high energy density of 26.2 W h kg−1 at a power density of 511.8 W kg−1. This work would provide an effective strategy to construct core/shell hybrid nanostructures for energy storage devices.
2. Experimental
In this work, the effective strategy for constructing hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs is schematically illustrated in Fig. 1. First, Zn–Co precursors were vertically grown on Ni foam by a hydrothermal reaction and ZnCo2O4 NWAs were formed after a post-annealing treatment. Then NixCo2x(OH)6x nanosheets were electrodeposited onto the ZnCo2O4 NWAs, resulting in core/shell nanostructures. | ||
| Fig. 1 Schematic illustration of the construction process of hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs on Ni foam. | ||
All the reagents were of analytical grade and used without further purification. Ni foam (100 pores per inch, ∼38 mg cm−2, ∼1.5 mm thick) was purchased from Lyrun New Material Co. Ltd. (Changsha, China).
2.1 Synthesis of ZnCo2O4 NWAs
First, Ni foam was ultrasonically cleaned with acetone, 3 M HCl solution, deionized water and ethanol for 15 min each to remove the impurities and oxide layer. In a typical process, 0.5 mmol Zn(NO3)2·6H2O, 1 mmol Co(NO3)2·6H2O, 1 mmol NH4F and 2.5 mmol urea were dissolved in 35 mL deionized water and magnetically stirred for about 15 min until it formed a homogeneous solution. The obtained solution was transferred into a 50 mL Teflon-lined stainless-steel autoclave and a piece of cleaned Ni foam (2.5 cm × 4.0 cm) was vertically placed into the solution against the bottom of the autoclave. Then the autoclave with the solution was maintained at 120 °C for 5 h. After cooling down to room temperature naturally, the products were taken out and rinsed with deionized water and ethanol several times. The precursors were dried at 60 °C for 10 h and finally annealed in a quartz tube at 400 °C for 2 h. The mass loading of ZnCo2O4 NWAs on Ni foam is ∼1.8 mg cm−2.2.2 Electrodeposition of NixCo2x(OH)6x nanosheets
The NixCo2x(OH)6x nanosheets were deposited on ZnCo2O4 NWAs using an electrochemical workstation (CS310, Wuhan Corrtest Instruments Co. Ltd., China) with a three-electrode configuration. In this procedure, the as-prepared ZnCo2O4 NWAs were used as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum plate as the counter electrode, respectively. The electrodeposition was carried out in 50 mL aqueous electrolyte consisting of 4 mM Ni(NO3)2 and 8 mM Co(NO3)2 using the potential static technique at −1.0 V for 10 min at room temperature. The obtained products were carefully rinsed with deionized water and dried in a vacuum oven at 60 °C for 12 h. The final mass loading of ZnCo2O4@NixCo2x(OH)6x NWAs on Ni foam is ∼2.5 mg cm−2. For comparison, the NixCo2x(OH)6x nanosheets were deposited on Ni foam for 14 min to obtain the same mass loading (∼0.7 mg cm−2) as that on ZnCo2O4 NWAs.2.3 Material characterization
The morphology and microstructure were characterized with a scanning electron microscope (SEM, Tescan MIRA 3 XMU) and a transmission electron microscopy (TEM, FEI Tecnai F30) equipped with an energy dispersive X-ray spectrometer (EDX, AMETEK, USA). The phase structure was identified by X-ray diffraction (XRD, Philips X'pert pro) with Cu Kα radiation (0.154056 nm) and the chemical composition was examined with an X-ray photoelectron spectrometer (XPS, PHI-5702), using Mg Kα X-rays (hν = 1253.6 eV) as the excitation source. The loaded mass of the active materials was measured using a microbalance (Mettler, XS105DU).2.4 Electrochemical measurements
All the electrochemical measurements were carried out using the same electrochemical workstation (CS310, Wuhan Corrtest Instruments Co. Ltd., China) in 2 M KOH aqueous electrolyte at room temperature. The samples were investigated in a three-electrode configuration with the nanostructures grown on Ni foam (1.0 cm × 1.0 cm) as the working electrodes, a SCE as the reference electrode and a platinum plate as the counter electrode. Cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) measurements were carried out to evaluate the electrochemical performance of the electrodes. Electrochemical impedance spectroscopy (EIS) analysis was carried out with a superimposed 5 mV sinusoidal voltage in the frequency range of 100 kHz to 0.01 Hz.2.5 Fabrication of a hybrid supercapacitor
A hybrid supercapacitor was fabricated by using the ZnCo2O4@NixCo2x(OH)6x NWAs as the positive electrode, AC as the negative electrode, cellulose paper as the separator and 2 M KOH as the aqueous electrolyte. The AC electrode was prepared by a typical procedure.18 In brief, AC, acetylene black and polyvinylidene fluoride (PVDF) with a mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were mixed in N-methylpyrrolidinone (NMP) to obtain homogeneous slurry. Then the slurry was coated onto a piece of cleaned Ni foam (1.0 cm × 1.0 cm) with a blade and dried under vacuum at 80 °C for 12 hours. Two-electrode tests were conducted to evaluate the electrochemical performance of the hybrid device.
