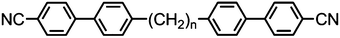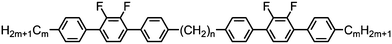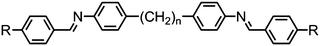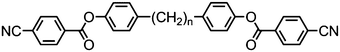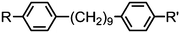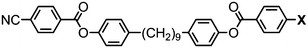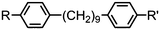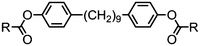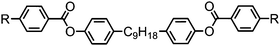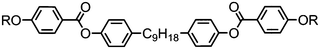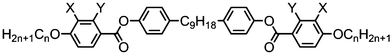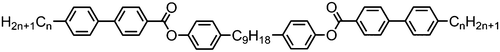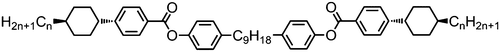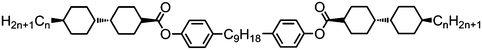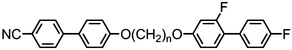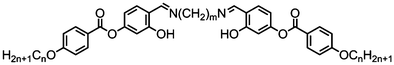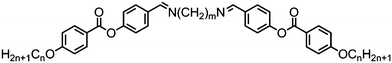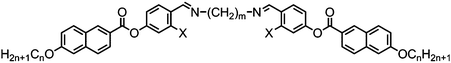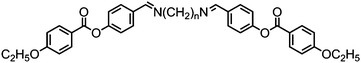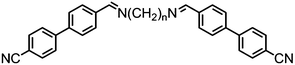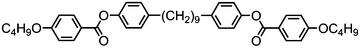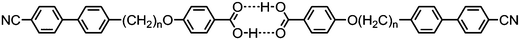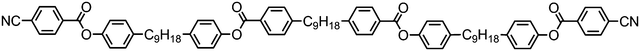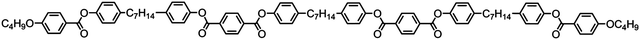Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The dependency of twist-bend nematic liquid crystals on molecular structure: a progression from dimers to trimers, oligomers and polymers
Richard J.
Mandle

Department of Chemistry, University of York, York, YO10 5DD, UK. E-mail: richard.mandle@york.ac.uk
First published on 25th August 2016
Abstract
This article gives an overview on recent developments concerning the twist-bend nematic phase. The twist-bend nematic phase has been discussed as the missing link between the uniaxial nematic mesophase (N) and the helical chiral nematic phase (N*). After an introduction discussing the key physical properties of the NTB phase and the methods used to identify the twist-bend nematic mesophase this review focuses on structure property relationships and molecular features that govern the incidence of this phase.
1. Introduction
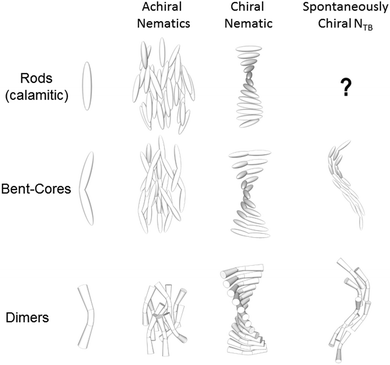
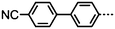
“Liquid crystal” is an umbrella term for a group of partially ordered, sometimes fluid, states of matter that exists in addition to the three ‘common’ states of matter, these being the solid, liquid and gaseous states. In addition to being the presently dominant display technology various liquid-crystalline (LC) mesophases are formed by DNA,1–6 carbohydrates and lipids,7–9 and peptides.10–12 Liquid-crystalline phases also occur on length scales beyond those of molecular dimensions, with mesophases being formed by (for example) suspensions of viral particles.13–15The uniaxial nematic phase is the simplest LC phase and possesses only an average orientational ordering of the molecules along a preferred direction (the director). The addition of chirality to a nematic LC, either through changes to molecular structure or via the addition of a supplementary chiral dopant to the host nematic phase, leads to the formation of a helical superstructure in the nematic phase (denoted N*, chiral nematic or cholesteric). For both bent-core liquid crystals and liquid-crystalline dimers an additional possibility exists; this being the formation of a mesophase with a helical local structure and thus locally chiral, formed by materials that are themselves achiral. Such a mesophase, termed the twist-bend nematic (NTB, Fig. 1) was predicted by Dozov16 to be formed by bent molecules and was later confirmed experimentally.17 This mesophase has been described as the “structural link” between the uniaxial nematic phase and the helical chiral nematic mesophase.18 It is well known that NTB phase is exhibited by both bent-core19 and liquid-crystalline dimers,17 however, no examples of twist-bend mesophases in rod-like (calamitic) liquid-crystalline materials are known and it is presently an open question whether or not such molecular structures can exhibit this unique mesophase.
Questions and reports concerning spontaneous breaking of mirror symmetry are of fundamental importance to a wide range of scientific disciplines,20–29 and so the formation of helical, chiral, nanoscale structures in liquid-crystalline bent-core compounds,30–32 dimers33–36 and trimers27,37–39 has prompted extensive study. When formed from a racemates or molecules lacking stereogenic centre it is the chirality that results from conformers that leads to the spontaneous breaking of mirror symmetry in the NTB phase. For each chiral conformer there exists its mirror image, which has the opposite sign of chirality and, in the absence of a biasing chiral dopant or environment, there exists equal probabilities of each of these and thus the bulk structure is achiral.34 Despite a number of theoretical treatments16,36,40–43 and comparative studies between experiment and theory,44–46 a general and comprehensive structure property relationship remains elusive to date, aside from the widely acknowledged requirement for a ‘bent’ molecular shape.47
2. Mesophase structure, physical properties and identification
The pitch length of the helical structure of the NTB phase is on the order of several nanometres and has been measured directly by freeze-fracture transmission electron microscopy (FFTEM, Fig. 2)18,19,48,49 and resonant carbon K-edge small angle X-ray scattering (SAXS)50 as well as indirectly, with measurement of the electroclinic effect51 and 2H NMR spectroscopy33 revealing the presence of an extremely short (i.e. several nanometers) helical pitch (PTB). In the case of resonant SAXS experiments the ability to measure the PTBin situ has allowed the temperature dependence of the pitch to be determined quantitatively for the first time.50 It is unfortunate that to date these techniques have only been applied to a small number of molecules, and so it is unknown how PTB varies with molecular structure.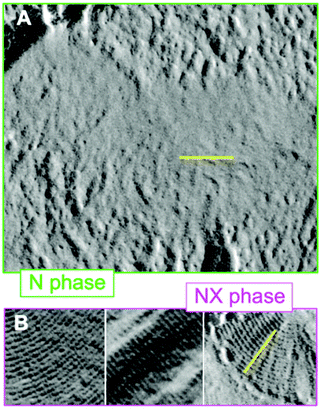 | ||
| Fig. 2 Comparison of the FFTEM image of CB7CB in the nematic phase quenched at 105 °C (A) and the NTB phase quenched at 95 °C (B). The scale bar corresponds to 100 nm. Reproduced from ref. 48, used with permission of the National Academy of Sciences of the United States of America, copyright 2013. | ||
The conical angle has been determined for CB7CB (2, Table 2) using birefringence measurements,522H NMR spectroscopy on CB7CB-d4, 129Xe NMR on CB7CB saturated with xenon and CB7CB doped with 8CB-d2 as a spin probe.53 As the conical angle remains less than the magic angle the NTB phase is optically uniaxial with positive birefringence; this has also been demonstrated by connoscopy.54 The orientational order parameters 〈P2〉 and 〈P4〉 for the CBnCB series (where n is 7, 8, 9 or 11, see Table 1), as determined by polarised Raman spectroscopy (PRS),55 are significantly higher for the dimer CB8CB (3), with even spacer parity, than for the odd parity dimers CB7CB, CB9CB and CB11CB (2, 4 and 6 respectively). Values determined by PRS for both odd- and even-parity dimers are in good agreement with those obtained previously by NMR using anthracene-d10 as a spin probe.56 Measurement of the dielectric permittivities of materials exhibiting the twist-bend nematic can provide values of the elastic constants, the conical ‘tilt’ angle in the NTB phase and in some cases order parameter data for both pure compounds and mixtures.57–61 Studies on the rheology of the twist-bend nematic phase of KA (0.2), a six component mixture of odd-parity bimesogens for which a pitch length of 10.5 nm was determined by FFTEM,58,62 reveal that the NTB phase is strongly shear thinning. At low shear stress (<1 Pa) the viscosity of the NTB phase was found to be 1000 times larger than that of the nematic phase in the same material, however, at higher shear stress (>10 Pa) the viscosity drops two orders of magnitude as the heliconical axis reorients due to shear-induced alignment.63
| No | Name | n | Cr | NTB | N | Iso | |||
|---|---|---|---|---|---|---|---|---|---|
| a The melting point of CB10CB was not reported. | |||||||||
| 1 | CB6CB | 6 | • | 183 | — | — | • | 230 | • |
| 2 | CB7CB | 7 | • | 102 | • | 104.5 | • | 116 | • |
| 3 | CB8CB | 8 | • | 175.0 | — | — | • | 195.9 | • |
| 4 | CB9CB | 9 | • | 83.0 | • | 105 | • | 119.8 | • |
| 5 | CB10CB | 10 | • | — | — | • | 174.1 | • | |
| 6 | CB11CB | 11 | • | 99.9 | • | 108.6 | • | 125.5 | • |
Although supported by a significant body of experimental evidence this view of the local structure has also been disputed, with some experimental evidence interpreted to be counter the heliconical model. Specifically a 2H NMR experiment was interpreted as showing that a helix is not present in the NTB/NX phase.67 Similar periodic length scales exist in the solid state of CB9CB (4) as those measured by FFTEM, with suggestions that the measured pitch may be due to surface freezing.68 In the model proposed by Dozov16 the spontaneous twit-bend deformation of the nematic director is a consequence of the bend elastic constant K33 falling below zero, conversely, experimental studies have shown that this is not the case for CB7CB.69 Most recently, the observation of direct isotropic liquid to NTB phases has provided a further challenge to future theoretical treatments.70,71 Presently, it seems that experimental evidence supports the heliconical model as the best descriptor of the local structure of the twist-bend mesophase.