10 were mixed in N-methylpyrrolidinone (NMP) to obtain homogeneous slurry. Then the slurry was coated onto a piece of cleaned Ni foam (1.0 cm × 1.0 cm) with a blade and dried under vacuum at 80 °C for 12 hours. Two-electrode tests were conducted to evaluate the electrochemical performance of the hybrid device.
The areal capacity (Qa) of the electrodes can be calculated from the discharge curves according to the following equation:
| Qa = (I × Δt)/A | (1) |
Energy density (E) and power density (P) of the device can be evaluated according to the following equations:
| E = ∫I × V(t)dt | (2) |
| P = E/Δt | (3) |
3. Results and discussion
Fig. 2a shows SEM images of ZnCo2O4 NWAs on Ni foam. It can be seen that numerous needle-like ZnCo2O4 nanowires were vertically grown on the surface of the Ni foam framework, leading to a stable and porous three-dimensional (3D) network with a large surface area and appropriate space (inset of Fig. 2a). A magnified SEM image (Fig. 2b) indicates that the ZnCo2O4 nanowires have an average diameter of 80 nm and a length of 4–6 μm. After electrodeposition, the slim ZnCo2O4 nanowires were uniformly decorated with interconnected NixCo2x(OH)6x nanosheets, forming unique forest-like core/shell arrays (Fig. 2c). Obviously, the nanosheets are ultrathin and wrinkled (Fig. 2d), which further increases the surface area of the electrode and provides more active sites for the redox reactions. | ||
| Fig. 2 SEM images of ZnCo2O4 NWAs (a and b) and ZnCo2O4@NixCo2x(OH)6x NWAs (c and d) on Ni foam at different magnifications. | ||
TEM was conducted to further detail the microstructures of the samples. As presented in Fig. 3a, the ZnCo2O4 nanowire has a diameter of ∼80 nm, similar to the SEM observations. The nanowire is composed of many interconnected nanoparticles, which may be attributed to the gas release during annealing. The high-resolution TEM (HRTEM) image (Fig. 3b) indicates a good crystallinity of the ZnCo2O4 nanowire. The inset of Fig. 3b shows a magnified image taken from the highlighted region by a white square. It demonstrates that the spacing of the lattice plane is about 0.286 nm, corresponding to the distance of the (220) planes of spinel ZnCo2O4 (JCPDS card no. 23-1390). Fig. 3c shows a selected area electron diffraction (SAED) pattern of the ZnCo2O4 nanowire. And the reflection rings correspond to (111), (311), (400) and (511) planes of spinel ZnCo2O4, which further confirms its crystal structure. As shown in Fig. 3d, the ZnCo2O4 nanowires were uniformly coated with interconnected nanosheets after electrodeposition. These nanosheets are ultrathin and consisted of numerous pores (several nanometers in size) throughout the surface (Fig. 3e), as previously reported.42,43 It is well noted that the hierarchical core/shell nanostructures would not only create an open and conductive network for electrolyte diffusion and electron transport, but also provide a large surface area and sufficient active sites for redox reactions. In addition, the EDX spectrum (Fig. 3f) of the ZnCo2O4@NixCo2x(OH)6x NWAs demonstrates that the core/shell hybrid structures are mainly composed of Co, Ni, Zn and O elements, except for Cu and C signals from the carbon supported Cu grid.
 | ||
| Fig. 3 TEM image (a), HRTEM image (b) and SAED pattern (c) of the ZnCo2O4 nanowire. TEM images (d and e) and EDX spectrum (f) of the ZnCo2O4@NixCo2x(OH)6x NWAs. | ||
XRD analysis was performed to identify the phase structures of the samples. To avoid the influence from Ni foam (Fig. S1a and S2†), the nanostructures were scratched from Ni foam. The XRD patterns of the obtained ZnCo2O4, NixCo2x(OH)6x and ZnCo2O4@NixCo2x(OH)6x nanostructures are displayed in Fig. 4. The strong peaks at 31.2°, 36.8°, 44.8°, 59.3° and 65.1° can respectively correspond to the (220), (311), (400), (511) and (440) planes of the spinel ZnCo2O4. The diffraction peaks of the NixCo2x(OH)6x nanosheets are weak and broad, indicating their low crystallinity, which can match with those of α-Ni(OH)2 (JCPDS card no. 38-0715) and Co(OH)2 (JCPDS card no. 30-0443). Besides, the Ni–Co hydroxides can convert into NiCo2O4 (JCPDS card no. 20-0781) after annealing at 400 °C for 2 h (Fig. S1b†), indicating that the molar ratio of Ni and Co in the Ni–Co hydroxides is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
 | ||
| Fig. 4 XRD patterns of the ZnCo2O4, NixCo2x(OH)6x and ZnCo2O4@NixCo2x(OH)6x nanostructures scratched from Ni foam. | ||
X-ray photoelectron spectroscopy (XPS) analysis was carried out to further confirm the chemical composition. A survey XPS spectrum of the ZnCo2O4@NixCo2x(OH)6x NWAs (Fig. 5a) reveals the presence of Zn, Ni, Co, O and C elements, which is in agreement with the EDX results. The high-resolution XPS spectrum of Zn 2p (Fig. 5b) demonstrates that the two major peaks at 1044.9 eV and 1021.9 eV are respectively ascribed to Zn 2p3/2 and Zn 2p1/2 of the Zn2+ oxidation state in the ZnCo2O4 nanowires.44 As shown in Fig. 5c, two peaks centered at 873.7 eV and 856.1 eV correspond to Ni 2p1/2 and Ni 2p3/2, indicating the Ni2+ oxidation state in Ni–Co hydroxides.20 Two kinds of Co species (Co2+ and Co3+) were detected in the Co 2p spectra (Fig. 5d). The strong peaks at 796.5 eV for Co 2p1/2 and 780.9 eV for Co 2p3/2 reveal the Co3+ oxidation state in the ZnCo2O4 phase.45 The peaks at 798.2 eV and 782.5 eV are ascribed to the presence of the Co2+ oxidation state in the NixCo2x(OH)6x nanosheets.46 The O 1s spectra (Fig. S3†) reveal the presence of metal–oxygen bonds in ZnCo2O4 nanowires and metal–hydrogen–oxygen bonds in NixCo2x(OH)6x nanosheets.20,47