Deuterium NMR spectroscopy is a powerful tool for the study of the NTB phase; the use of a suitably deuterated prochiral mesogen or solute as a spin probe (e.g. anthracene-d10,56 8CB-d272) allows the rapid distinction between the uniaxial nematic and the heliconical twist-bend nematic phases as follows. In the isotropic liquid the deuterium NMR spectrum of a pro-chiral methylene (–CD2–) is a singlet, in a uniaxial nematic phase the same deuterium environment is a quadrupolar doublet, however, as a consequence of the local chirality results the twist-bend nematic phase these two deuterons are no longer equivalent and so two quadrupolar doublets are observed. Example spectra are given in Fig. 3.
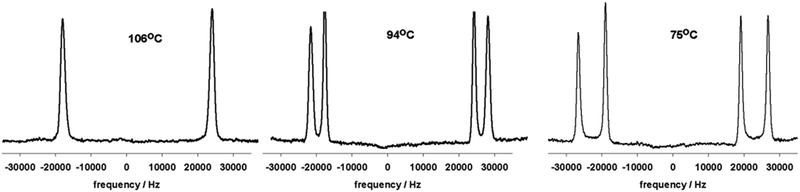 | ||
| Fig. 3 2H NMR spectra of 8CB-d2 dissolved in CB7CB and recorded in the nematic (106 °C, 46.0 MHz) and twist-bend nematic (94 °C and 75 °C, 61.4 MHz). Adapted with permission from ref. 72. Copyright American Chemical Society 2012. | ||
The optical textures of the twist-bend nematic phase are both distinctive and sufficiently different from other mesophases that they can be used for preliminary assignment. Typical optical textures obtained for the NTB phase on cooling from the schlieren texture of the nematic phase are presented in Fig. 4; immediately below the phase transition the birefringent regions of what was the schlieren texture in the nematic become blocky, further cooling yields significant changes in the birefringence. More detailed discussion of the optical textures of the NTB phase can be found elsewhere.66,73 The ability to draw freestanding films of the twist-bend nematic indicates that this mesophase has structural periodicity (Fig. 5).66
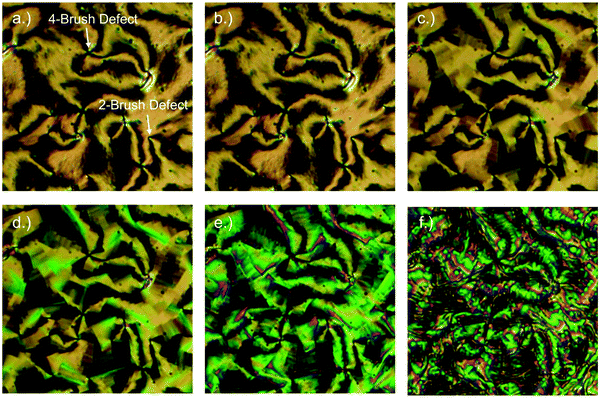 | ||
| Fig. 4 Polarized optical micrographs of CB11CB showing the textures observed for (a) the nematic phase at 107 °C; (b) the nematic phase at 106.8 °C; (c) the nematic to NTB transition at 106.5 °C; (d) the NTB phase at 106.2 °C; (e) the NTB phase at 105 °C and; (f) the NTB phase at 100.7 °C. The textures were obtained for a specimen contained in a 9 μm cell treated to give homeotropic alignment. Reproduced from ref. 66 with permission of the Royal Society of Chemistry. | ||
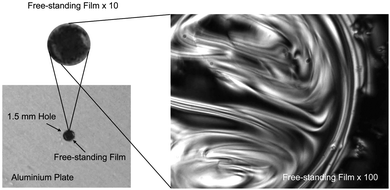 | ||
| Fig. 5 Photomicrograph of a free standing film of CB11CB drawn over a 1.5 mm aperture in an aluminium plate, with an exposure time of 500 milliseconds. Reproduced from ref. 66 with permission of the Royal Society of Chemistry. | ||
Calorimetric studies of the nematic to twist-bend nematic phase transition reveal it to be weakly first order and close to tricritical.17,59,60,66,74 In non-resonant small angle X-ray scattering experiments the pattern obtained for the twist-bend nematic phase is similar to that of the nematic phase; only diffuse scattering in both the wide and small angle regions as both the N and NTB phases lack electron density modulation, despite the spatial periodicity present in the latter.17,19,48,49,75–81 SAXS patterns obtained for CB11CB (6) with Cu Kα radiation (λ = 0.154 nm) for a magnetically aligned (field of ≈0.6 T at sample position) sample in the nematic (110 °C) and the twist-bend nematic (99 °C) are given in Fig. 6, using an experimental setup that has been described previously.76 Although non-resonant SAXS experiments are not diagnostic for the local structure of the twist-bend nematic phase, they are a useful tool for distinguishing between the NTB and smectic phases, in which diffuse layers of spacing dL lead to sharp scattering at 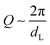 . As noted by Zhu et al., non-resonant SAXS experiments should be able to distinguish between the twist-bend and splay-bend nematic phases.50 For materials suspected of exhibiting a twist-bend nematic phase the construction of phase diagrams with twist-bend nematic materials (such as the CBnCB compounds) has proved to be a useful way of demonstrating unequivocally that novel materials exhibit this state of matter.17,44,59,76,81–84
. As noted by Zhu et al., non-resonant SAXS experiments should be able to distinguish between the twist-bend and splay-bend nematic phases.50 For materials suspected of exhibiting a twist-bend nematic phase the construction of phase diagrams with twist-bend nematic materials (such as the CBnCB compounds) has proved to be a useful way of demonstrating unequivocally that novel materials exhibit this state of matter.17,44,59,76,81–84
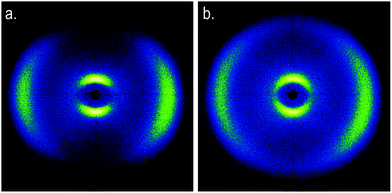 | ||
| Fig. 6 Two-dimensional small-angle X-ray scattering patterns obtained for a magnetically aligned sample of CB11CB (6) in the nematic phase at 110 °C (a) and the NTB phase at 99 °C (b). The synthesis of CB11CB (6) and the experimental setup used is described in ref. 66 and 76. | ||
The potential for stimuli-responsive behaviour in twist-bend nematic materials, as well as other LC phases with helical structures, has the potential to yield novel display technologies utilising switchable reflection,85–87 as well as in producing defined nanostructures that can be used for photonic applications.88–90
3. Molecular structure and the NTB phase
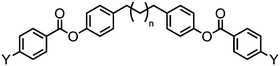
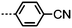
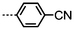
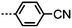
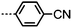
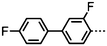
As with all mesophases the occurrence of the twist-bend nematic phase in a bulk material has its origin in molecular structure, and since the identification of the lower temperature nematic phase exhibited by CB7CB (2) by Cestari et al. as the twist-bend nematic phase there has been a resurgence of interest in the structure property relationships of liquid-crystalline dimers.17 We can subdivide the structure of a liquid crystal dimer into sub sections (Fig. 7) as follows. Firstly, there are two mesogenic units that are comprised of rigid, cyclic ring structures (such as phenyl, hetroaryl, cyclohexyl, bicyclooctyl) and these may or may not contain either lateral substituents and/or ring-to-ring link groups such as esters,83 imines,91,92 azo groups,54,93 and such. In terms of the choice of terminal group there are examples of polar,83 apolar76 and mixed polar/apolar units being employed.94,95 The mesogenic units are attached to a spacer which, in the context of the NTB phase, is almost always a hydrocarbon of odd parity. The chemical nature of the linking group between the mesogenic units and the spacer is also important, with methylene linking groups being dominant but a large and significant number of imine,91,92 ether,79,82 ester54,96 (and combinations thereof) being known. Lastly, the NTB phase is known to exist in trimers49,97 and tetramers,77 and although no such reports have been made to date, it seems highly probable that this phase will also be observed in higher oligomers.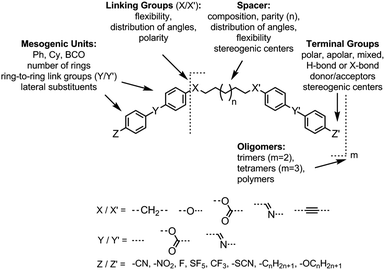 | ||
| Fig. 7 General structure of materials that exhibit the twist-bend nematic phase and possible variations to molecular structure leading from simple bimesogens to more complex oligomeric structures. | ||
A ‘bent’ molecular shape is a prerequisite for a material to exhibit the twist-bend nematic phase and this is most commonly achieved by using a spacer of odd parity, although as will be discussed, exceptions exist to this rule where even parity spacers are used and the molecular bend is provided by some other structural unit. When the spacer parity is odd then the material is bent; conversely an even spacer parity affords a near linear material and thus for simple dimers there is a strong even effect due to the significantly different shape; this is well illustrated by comparison of the properties of the CBnCB series of materials (1–6, Table 1). Replacement of both of the methylene linking groups with esters and ethers leads to loss of the NTB phase, however the resulting materials still exhibit the nematic phase.81,98