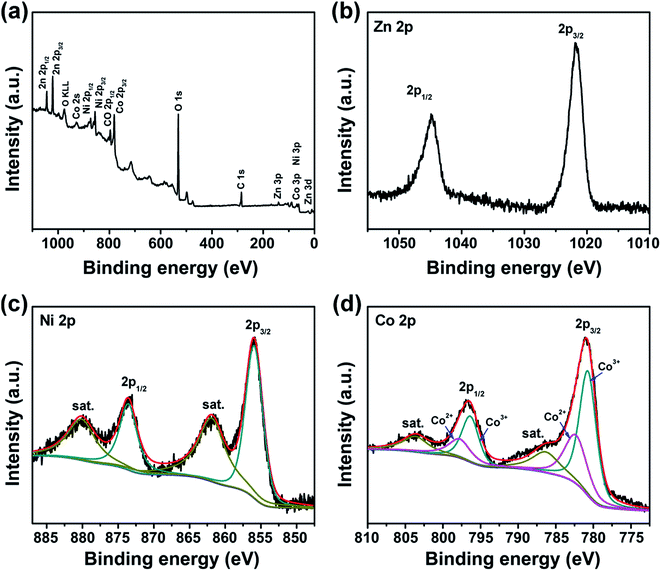 | ||
| Fig. 5 XPS spectra of the ZnCo2O4@NixCo2x(OH)6x core/shell NWAs: survey spectrum (a) and high-resolution spectra of Zn 2p (b), Ni 2p (c) and Co 2p (d). | ||
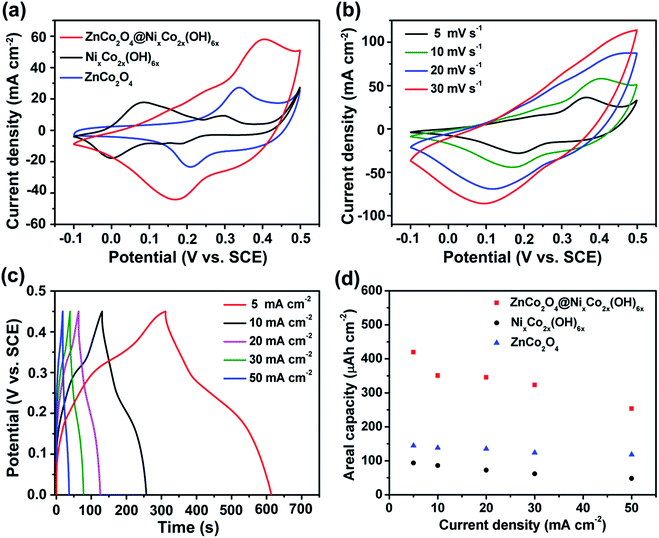
Three-electrode measurements were conducted to investigate the electrochemical performance of electrodes. Fig. 6a shows a comparison of cyclic voltammetry (CV) curves of the prepared electrodes within the potential window of −0.1 to 0.5 V at the same scan rate of 10 mV s−1. It can be seen that all the CV curves have obvious redox peaks, revealing the faradic nature of the battery-type electrodes. For the ZnCo2O4 electrode, a pair of strong redox peaks are attributed to the faradaic redox reactions assigned to the Co(OH)2/CoOOH redox couple prior to the onset of oxygen evolution.27,29 For the NixCo2x(OH)6x electrode, the redox peaks are attributed to the faradaic reactions of the Ni–Co hydroxides with the electrolyte ions. In contrast, the CV curve of the ZnCo2O4@NixCo2x(OH)6x electrode with stronger redox peaks indicates combinative faradic characteristics of the two materials. The redox reactions in the alkaline electrolyte for the core/shell hybrid electrode can be described as the following equations:43,48
| Co2O42− + 2H2O + OH− ↔ 2CoOOH + e− | (4) |
| Co(OH)2 + OH− ↔ CoOOH + H2O + e− | (5) |
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− | (6) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (7) |
The hybrid electrode shows a significantly larger integrated CV area which implies its higher capacity. The integrated area of the cleaned Ni foam is limited when compared with that of the hybrid electrode, suggesting that the capacity contribution from the cleaned Ni foam is negligible (Fig. S4†). Moreover, the CV curves of the ZnCo2O4@NixCo2x(OH)6x electrode at the scan rates of 5–50 mV s−1 are shown in Fig. 6b. With increasing scan rate, the position of the cathodic peak gradually shifts towards a more cathodic position and the anode peak towards a more anodic direction, implying a good rate capability of the hybrid electrode.
Fig. 6c shows the galvanostatic charge–discharge (GCD) curves of the ZnCo2O4@NixCo2x(OH)6x electrode at current densities ranging from 5 to 50 mA cm−2 within the potential window of 0–0.45 V. Obviously, the potential plateaus between 0.15 and 0.25 V can be seen in all curves and they reveal the battery-type characteristic of the hybrid electrode, which is consistent with the CV curves. Recently, Brousse et al. proposed that it would be most appropriate and meaningful to evaluate the capacity of battery-type electrodes and it would be essential to build a full device with a capacitive negative electrode.8 The areal capacity of the electrodes can be calculated by using the GCD curves (Fig. 6c and S5†) and eqn (1). The mass loading of NixCo2x(OH)6x nanosheets coated on ZnCo2O4 nanowires can be controlled by varying the electrodeposition time. It is found that the hybrid electrode with an electrodeposition time of 10 min would have the highest areal capacity (Fig. S6†). The limited capacity of cleaned Ni foam is further confirmed by using the GCD curves (Fig. S4b†). As shown in Fig. 6d, the areal capacity of the optimized ZnCo2O4@NixCo2x(OH)6x electrode is up to 419.1 μA h cm−2 at 5 mA cm−2, which is nearly 3 times as that of the pristine ZnCo2O4 electrode (144.5 μA h cm−2) and over 4 times as the value of the NixCo2x(OH)6x electrode (93.5 μA h cm−2) under the same conditions. Such a high areal capacity is comparable to those of reported core/shell electrodes, such as Co3O4@NiCo2O4 (∼311.7 μA h cm−2),49 ZnO@Ni3S2 (∼318.5 μA h cm−2),50 ZnCo2O4@Ni(OH)2 (∼388.9 μA h cm−2)24 and NiCo2S4/CoxNi1−x(OH)2 (∼397.2 μA h cm−2).41 Moreover, it can be found that the areal capacity gradually decreases with the current density and there still remains 253.4 μA h cm−2 for the ZnCo2O4@NixCo2x(OH)6x electrode at a high current density of 50 mA cm−2 (60.6% retention of that at 5 mA cm−2), demonstrating a good rate capability. These results highlight the advantages of the core/shell nanostructures.
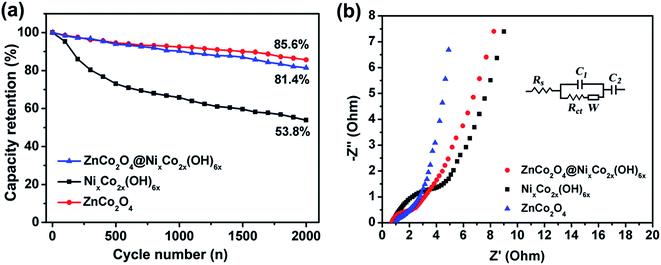
Long-life cycling performance of the prepared electrodes was evaluated by a repeated charge–discharge process at a current density of 20 mA cm−2 and the results are presented in Fig. 7a. For all the curves, the capacity retention slowly decreases with the cycle number. After 2000 cycles, the NixCo2x(OH)6x electrode retains only 53.8% of its initial capacity. While the retention of areal capacity of the ZnCo2O4 electrode is as high as 85.6%, exhibiting good cycling stability. With the rational combination of ZnCo2O4 nanowires and NixCo2x(OH)6x nanosheets, the overall retention of the hybrid electrode is still 81.4% after 2000 cycles, which is much better than that of the NixCo2x(OH)6x electrode and verifies its superiority as an advanced electrode. Electrochemical impedance spectroscopy (EIS) was carried out to investigate the electrical conductivity and ion diffusion. The corresponding Nyquist plots (Fig. 7b) can be fitted with an equivalent circuit (inset of Fig. 7b). At low frequency, the slope of the curve demonstrates the Warburg resistance (W), representing the electrolyte diffusion into the electrode. It is found that the W values of all the three electrodes are almost the same. At high frequency, the intersection point on the real axis shows the bulk resistance (Rs) of the electrochemical system and a higher Rs value indicates a lower electrical conductivity of the sample and vice versa. The semicircle displays the charge-transfer resistance (Rct) which results from the faradaic reactions and the double-layer capacitance on the electrode surface.6,36,51 The fitted values of Rs, Rct and W of the three electrodes are listed in Table S1.