3.1. Methylene linked dimers
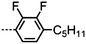
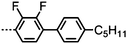
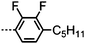
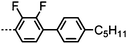
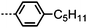
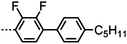
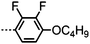
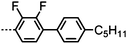
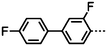
Dimers containing two 4′′-alkyl-2′,3′-difluoroterphenyl mesogenic units joined by either a heptamethylene or undecamethylene spacer were also found to exhibit the twist-bend nematic phase (Table 2), with 7 being one of only a handful of materials exhibiting a twist-bend to smectic phase transition.99 Compound 8 was found to exhibit a linear (polar) microsecond optical response to applied electric fields, with domains of opposite handedness exhibiting opposite responses when confined in planar cells. One possible interpretation of this phenomenon was noted by the authors as being a ‘very short helical pitch’.84As depicted in Fig. 7 it is possible to include ring-to-ring linking units in the mesogenic units, resulting in predictable changes to the shape and polarity of the resulting material. Concerning the N-phenylbenzimine dimers reported by Henderson et al. (Table 3), materials with terminal cyano, methoxy or ethoxy groups and a central pentamethylene spacer (10–12) exhibit nematic and NTB phases (identified as Sm in ref. 101).102 In a similar vein to the CBnCB compounds, analogous dimers with even spacer parity (13–14) are solely nematogenic and exhibit large increases in clearing point relative to the odd parity materials.101
As with the CBnCB series of materials the even-parity members of the PCBnPCB series (16, 18; Table 4) were found to have significantly higher melting and clearing points than the odd-parity materials (15, 17, 19, Table 4). However, whereas the odd-parity materials exhibit the twist-bend nematic phase the even-parity homologues lack the perquisite bent shape and thus do not exhibit this phase. Additionally the even-parity materials, as a consequence of their anticipated liner shape, have significantly higher melting and clearing points than the odd-parity materials. The identity of the NTB phase of compounds 15, 17 and 19 was confirmed by miscibility with CB11CB.83
Reversal of the ester linking units of 17 affords compound 20 (Table 5), which has marginally reduced NTB–N and N–Iso transition temperatures. The positioning of a lateral fluoro group in either the 2- or 3-positions of the outermost ring leads to reduced clearing points and N–NTB transition temperatures (in the case of 21 it occurs simultaneously with crystallisation at around 75 °C). The use of a ‘three-ring’ mesogenic unit (23) as opposed to a two ring unit (7) leads to significant increases to the NTB–N transition temperature as well as a 180 °C increase in the clearing point; this indicates that the aspect ratio of the mesogenic units (i.e. length to breadth ratio) of the dimer can significantly change the thermal behaviour of the material, as was demonstrated with compounds 17 and 20.
| No | Name | R | Cr | NTB | N | Iso | |||
|---|---|---|---|---|---|---|---|---|---|
| a The NTB phase could be observed with rapid uncontrolled cooling, however this precludes accurate determination of the transition temperature. | |||||||||
| 4 | CB9CB |
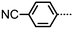
|
• | 83 | • | 105 | • | 119.8 | • |
| 17 | PCB9PCB |
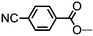
|
• | 157.6 | (• | 114.5 | • | 146.6) | • |
| 20 | CPB9CPB |
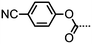
|
• | 125.2 | (• | 95.0) | • | 138.4 | • |
| 21 | n/a |
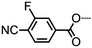
|
• | 108.4 | (• | —a | • | 97.8) | • |
| 22 | n/a |
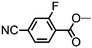
|
• | 150.0 | (• | 108.6 | • | 128.5) | • |
| 23 | n/a |
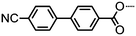
|
• | 165.5 | • | 179.6 | • | 324.0 | • |
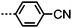
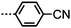
The effect of polar terminal groups on the NTB was also explored by Goodby et al., who synthesised a number of methylene-linked dimers consisting of a heptamethylene or nonamethylene spacer, two phenyl benzoate mesogenic units and one of six polar terminal groups; cyano (7, 9), fluoro (24, 25), trifluoromethyl (26, 27), isothiocyanate (28, 29), nitro (30, 31) and pentafluorosulfanyl (32, 33). Many of these materials were non mesogenic (see Table 6), although the compounds featuring CN and SCN terminal groups exhibited both nematic and NTB phases. Comparing the compounds in Tables 5 and 6 it was noted that the neither the incidence nor thermal stability of the twist-bend nematic phase depends on the dipole moment.45
| No. | Y | n | Cr | NTB | N | I | |||
|---|---|---|---|---|---|---|---|---|---|
| 15 | CN | 5 | • | 149 | (• | 120.0 | • | 139.0) | • |
| 17 | CN | 7 | • | 157.6 | (• | 114.5 | • | 146.6) | • |
| 24 | F | 5 | • | 91.5 | — | — | — | — | • |
| 25 | F | 7 | • | 97.6 | — | — | — | — | • |
| 26 | CF3 | 5 | • | 114.9 | — | — | — | — | • |
| 27 | CF3 | 7 | • | 102.4 | — | — | — | — | • |
| 28 | SCN | 5 | • | 105.1 | (• | 103.3) | • | 120.5 | • |
| 29 | SCN | 7 | • | 97.7 | • | 103.7 | • | 127.4 | • |
| 30 | NO2 | 5 | • | 113.3 | — | — | — | — | • |
| 31 | NO2 | 7 | • | 105.4 | — | — | (• | 97.8) | • |
| 32 | SF5 | 5 | • | 126.5 | — | — | — | — | • |
| 33 | SF5 | 7 | • | 123.0 | — | — | — | — | • |
In unsymmetrical liquid-crystalline bimesogens with varying polar terminal units it was found that functional groups that are normally detrimental to mesophase stability (such as CF3, SF5) could be incorporated into the molecular structure, with the resulting material still retaining the nematic and twist-bend phases (Table 7). When studied by SAXS no significant differences in the obtained patterns were observed between the symmetrical material 7 and unsymmetrical compounds such as 35, with the diffuse small-angle scattering peak being approximately one half of the all trans molecular length and therefore indicating that the two distinct polar groups are not segregated in either the nematic or nematic twist-bend phases.
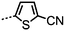
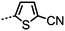
Ahmed et al. reported several unsymmetrical bimesogens (Table 8), several of which were found to exhibit the twist-bend nematic phase. Ahmed et al. suggested that the position of polar groups (i.e. lateral, terminal or absent) is not critical for the formation of the NTB phase, as was also suggested earlier.45 As shown in Table 8, there is a marked increase in clearing points with increasing aspect ratio, whilst reducing the symmetry of the molecule was found to lead to lower melting points. The replacement of a single phenyl ring in CB9CB with thiophene (to afford 39) leads to a small drop in both the clearing point and N–NTB transition temperature, presumably due to the non-linearity of a 2,5-disubstitued thiophene unit. Compound 46 exhibits a transition from the NTB phase into an as yet unidentified, highly ordered smectic (SmX) mesophase.
In ‘polar’ dimers and bimesogens, i.e. materials exhibiting well-defined polar terminal or lateral substituents (such as cyano, nitro, fluoro groups etc.), the NTB phase does not appear to exhibit any dependency on the molecular dipole moment.83,94,103,104 For unrelated materials with similar clearing points the N–NTB transition temperatures are, apparently, almost identical; for example compounds 22 and 29, 45 and 46.