† Impressively, the ZnCo2O4@NixCo2x(OH)6x electrode exhibits a lower bulk resistance (0.75 Ω) and charge-transfer resistance (1.12 Ω) than the NixCo2x(OH)6x electrode (0.82 Ω and 2.02 Ω, respectively), which is due to the good conductivity of the ZnCo2O4 nanowires. The hybrid electrode displays a little higher charge transfer resistance than the pristine ZnCo2O4 electrode. This is due to the influence from the low conductivity of the NixCo2x(OH)6x nanosheet shell. Furthermore, the Bode plots (Fig. S7†) reveal that the hybrid electrode can show slower response time than the NixCo2x(OH)6x electrode.
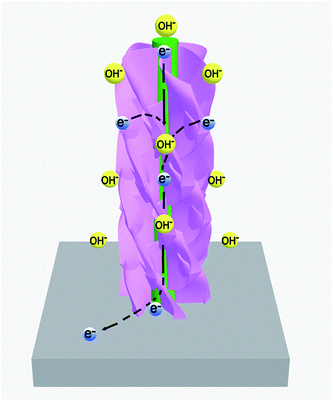
As expected, the ZnCo2O4@NixCo2x(OH)6x electrode shows high areal capacity, good rate capability and cycling stability, which mainly benefits from the core/shell structures and the synergetic effect between each component. Fig. 8 illustrates the advantages of the hierarchical ZnCo2O4@NixCo2x(OH)6x NWAs. Firstly, the ZnCo2O4 NWAs, tightly attaching on the surface of conductive Ni foam and serving as backbones, can create a porous and stable 3D network. This conductive network would facilitate electrolyte ion diffusion and electron transport during the reversible electrochemical reactions. The interconnected NixCo2x(OH)6x nanosheets, coated on the ZnCo2O4 NWA backbones, are ultrathin and porous, which can provide a larger surface area and more active sites. Finally, the rational construction can make the best of each component resulting in a synergetic effect. These inherent advantages are expected to make the hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs particularly attractive.
To further evaluate the practical performance of the ZnCo2O4@NixCo2x(OH)6x NWAs, we have fabricated a hybrid supercapacitor with the hierarchical core/shell NWAs as the positive electrode, AC as the negative electrode and a piece of cellulose paper as the separator, as illustrated in Fig. 9a. The electrochemical performance of the device can be optimized by balancing the charges stored in the positive (Q+) and negative (Q−) electrodes. To obtain Q+ = Q−, the charge can be balanced based on the following equation:
 | (8) |
4. Conclusions
In summary, hierarchical ZnCo2O4@NixCo2x(OH)6x core/shell NWAs have been successfully designed and synthesized combining a hydrothermal method and an electrodeposition process. The ZnCo2O4 NWAs exhibit a good conductivity and stability, serving as backbones. Interconnected NixCo2x(OH)6x nanosheets were subsequently electrodeposited on the nanowire backbones, forming unique hierarchical core/shell nanowire arrays. The core/shell NWAs can make use of the hierarchical structures and the synergetic effect between each component. The novel electrode based on the core/shell structures exhibited a high areal capacity of 419.1 μA h cm−2, as well as good rate capability and cycle performance. We have assembled a hybrid supercapacitor using the ZnCo2O4@NixCo2x(OH)6x NWAs as the positive electrode and AC as the negative electrode. The hybrid device can show a high energy density of 26.2 W h kg−1 at a power density of 511.8 W kg−1, revealing that the hierarchical ZnCo2O4@NixCo2x(OH) NWAs have great potential for applications in hybrid supercapacitors and this effective strategy also casts new light on the design and synthesis of core/shell hybrid nanostructures for energy storage devices.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 61176058 and 61404066).References
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Chem. Soc. Rev., 2013, 42, 3127–3171 RSC.