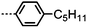
Moving now to materials lacking polar functional groups in either the terminal or lateral positions of the mesogenic units, the nonamethylene linked phenyl 4-pentylbenzoate dimer 47 was found to exhibit both nematic and twist-bend nematic mesophases, although both were monotropic. Replacement of the outermost phenyl ring of 47 (Table 9) with trans cyclohexyl affords a small increase in melting point and a large increase in clearing point, however the NTB phase is lost. Replacement of the phenyl ring with [2,2,2]bicyclooctane (BCO, 49) again affords an increase in clearing point, analogous to the behaviour of calamitic materials,105 however in this case the material still exhibits the NTB phase with an accompanying increase in thermal stability of over 30 °C.
Turning now to analogues of 47 with varying terminal chain length, the melting points, NTB–N and N–Iso transition temperatures do not show significant variation aside from a small odd–even effect. All phase transitions are monotropic, and in the case of 53 and 55 recrystallisation occurs directly from the isotropic liquid due to the short range of supercooling.
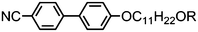
Incorporating an alkoxy terminal chain in lieu of an alkyl (Table 11) leads to increased melting points, but also significant increases in the N–I and N–NTB transition temperatures to the point where 58, 59 and 60 exhibit enantiotropic NTB phases. Compound 60 exhibits a transition from the NTB phase into an anticlinic smectic C phase – this is the only known example of this phase transition that is currently known. The associated enthalpy, as measured by differential scanning calorimetry, of the SmCA–NTB transition of 60 is an order of magnitude larger than the NTB–N phase transition. Study of 60 by small angle X-ray scattering reveals that at the phase transition the correlation length increases from roughly 5.5 nm in the NTB phase to 27 nm in the SmCA phase. The SmCA phase has an average layer spacing equal to 0.5 molecular lengths, and thus is extensively intercalated. The C7 homologue (61) exhibits a nematic to SmCA transition, whilst at a terminal chain length of C8 (62) or greater neither the nematic nor NTB phase are observed, instead a direct isotropic to anticlinic smectic C transition occurs (Fig. 8).
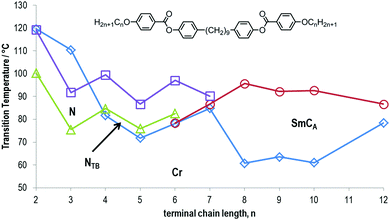 | ||
| Fig. 8 Plot of transition temperatures (°C) as a function of the length of the terminal alkoxy chain for compounds 56–65.49 | ||
As was reported for compounds 22 and 23 the incorporation of one or two lateral fluoro group in either the 2- or 3-positions of the outermost ring leads to reduced clearing points and N–NTB transition temperatures; in the case of compound 67 the material does not exhibit the twist-bend nematic phase (Table 12). Compound 65 exhibits a direct isotropic to smectic CA phase transition, however, incorporating a methyl or methoxy group in the 2-position of the outermost ring (to afford 68 and 69 respectively) disrupts the packing that leads to the SmCA phase and the materials instead exhibit nematic and twist-bend nematic mesophases, albeit with significantly (>50 °C) reduced clearing points. The steric footprint of fluorine is much smaller than that of a methyl or methoxy group, and so when it is used as a lateral substituent the depression in clearing point is not as pronounced and the Iso–SmCA phase transition is retained.
Increasing the aspect ratio of the phenyl 4-alkylbenzoate dimers (Table 10) to afford the phenyl 4-alkylbiphenyl-4′-carboxylate materials (Table 13) leads to significant increases in both clearing and melting points, with shorter homologues (71 and 72) exhibiting nematic and NTB mesophases, with longer homologues (73 and 74) exhibit an anticlinic smectic C phase. Compound 72, with terminal propyl chains, exhibits a nematic to unknown-smectic mesophase transition, whereas 74 exhibits an additional monotropic smectic B phase.76,78,106
| No | R | Cr | SmCA | NTB | N | Iso | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 56 | C2H5 | • | 119.6 | — | — | (• | 100.4 | • | 119.2 | •) |
| 57 | C3H7 | • | 110.4 | — | — | (• | 75.5 | • | 91.8 | •) |
| 58 | C4H9 | • | 83.3 | — | — | • | 84.7 | • | 99.5 | • |
| 59 | C5H11 | • | 71.8 | — | — | • | 76.0 | • | 86.6 | • |
| 60 | C6H13 | • | 78.2 | • | 78.4 | • | 85.2 | • | 97.1 | • |
| 61 | C7H15 | • | 84.8 | • | 86.6 | — | — | • | 90.2 | • |
| 62 | C8H17 | • | 60.8 | • | 95.7 | — | — | — | — | • |
| 63 | C9H19 | • | 63.5 | • | 92.2 | — | — | — | — | • |
| 64 | C10H21 | • | 61.0 | • | 92.6 | — | — | — | — | • |
| 65 | C12H25 | • | 78.5 | • | 86.6 | — | — | — | — | • |
The behaviour of the phenyl 4-alkylbiphenyl-4′-carboxylate materials (Table 13) is largely mirrored by the analogous phenyl trans 4-(4-alkylcycohexyl)benzoate dimers (Table 14), with the ethyl- and propy-terminated materials (75 and 76) exhibiting both nematic and NTB mesophases, and the butyl- and pentyl-terminated homologues exhibiting nematic and SmCA phases (77 and 78). However, the trans, trans 4-(4-alkylcycohexyl)cyclohexylcarboxylate dimers (Table 15) differ significantly in terms of their liquid-crystalline behaviour; all materials (79–82) exhibit nematic and smectic B mesophases, with butyl and pentyl terminated derivatives also exhibiting additional smectic phases.
When studied by small-angle X-ray scattering, the various smectic phases of 71–82 were found to be intercalated, that is, the layer spacing is roughly one half of the all trans molecular length regardless of the mesophase.106 Comparing the behaviour of 71–82. The NTB phase becomes less stable in comparison to intercalated smectic phases (both SmCA and SmB) as the proportionality of σ to π-bonded structure is increased, mirroring the trends seen for conventional calamitic materials.107
3.2. Dimers with other linking groups
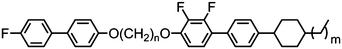
As shown in Table 16, and depending on the parity of the central spacer as well as the terminal chain length, the so-called FBOnODFCBm materials exhibit twist-bend or smectic A mesophases, the latter being identified as the subtype A2 by SAXS and the NTB phase being identified unequivocally by miscibility studies with CB11CB.79 Compound 85 remains the only example to date of a material exhibiting a twist-bend nematic to smectic A phase transition, with this polymorphism being extremely sensitive to even small changes in molecular structure; any increase or decrease in the length of the terminal chain results in the loss of the NTB phase. In all cases, replacement of the 4-fluorobiphenyl mesogenic unit with a 4-cyanobiphenyl and several fluorinated biphenyls was found to suppress the formation of both the ‘X’ and SmA2 phases exhibited by 85 (Table 17). However, in all cases the nematic phase was retained, while all but 90 and 91 still exhibit the NTB phase.79,82
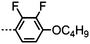
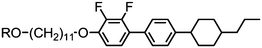
Sebastián et al. presented a thorough reinvestigation into 95 (FFO9OCB) and demonstrated that this material exhibits a twist-bend nematic phase, which had not been reported in previous works concerning this material.59,109,110 Analysis of the dielectric behaviour of 95 according to the model of Stocchero et al.111 suggests that the molecules are almost entirely (94% at TN–I, 98% at TN–I – 45 °C) in their all trans conformation. The octamethylene homologue 96 (FFO8OCB) exhibits significantly higher melting and clearing points and does not exhibit the NTB phase,112 similar behaviour can be seen for even members of the CBnCB series. Later, the analogous 97 (FFO11OCB) was also found to exhibit a twist-bend nematic phase,79 with the transition temperatures being only marginally different to the nonamethylene material initially reported by Sebastián et al. It is possible, if not probable, that other members of the FFOnOCB series of materials will be found to exhibit the NTB phase (Table 18).113
Replacement of the 2,4′-difluorobiphenyl unit employed in 97 (FFO11OCB) with other fluorinated biphenyls (Table 19) has some impact on the transition temperatures, with all but 101 exhibiting the nematic and nematic twist-bend phases. Clearly there are a significant number of combinations of mesogenic unit and spacer length that can give rise to the twist-bend phase in ether-linked materials.
| No | Name | n | m | Cr | MX | Colrec | Ncol | N | Iso | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 102 | 5-4 | 4 | 5 | • | 106 | — | — | — | — | — | — | (• | 102) | • |
| 103 | 5-6 | 6 | 5 | • | 113 | — | — | — | — | (• | 87 | • | 97) | • |
| 104 | 5-8 | 8 | 5 | • | 103 | — | — | (• | 99) | — | — | (• | 102) | • |
| 105 | 5-10 | 10 | 5 | • | 105 | — | — | • | 111 | — | — | — | — | • |
| 106 | 5-12 | 12 | 5 | • | 107 | — | — | • | 116 | — | — | — | — | • |
| 107 | 5-14 | 14 | 5 | • | 105 | • | 114 | • | 120 | — | — | — | — | • |
| 108 | 7-4 | 4 | 7 | • | 119 | — | — | — | — | — | • | 121 | • | |
| 109 | 7-6 | 6 | 7 | • | 98 | — | — | — | — | (• | 93) | • | 113 | • |
| 110 | 7-8 | 8 | 7 | • | 103 | — | — | — | — | — | — | • | 110 | • |
| 111 | 7-10 | 10 | 7 | • | 97 | — | — | • | 106 | — | — | • | 108 | • |
| 112 | 7-12 | 12 | 7 | • | 100 | — | — | • | 112 | — | — | — | — | • |
| 113 | 7-14 | 14 | 7 | • | 99 | — | — | • | 117 | — | — | — | — | • |
Šepelj et al. later reported a homologous series of symmetrical, imine-linked phenyl 4-alkoxybenzoate dimers (Table 21), the majority of which exhibited the columnar-like B6′ mesophase. Compounds 114 (5-4) and 120 (7-4), which have the shortest terminal alkoxy chains in the series, also exhibit an additional nematic phase, with 120 exhibiting a nematic to nematic (termed NX in ref. 92) transition. Ivšić et al. revisited compound 120 and identified the lower temperature nematic phase as the NTB phase,104 although on the basis of a 1H–1H NOESY experiment they suggest an alternate structural model for this phase featuring local domains of syn packed dimers. The identification of the NTB phase in compounds 114 and 120 supports the idea that the lower-temperature nematic phase exhibited by the structurally similar materials in Table 20 is the NTB rather than a columnar nematic phase.
| No | Name | n | m | Cr | B6′ | NTB | N | Iso | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 114 | 5-4 | 4 | 5 | • | 114.0 | (• | 99.1 | — | — | • | 102.0) | • |
| 115 | 5-6 | 6 | 5 | • | 123.5 | (• | 116.9) | — | — | — | — | • |
| 116 | 5-8 | 8 | 5 | • | 94.2 | • | 121.0 | — | — | — | — | • |
| 117 | 5-10 | 10 | 5 | • | 88.5 | • | 109.5 | — | — | — | — | • |
| 118 | 5-12 | 12 | 5 | • | 96.2 | • | 96.1 | — | — | — | — | • |
| 119 | 5-14 | 14 | 5 | • | 101.2 | — | — | — | — | — | — | • |
| 120 | 7-4 | 4 | 7 | • | 112.2 | (• | 84.4 | • | 96.6) | • | 115.0 | • |
| 121 | 7-6 | 6 | 7 | • | 96.4 | • | 114.7 | — | — | — | — | • |
| 122 | 7-8 | 8 | 7 | • | 111.2 | • | 119.5 | — | — | — | — | • |
| 123 | 7-10 | 10 | 7 | • | 100.1 | • | 110.1 | — | — | — | — | • |
| 124 | 7-12 | 12 | 7 | • | 90.9 | • | 99.3 | — | — | — | — | • |
| 125 | 7-14 | 14 | 7 | • | 73.3 | — | — | — | — | — | — | • |
Incorporation of a naphthyl unit in place of the outermost phenyl ring of the materials presented in Tables 20 and 21 affords 126–129 (Table 22). In all cases the clearing points and melting points have increased relative to the parent phenyl materials as a consequence of the now increased aspect ratio of the individual mesogenic units. Only one material (128) was found to exhibit the twist-bend nematic phase, with others exhibiting B6 and rectangular columnar mesophases. Materials with even spacer parity (i.e. m = 6, m = 8) were also prepared and found to exhibit both modulated and intercalated smectic A and C mesophases.
Knowing that shorter terminal chains favour nematic and NTB mesophases Dawood et al. reported the synthesis and characterisation of five symmetrical, imine linked phenyl 4-ethoxybenzoate dimers with varying central spacer length (Table 23). The homologue with the shortest central spacer (130, 2O-3-O2) exhibits a direct isotropic to twist-bend nematic phase transition, this being the first observation of this transition in a pure compound, which had first been observed in a binary mixture.70
Three symmetrical dimers with cyanobiphenyl mesogenic units and imine linking groups have been reported. For these compounds there are sizable increases in the melting point, clearing point and N–NTB transition temperature when compared to the analogous CBnCB materials can be directly attributed to the change in conformational distribution that results from the incorporation of an imine (Table 24).104,106,115
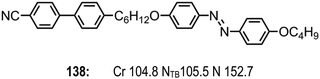 | ||
| Fig. 9 The molecular structure and transition temperature of 138 (CB6OABOBu), reported by Paterson et al.80 | ||
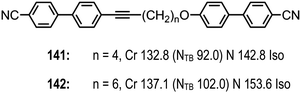
Replacement of a single methylene unit in CB7CB to afford 139 (CB6OCB) or CB9CB to afford 140 (CB8OCB) leads to significant increases in both melting and clearing points, with a small increase in the nematic to twist-bend nematic transition temperature (Table 25). For 139 the helical pitch was measured to be 8.9 nm by FFTEM, which is close to the structurally related CB7CB (∼8 nm),116 however for 140 such data has not been reported. For both materials the clearing points and nematic to NTB transition temperatures are essentially identical.
A dimethylene group has some degree of flexibility whereas an alkyne is rigid and the replacement of the first two methylene units of compounds 139 and 140 with an alkyne affords compounds 141 and 142 respectively (Fig. 10), the latter having significantly increased melting points compared to those of the parent dimethylene materials. For 141 the clearing point and N–NTB transition temperature are significantly reduced when compared to 139, while for 142 there is only a marginally reduced N–NTB transition temperature with the clearing point being essentially identical to that of 140. The large difference in the N–NTB transition temperatures of compounds 141 and 142, which is not present in the analogous 139 and 140, indicates that the flexibility of the spacer is significant – the inflexible alkyne occupying a greater proportion of the spacer length in 141 than in 142. This apparent sensitivity of the twist-bend nematic phase to the flexibility of the spacer supports the observation that the NTB phase is relatively common in mesogenic dimers and relatively rare in bent-core liquid crystals, the latter being typically regarded as rigid.31
3.3. Bent core systems
A series of asymmetric, rigid bent-core materials with a central phenyl piperazine group were prepared by Schroder et al.,118 with shorter homologues exhibiting a nematic–nematic phase transition. Later, compound 145 was revisited and the second nematic phase identified as being the NTB phase, the helical pitch (PTB) was measured by FFTEM to be 14 nm – larger than CB7CB, but still extremely short.19 Based on this it seems likely that the lower temperature nematic phase exhibited by compounds 143 and 144 is also the NTB phase. To date these, along with compound 153 (Fig. 11), are the only known examples of bent-core materials that exhibit the twist-bend nematic phase, although others have been suggested previously (Table 26).119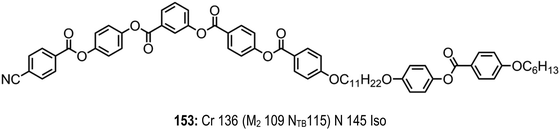 | ||
| Fig. 11 Chemical structure and transition temperatures of the hybrid bent-core/calamitic dimer reported by Tamba et al.120 Phase transitions are presented in parenthesis are monotropic. | ||
| No | n | Cr | SmCP | ColX | NX/NTB | N | Iso | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 143 | 4 | • | 201 | — | — | — | — | (• | 193) | • | 212 | • |
| 144 | 5 | • | 187 | — | — | — | — | (• | 172) | • | 192 | • |
| 145 | 6 | • | 176 | — | — | (• | 157 | • | 169) | • | 188 | • |
| 146 | 7 | • | 169 | — | — | — | — | — | — | • | 186 | • |
| 147 | 8 | • | 177 | • | 180 | — | — | — | — | • | 180.5 | • |
| 148 | 9 | • | 167 | • | 185 | — | — | — | — | — | — | • |
| 149 | 10 | • | 162 | • | 191 | — | — | — | — | — | — | • |
| 150 | 11 | • | 161 | • | 194 | — | — | — | — | — | — | • |
| 151 | 12 | • | 165 | • | 201 | — | — | — | — | — | — | • |
| 152 | 16 | • | 142 | • | 193 | — | — | — | — | — | — | • |
Tamba et al. reported a hybrid, ether-linked bent-core/calamitic dimer that exhibited the twist-bend nematic phase as well as an unidentified ‘M2’ mesophase.120 While variants featuring other spacer lengths (trimethylenoxy or hexamethylenoxy) or a dodecyloxy terminal chain in lieu of the nitrile unit employed in 153 were still liquid-crystalline, these no longer exhibit the twist-bend nematic phase.