- X. Peng, L. Peng, C. Wu and Y. Xie, Chem. Soc. Rev., 2014, 43, 3303–3323 RSC.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- J. Chang, M. Jin, F. Yao, T. H. Kim, V. T. le, H. Yue, F. Gunes, B. Li, A. Ghosh, S. Xie and Y. H. Lee, Adv. Funct. Mater., 2013, 23, 5074–5083 CrossRef CAS.
- F. Zhang, T. Zhang, X. Yang, L. Zhang, K. Leng, Y. Huang and Y. Chen, Energy Environ. Sci., 2013, 6, 1623–1632 CAS.
- T. Brousse, D. Belanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef CAS.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gomez-Romero, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- Q. Ke, M. Zheng, H. Liu, C. Guan, L. Mao and J. Wang, Sci. Rep., 2015, 5, 13940 CrossRef PubMed.
- J.-K. Chang, C.-M. Wu and I. W. Sun, J. Mater. Chem., 2010, 20, 3729–3735 RSC.
- X. Li, S. Xiong, J. Li, J. Bai and Y. Qian, J. Mater. Chem., 2012, 22, 14276–14283 RSC.
- M. Yu, W. Wang, C. Li, T. Zhai, X. Lu and Y. Tong, NPG Asia Mater., 2014, 6, e129 CrossRef CAS.
- W. Fu, C. Zhao, W. Han, Y. Liu, H. Zhao, Y. Ma and E. Xie, J. Mater. Chem. A, 2015, 3, 10492–10497 CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- B. G. Choi, M. Yang, S. C. Jung, K. G. Lee, J.-G. Kim, H. Park, T. J. Park, S. B. Lee, Y.-K. Han and Y. S. Huh, ACS Nano, 2013, 7, 2453–2460 CrossRef CAS PubMed.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- Y.-Z. Su, K. Xiao, N. Li, Z.-Q. Liu and S.-Z. Qiao, J. Mater. Chem. A, 2014, 2, 13845–13853 CAS.
- J. Li, M. Yang, J. Wei and Z. Zhou, Nanoscale, 2012, 4, 4498–4503 RSC.
- C. Shang, S. Dong, S. Wang, D. Xiao, P. Han, X. Wang, L. Gu and G. Cui, ACS Nano, 2013, 7, 5430–5436 CrossRef CAS PubMed.
- L. Qu, Y. Zhao, A. M. Khan, C. Han, K. M. Hercule, M. Yan, X. Liu, W. Chen, D. Wang, Z. Cai, W. Xu, K. Zhao, X. Zheng and L. Mai, Nano Lett., 2015, 15, 2037–2044 CrossRef CAS PubMed.
- H. Chen, Q. Zhang, J. Wang, Q. Wang, X. Zhou, X. Li, Y. Yang and K. Zhang, Nano Energy, 2014, 10, 245–258 CrossRef CAS.
- T. W. Kim, M. A. Woo, M. Regis and K.-S. Choi, J. Phys. Chem. Lett., 2014, 5, 2370–2374 CrossRef CAS PubMed.
- H. X. Chuo, H. Gao, Q. Yang, N. Zhang, W. B. Bu and X. T. Zhang, J. Mater. Chem. A, 2014, 2, 20462–20469 CAS.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS.
- Y. Qiu, S. Yang, H. Deng, L. Jin and W. Li, J. Mater. Chem., 2010, 20, 4439–4444 RSC.
- B. Liu, B. Liu, Q. Wang, X. Wang, Q. Xiang, D. Chen and G. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011–10017 CAS.
- S. Wang, J. Pu, Y. Tong, Y. Cheng, Y. Gao and Z. Wang, J. Mater. Chem. A, 2014, 2, 5434–5440 CAS.