3.4. Chiral systems
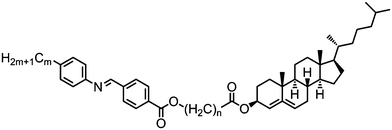
Several unsymmetrical, ester-linked cholesterol/N-phenyl-4-alkylbenzimine bimesogens with odd-spacer parity were found to form nematic and twist-bend nematic phases, with even-parity homologues being solely nematogenic (i.e. they only exhibit a nematic phase) or exhibiting an additional smectic A phase.The mesophase behaviour of compounds 154–161 is more complex than depicted in Table 27; while on heating the materials exhibit chiral nematic phases on cooling blue phases are seen instead, leading to direct BP–NTB phase transitions. In the case of compound 161 a total of six distinct phase transitions are seen by DSC over a 2 °C temperature range at the N*–NTB phase transition (Fig. 12). The NTB pitch length of 154 was measured by AFM and reported to be 50 nm; significantly larger than that reported for CB7CB, but despite this difference in pitch, these two materials were found to be miscible at all concentrations.54,96
| No | Name | n | m | Cr | SmA | NTB | N* | Iso | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 154 | SB 1/3 | 3 | 1 | • | 112.3 | — | — | (• | 55.1 | • | 67.7) | • |
| 155 | SB 1/4 | 4 | 1 | • | 127.9 | • | 146.9 | — | — | • | 191.8 | • |
| 156 | SB 1/5 | 5 | 1 | • | 92.5 | — | — | (• | 67.9) | • | 102.3 | • |
| 157 | SB 1/6 | 6 | 1 | • | 152.3 | (• | 120.0) | — | — | • | 158.4 | • |
| 158 | SB 1/7 | 7 | 1 | • | 83.0 | — | — | (• | 75.7) | • | 110.8 | • |
| 159 | SB 1/9 | 9 | 1 | • | 81.2 | — | — | (• | 75.7) | • | 113.6 | • |
| 160 | SB 1/10 | 10 | 1 | • | 82.7 | — | — | — | — | • | 137.5 | • |
| 161 | SB 1/15 | 15 | 1 | • | 62.9 | — | — | (• | 63.2) | • | 107.9 | • |
| 162 | SB 2/5 | 5 | 2 | • | 74.0 | — | — | (• | 60.6) | • | 93.6 | • |
| 163 | SB 3/5 | 5 | 3 | • | 77.5 | — | — | (• | 62.2) | • | 98.8 | • |
| 164 | SB 4/5 | 5 | 4 | • | 44.2 | • | 71.9 | — | — | • | 99.3 | • |
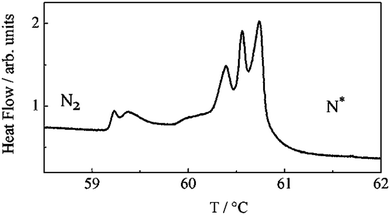 | ||
| Fig. 12 High-resolution DSC thermogram for 161, showing multiple phase transitions between the N* and N2 (NTB) mesophases. Reproduced from ref. 96 with permission of the Royal Society of Chemistry. | ||
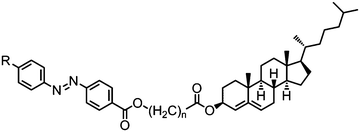
In general, azo linked materials (Table 28) have lower melting points than the analogous imine linked materials (e.g.156/165, 158/168, 159/169 and 161/170), while the clearing points and N–NTB transitions remain approximately the same. In the case of compound 170 a transition from the nematic into an unknown smectic mesophase (SmX) was reported, whereas the parent imine material exhibits a twist-bend nematic phase. The photochemical behaviour of these materials was not commented upon; however, it would appear reasonable to speculate that the photo-isomerisable azo linkage will result in a isothermal twist-bend nematic phase transition.80
| No | Name | n | R | Cr | SmX | NTB | N | Iso | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 165 | Azo 1/5 | 5 | –CH3 | • | 83.4 | — | — | (• | 67.1) | • | 105.5 | • |
| 166 | Azo O1/5 | 5 | –OCH3 | • | 84.3 | — | — | (• | 80.8) | • | 124.9 | • |
| 167 | Azo O2/5 | 5 | –OC2H5 | • | 107.0 | (• | 97.6) | — | — | • | 134.4 | • |
| 168 | Azo 1/7 | 7 | –CH3 | • | 63.9 | — | — | • | 74.0 | • | 111.5 | • |
| 169 | Azo 1/9 | 9 | –CH3 | • | 69.6 | — | — | • | 75.8 | • | 113.6 | • |
| 170 | Azo 1/15 | 15 | –CH3 | • | 60.5 | (• | 60.3) | — | — | • | 100.3 | • |
Archibald et al. constructed a binary phase diagram between the dimer 58 and the chiral dopant BDH1281 (Fig. 13), at and above a dopant concentration of 5.5 wt% a direct isotropic to NTB phase transition was observed.43 Additionally at and above 5.2 wt% of BDH1281 a transition from the twist-bend nematic to a lower temperature mesophase lacking lamellar order (hence NX) was also observed; the possibility of this mesophase being a splay-bend nematic was hinted at, but this is speculative and lacking experimental confirmation presently, perhaps by small angle or grazing incidence X-ray scattering as suggested by Zhu.50 The dopant/dimer mixtures also exhibit an apparent phase transition between two isotropic liquids which was postulated to be due to the formation of supermolecular aggregates.70 Mixtures containing below 5.5 wt% of the chiral dopant exhibit wide temperature range (>5 °C) blue phases in analogy to those reported previously for mixtures of bimesogens and the dopant BDH1281 (Table 29).121
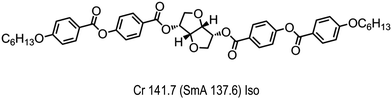 | ||
| Fig. 13 Molecular structure and transition temperatures (°C) of the dianhydro-D-glucitol derived chiral dopant (BDH1281) used by Archibald et al.70 Monotropic phase transitions are presented in parenthesis. | ||
| Wt% BDH1281 | Cr | NX | NTB | N* | BPIII | Iso1 | Iso2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Note, the nematic phase exhibited in the absence of a chiral dopant is achiral. Monotropic phase transitions are presented in parenthesis. | |||||||||||||
| 0a | • | 83.3 | — | — | • | 84.7 | • | 99.5 | — | — | • | — | — |
| 0.6 | • | 80.9 | — | — | • | 82.2 | • | 83.4 | • | 95.3 | • | 98.6 | • |
| 2.1 | • | 80.5 | — | — | • | 82.4 | • | 82.5 | • | 95.3 | • | 98.4 | • |
| 4.7 | • | 79.5 | — | — | • | 82.1 | • | 82.3 | • | 91.6 | • | 98.1 | • |
| 5.2 | • | 79.5 | (• | 62.1) | • | 83.5 | • | 83.6 | • | 92.7 | • | 97.3 | • |
| 5.4 | • | 79.5 | (• | 66.1) | • | 83.3 | • | 83.4 | • | 88.6 | • | 96.6 | • |
| 5.5 | • | 79.4 | (• | 67.2) | • | 83.2 | — | — | — | — | • | 96.8 | • |
| 6.5 | • | 79.3 | (• | 66.4) | • | 83.4 | — | — | — | — | • | 96.4 | • |
| 7.1 | • | 79.4 | (• | 67.2) | • | 82.3 | — | — | — | — | • | 96.4 | • |
| 8.4 | • | 79.1 | (• | 68.3) | • | 81.9 | — | — | — | — | • | 95.3 | • |
| 9.8 | • | 79.2 | (• | 69.6) | • | 79.2 | — | — | — | — | • | 96.5 | • |
3.5. Oligomers: trimers and tetramers
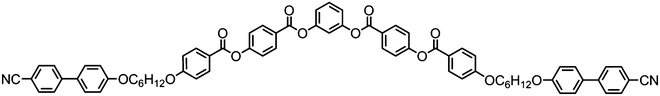
Liquid-crystalline trimers are aptly named, being molecular entities comprised of three interconnected mesogenic units.122,123 The manner of the attachment need not be linear,124 and the mesogenic units are not strictly limited to rod-like groups.125 Liquid-crystalline tetramers can be defined as discrete molecules containing four interconnected mesogenic units. Whereas there are numerous examples of liquid-crystalline trimers, relatively few tetramers have been reported.126–129 Linear higher oligomers pose synthetic challenges, chiefly as efficient chromatographic purification is challenging, however some examples of pentamers exist.130,131In the context of the twist-bend nematic phase relatively few oligomeric materials are known to exhibit this state of matter. The hydrogen-bonded trimer formed spontaneously by 172 (CB6OBA, Table 30) was the first trimeric twist-bend nematogen to be reported; the analogue with even spacer parity, 171, (CB5OBA) does not exhibit the twist-bend nematic phase. As the carboxylic acid group contains both a hydrogen bond donor and acceptor both ‘open’ and ‘closed’ H-bonded structures are possible. Variable temperature FT-IR has been used to examine the dynamic equilibrium that exists between free acid, ‘open’ dimers with one H-bond and ‘closed’ dimers with two H-bonds.93,132
As far as we are aware, the hybrid calamitic/bent core trimer 173 (Table 31) is only the second example of a twist-bend nematic material where the prerequisite bent shape arises not from a flexible spacer but rather a rigid unit (the other being 145). Values of the orientational order parameters 〈P2〉 and 〈P4〉 of 173 were determined by SAXS using the method of Davidson,133 which is incidentally known to be flawed,134 with the behaviour as a function of temperature across both the nematic and twist-bend nematic phases being to be consistent with those later obtained by PRS by Zhang et al. for the CBnCB materials.55 The d-spacing of the diffuse small angle peak in both the nematic and NTB phases was approximately one third of the molecular length, indicating that both phases are intercalated. The pitch of the NTB phase of 173 was determined by FFTEM according to the method used by Chen et al.,48 giving a value of ∼19 nm which corresponds to approximately four molecular lengths.49 When one of the cyanobiphenyl units is replaced with a decyloxy chain the resulting material exhibits nematic and biaxial smectic A mesophases, but not the twist-bend nematic.135
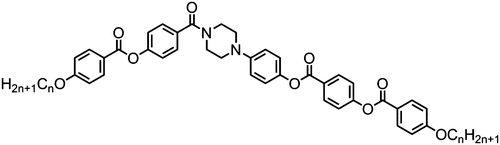
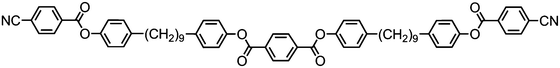
The symmetrical, linear trimer 174 (Table 32) exhibits nematic and twist-bend nematic mesophases, as does the related tetrameric compound 176 (T49, Table 33).77,95 When studied by SAXS the diffuse small angle scattering peak observed in both the nematic and NTB phases occurs at roughly 0.33 molecular lengths for 174 and 0.25 molecular lengths for 176 (58.0 Å and 62.8 Å respectively),95 confirming that for both materials the lower temperature mesophase is not lamellar but nematic as well as demonstrating that the mesophases are extensively intercalated, as was reported by Wang et al. for the trimer 173.49 The associated enthalpy of the NTB–N transition is typically much smaller than the N–Iso transition, and this is also observed for the trimer 175. In the case of the tetramer 176 the associated enthalpy of the NTB–N transition is somewhat larger than that of the clearing point, with both transitions being strongly first-order.
The tetramer 177 was also found to exhibit nematic and twist-bend nematic mesophases. Unlike 176 this material lacks strongly polar terminal groups, instead having butoxy chains, while the spacers used are slightly shorter (C7vs. C9). Additionally the two ‘central’ mesogenic units have different aspect ratios to the ‘outer’ units. When studied by SAXS, as with other oligomeric twist-bend materials, the diffuse small angle scattering peak that corresponds to the molecular length is approximately 1/4th of the molecular length, demonstrating that both the nematic and NTB phases are extensively intercalated, while the lack of sharp peaks confirms the two mesophases are not lamellar. It seems reasonable to conclude that many combinations of terminal chain length, central spacer length and mesogenic unit composition will give rise to the twist-bend nematic phase in oligomeric materials (Table 34).
4. Summary and outlook
The experimental observation of the predicted twist-bend nematic phase has led to a renewed interest in liquid-crystalline dimers. The number of materials known to exhibit a twist-bend nematic phase has increased significantly since the experimental discovery of this state of matter, with around 90 materials, many of which were prepared by the materials group at York, now known to exhibit this mesophase. Several examples of twist-bend nematic phases formed by lower oligomers (trimers and tetramers), and there is every reason to believe that examples of higher oligomers and polymers that generate the NTB phase will be found. Indeed, the lower temperature nematic phase observed in copolymers exhibiting a nematic–nematic phase transition has been suggested to be a twist-bend nematic phase.137 The synthesis of the tetramer 177 is performed in such a way that the individual mesogenic units are installed one-by-one, meaning that the synthesis of higher oligomers of analogous molecular structure may be achievable. A general and definitive structure property relationship remains elusive; however, a gross bent molecular shape is seemingly required and the molecular flexibility appears important as well. The incidence of the twist-bend nematic phase is not especially dependent on combinations of functional groups etc., provided the prerequisite bent shape is satisfied.The largest sub-group to exhibit the twist-bend nematic phase are methylene linked with a nonamethylene spacer and mesogenic units constructed of two rigid cyclic units, with around 40 materials known in total. For these materials that there is linear relationship between the N–I transition temperature (TN–I) and the NTB–N transition temperature (TN–NTB, plot given in Fig. 14). This plot contains materials having both polar and non-polar terminal groups as well as both symmetric and unsymmetrical dimers. It must also be noted that in the case of materials having terminal alkyl/alkoxy chains the length of these groups has, apparently, no significant influence on the relationship between TN–I and TN–NTB. Analogous materials with different spacer lengths or different aspect ratios for the individual mesogenic units yield different slopes, however the liner relationship is retained. In essence, the temperature at which the nematic or twist-bend nematic phase transition occurs are dependent on one another and are therefore somewhat independent of molecular structure, i.e. the exact values of each phase transition depend on the chemical makeup of a given material but the relationship between these transitions does not.
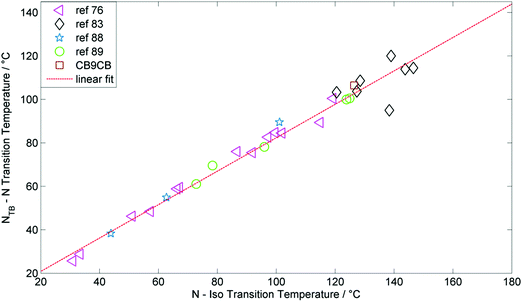 | ||
| Fig. 14 Plot of the nematic to isotropic transition temperature (°C) versus the twist-bend nematic to nematic transition temperature (°C) for dimers and bimesogens with ‘two-ring’ mesogenic units and nonamethylene spacer. The R2 value of the linear fit is 0.975. Data was taken from ref. 55, 76, 83, 94 and 95. | ||
Transitions from the twist-bend nematic to smectic phases are rare, although examples exist, and it is worth speculating as to whether or not re-entrant NTB phases can exist; examples of both re-entrant nematic and re-entrant smectic phases in dimers have been reported,138,139 but to date such behaviour has not been observed for the NTB phase.
Acknowledgements
I would like to thank Professor John Goodby FRS, Dr Edward Davis, Dr Stephen Cowling, Dr Mark Sims and Mr Craig Archbold for many useful discussions concerning dimers and oligomers in the context of the NTB phase, as well as for assistance in preparing this article. I would also like to extend this gratitude to my current and former students, namely Constantin Voll, Sam Lobato, Dan Lewis, Phillip Foeller, Jess Andrews, Frank Simpson and Matthew Stevens, for undertaking projects work on dimers and twist-bend nematics.References
- A. C. Neville and B. M. Luke, J. Cell Sci., 1971, 8, 93–109 CAS.
- Y. M. Yevdokimov, S. G. Skuridin and V. I. Salyanov, Liq. Cryst., 1988, 3, 1443–1459 CrossRef.
- F. Livolant, A. M. Levelut, J. Doucet and J. P. Benoit, Nature, 1989, 339, 724–726 CrossRef CAS PubMed.
- F. P. Booy, W. W. Newcomb, B. L. Trus, J. C. Brown, T. S. Baker and A. C. Steven, Cell, 1991, 64, 1007–1015 CrossRef CAS PubMed.
- Z. Reich, E. J. Wachtel and A. Minsky, Science, 1994, 264, 1460–1463 CAS.
- S. Mangenot, A. Leforestier, D. Durand and F. Livolant, J. Mol. Biol., 2003, 333, 907–916 CrossRef CAS PubMed.
- P. Bault, P. Gode, G. Goethals, J. W. Goodby, J. A. Haley, S. M. Kelly, G. H. Mehl, G. Ronco and P. Villa, Liq. Cryst., 1998, 25, 31–45 CrossRef CAS.
- J. W. Goodby, Liq. Cryst., 2006, 33, 1229–1237 CrossRef CAS.
- R. Xu, F. Ali-Rachedi, N. M. Xavier, S. Chambert, F. Ferkous, Y. Queneau, S. J. Cowling, E. J. Davis and J. W. Goodby, Org. Biomol. Chem., 2015, 13, 783–792 CAS.
- J. Watanabe, Y. Fukuda, R. Gehani and I. Uematsu, Macromolecules, 1984, 17, 1004–1009 CrossRef CAS.
- J. Watanabe, H. Ono, I. Uematsu and A. Abe, Macromolecules, 1985, 18, 2141–2148 CrossRef CAS.
- E. Iizuka, S. Inoue, K. Hanabusa and H. Shirai, Mol. Cryst. Liq. Cryst., 1987, 149, 61–77 CrossRef CAS.
- S. Fraden, G. Maret, D. L. D. Caspar and R. B. Meyer, Phys. Rev. Lett., 1989, 63, 2068–2071 CrossRef CAS PubMed.