- G. Zhou, J. Zhu, Y. Chen, L. Mei, X. Duan, G. Zhang, L. Chen, T. Wang and B. Lu, Electrochim. Acta, 2014, 123, 450–455 CrossRef CAS.
- W. Fu, X. Li, C. Zhao, Y. Liu, P. Zhang, J. Zhou, X. Pan and E. Xie, Mater. Lett., 2015, 149, 1–4 CrossRef CAS.
- I. K. Moon and K.-Y. Chun, RSC Adv., 2015, 5, 807–811 RSC.
- Z. Sun, W. Ai, J. Liu, X. Qi, Y. Wang, J. Zhu, H. Zhang and T. Yu, Nanoscale, 2014, 6, 6563–6568 RSC.
- M. Huang, Y. Zhang, F. Li, L. Zhang, Z. Wen and Q. Liu, J. Power Sources, 2014, 252, 98–106 CrossRef CAS.
- W. Ma, H. Nan, Z. Gu, B. Geng and X. Zhang, J. Mater. Chem. A, 2015, 3, 5442–5448 CAS.
- X. Xia, J. Tu, Y. Zhang, J. Chen, X. Wang, C. Gu, C. Guan, J. Luo and H. J. Fan, Chem. Mater., 2012, 24, 3793–3799 CrossRef CAS.
- D. Cai, B. Liu, D. Wang, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li and T. Wang, J. Mater. Chem. A, 2014, 2, 4954–4960 CAS.
- W. Yang, Z. Gao, J. Ma, X. Zhang, J. Wang and J. Liu, J. Mater. Chem. A, 2014, 2, 1448–1457 CAS.
- J. Wang, D. Chao, J. Liu, L. Li, L. Lai, J. Lin and Z. Shen, Nano Energy, 2014, 7, 151–160 CrossRef CAS.
- J. Zhu, L. Huang, Y. Xiao, L. Shen, Q. Chen and W. Shi, Nanoscale, 2014, 6, 6772–6781 RSC.
- L. Yu, G. Zhang, C. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137–139 RSC.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- L. Huang, D. Chen, Y. Ding, Z. L. Wang, Z. Zeng and M. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 11159–11162 CAS.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- W. Luo, X. Hu, Y. Sun and Y. Huang, J. Mater. Chem., 2012, 22, 8916–8921 RSC.
- T. F. Hung, S. G. Mohamed, C. C. Shen, Y. Q. Tsai, W. S. Chang and R. S. Liu, Nanoscale, 2013, 5, 12115–12119 RSC.
- U. M. Patil, M. S. Nam, J. S. Sohn, S. B. Kulkarni, R. Shin, S. Kang, S. Lee, J. H. Kim and S. C. Jun, J. Mater. Chem. A, 2014, 2, 19075–19083 CAS.
- L. Hu, B. Qu, C. Li, Y. Chen, L. Mei, D. Lei, L. Chen, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 5596–5602 CAS.
- X. Liu, S. Shi, Q. Xiong, L. Li, Y. Zhang, H. Tang, C. Gu, X. Wang and J. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790–8795 CAS.
- G. Zhang, T. Wang, X. Yu, H. Zhang, H. Duan and B. Lu, Nano Energy, 2013, 2, 586–594 CrossRef CAS.
- Z. Xing, Q. Chu, X. Ren, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, J. Power Sources, 2014, 245, 463–467 CrossRef CAS.
- C.-W. Huang and H. Teng, J. Electrochem. Soc., 2008, 155, A739–A744 CrossRef CAS.
- X.-F. Lu, D.-J. Wu, R.-Z. Li, Q. Li, S.-H. Ye, Y.-X. Tong and G.-R. Li, J. Mater. Chem. A, 2014, 2, 4706–4713 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns, additional results of electrochemical tests. See DOI: 10.1039/c5ta07965a |
| This journal is © The Royal Society of Chemistry 2016 |