- S. Fraden, G. Maret and D. L. D. Caspar, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1993, 48, 2816–2837 CrossRef CAS.
- S. Zhou, A. Sokolov, O. D. Lavrentovich and I. S. Aranson, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1265–1270 CrossRef CAS PubMed.
- I. Dozov, Europhys. Lett., 2001, 56, 247–253 CrossRef CAS.
- M. Cestari, S. Diez-Berart, D. A. Dunmur, A. Ferrarini, M. R. de la Fuente, D. J. B. Jackson, D. O. Lopez, G. R. Luckhurst, M. A. Perez-Jubindo, R. M. Richardson, J. Salud, B. A. Timimi and H. Zimmermann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031704 CrossRef CAS PubMed.
- V. Borshch, Y. K. Kim, J. Xiang, M. Gao, A. Jakli, V. P. Panov, J. K. Vij, C. T. Imrie, M. G. Tamba, G. H. Mehl and O. D. Lavrentovich, Nat. Commun., 2013, 4, 1–8 Search PubMed.
- D. Chen, M. Nakata, R. F. Shao, M. R. Tuchband, M. Shuai, U. Baumeister, W. Weissflog, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89, 022506 CrossRef PubMed.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767–768 CrossRef CAS.
- V. Avetisov and V. Goldanskii, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 11435–11442 CrossRef CAS.
- S. J. Sowerby, W. M. Heckl and G. B. Petersen, J. Mol. Evol., 1996, 43, 419–424 CrossRef CAS PubMed.
- M. Bolli, R. Micura and A. Eschenmoser, Chem. Biol., 1997, 4, 309–320 CrossRef CAS PubMed.
- J. Bailey, A. Chrysostomou, J. H. Hough, T. M. Gledhill, A. McCall, S. Clark, F. Menard and M. Tamura, Science, 1998, 281, 672–674 CrossRef CAS PubMed.
- C. Viedma, Astrobiology, 2007, 7, 312–319 CrossRef CAS PubMed.
- M. Alaasar, M. Prehm and C. Tschierske, Chem. – Eur. J., 2016, 22, 6583–6597 CrossRef CAS PubMed.
- H. Sasaki, Y. Takanishi, J. Yamamoto and A. Yoshizawa, Soft Matter, 2016, 12, 3331–3339 RSC.
- J. Sniechowska, P. Paluch, G. Bujacz, M. Gorecki, J. Frelek, D. T. Gryko and M. J. Potrzebowski, CrystEngComm, 2016, 18, 3561–3565 RSC.
- C. Tschierske and G. Ungar, ChemPhysChem, 2016, 17, 9–26 CrossRef CAS PubMed.
- D. R. Link, G. Natale, R. Shao, J. E. Maclennan, N. A. Clark, E. Korblova and D. M. Walba, Science, 1997, 278, 1924–1927 CrossRef CAS PubMed.
- R. A. Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907–961 RSC.
- H. Takezoe and Y. Takanishi, Jpn. J. Appl. Phys., Part 1, 2006, 45, 597–625 CrossRef CAS.
- L. Beguin, J. W. Emsley, M. Lelli, A. Lesage, G. R. Luckhurst, B. A. Timimi and H. Zimmermann, J. Phys. Chem. B, 2012, 116, 10407 CrossRef CAS.
- J. W. Emsley, M. Lelli, A. Lesage and G. R. Luckhurst, J. Phys. Chem. B, 2013, 117, 6547–6557 CrossRef CAS PubMed.
- J. W. Emsley, P. Lesot, G. R. Luckhurst, A. Meddour and D. Merlet, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 040501 CrossRef PubMed.
- C. Greco, G. R. Luckhurst and A. Ferrarini, Phys. Chem. Chem. Phys., 2013, 15, 14961–14965 RSC.
- H. Takezoe, Top. Curr. Chem., 2012, 318, 303–330 CrossRef CAS PubMed.
- A. Yoshizawa, Y. Kato, H. Sasaki, Y. Takanishi and J. Yamamoto, Soft Matter, 2015, 11, 8827–8833 RSC.
- A. Yoshizawa, Y. Kato, H. Sasaki, Y. Takanishi and J. Yamamoto, J. Phys. Chem. B, 2016, 120, 4843–4851 CrossRef CAS PubMed.
- C. Greco, G. R. Luckhurst and A. Ferrarini, Soft Matter, 2014, 10, 9318–9323 RSC.
- C. Greco and A. Ferrarini, Phys. Rev. Lett., 2015, 115, 147801 CrossRef PubMed.
- C. Meyer and I. Dozov, Soft Matter, 2016, 12, 574–580 RSC.
- A. G. Vanakaras and D. J. Photinos, Soft Matter, 2016, 12, 2208–2220 RSC.
- D. O. Lopez, B. Robles-Hernandez, J. Salud, M. R. de la Fuente, N. Sebastian, S. Diez-Berart, X. Jaen, D. A. Dunmur and G. R. Luckhurst, Phys. Chem. Chem. Phys., 2016, 18, 4394–4404 RSC.
- K. S. Krishnamurthy, P. Kumar, N. B. Palakurthy, C. V. Yelamaggad and E. G. Virga, Soft Matter, 2016, 12, 4967–4978 RSC.
- S. A. Pardaev, S. M. Shamid, M. G. Tamba, C. Welch, G. H. Mehl, J. T. Gleeson, D. W. Allender, J. V. Selinger, B. Ellman, A. Jakli and S. Sprunt, Soft Matter, 2016, 12, 4472–4482 RSC.
- C. T. Imrie and P. A. Henderson, Chem. Soc. Rev., 2007, 36, 2096–2124 RSC.
- D. Chen, J. H. Porada, J. B. Hooper, A. Klittnick, Y. Q. Shen, M. R. Tuchband, E. Korblova, D. Bedrov, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15931–15936 CrossRef CAS PubMed.
- Y. Wang, G. Singh, D. M. Agra-Kooijman, M. Gao, H. K. Bisoyi, C. M. Xue, M. R. Fisch, S. Kumar and Q. Li, CrystEngComm, 2015, 17, 2778–2782 RSC.
- C. H. Zhu, M. R. Tuchband, A. Young, M. Shuai, A. Scarbrough, D. M. Walba, J. E. Maclennan, C. Wang, A. Hexemer and N. A. Clark, Phys. Rev. Lett., 2016, 116, 147803 CrossRef PubMed.
- C. Meyer, G. R. Luckhurst and I. Dozov, Phys. Rev. Lett., 2013, 111, 067801 CrossRef CAS PubMed.
- C. Meyer, G. R. Luckhurst and I. Dozov, J. Mater. Chem. C, 2015, 3, 318–328 RSC.
- J. P. Jokisaari, G. R. Luckhurst, B. A. Timimi, J. F. Zhu and H. Zimmermann, Liq. Cryst., 2015, 42, 708–721 CAS.
- E. Gorecka, N. Vaupotic, A. Zep, D. Pociecha, J. Yoshioka, J. Yamamoto and H. Takezoe, Angew. Chem., Int. Ed., 2015, 54, 10155–10159 CrossRef CAS PubMed.
- Z. P. Zhang, V. P. Panov, M. Nagaraj, R. J. Mandle, J. W. Goodby, G. R. Luckhurst, J. C. Jones and H. F. Gleeson, J. Mater. Chem. C, 2015, 3, 10007–10016 RSC.
- P. J. Barnes, A. G. Douglass, S. K. Heeks and G. R. Luckhurst, Liq. Cryst., 1993, 13, 603–613 CrossRef CAS.
- D. O. Lopez, N. Sebastian, M. R. de la Fuente, J. C. Martinez-Garcia, J. Salud, M. A. Perez-Jubindo, S. Diez-Berart, D. A. Dunmur and G. R. Luckhurst, J. Chem. Phys., 2012, 137, 034502 CrossRef CAS PubMed.
- R. R. R. de Almeida, C. Zhang, O. Parri, S. N. Sprunt and A. Jakli, Liq. Cryst., 2014, 41, 1661–1667 CrossRef.
- N. Sebastian, D. O. Lopez, B. Robles-Hernandez, M. R. de la Fuente, J. Salud, M. A. Perez-Jubindo, D. A. Dunmur, G. R. Luckhurst and D. J. B. Jackson, Phys. Chem. Chem. Phys., 2014, 16, 21391–21406 RSC.
- B. Robles-Hernandez, N. Sebastian, M. R. de la Fuente, D. O. Lopez, S. Diez-Berart, J. Salud, M. B. Ros, D. A. Dunmur, G. R. Luckhurst and B. A. Timimi, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2015, 92, 062505 CrossRef PubMed.
- S. Parthasarathi, D. S. Rao, N. B. Palakurthy, C. V. Yelamaggad and S. Krishna Prasad, J. Phys. Chem. B, 2016, 120, 5056–5062 CrossRef CAS PubMed.
- K. Adlem, M. Copic, G. R. Luckhurst, A. Mertelj, O. Parri, R. M. Richardson, B. D. Snow, B. A. Timimi, R. P. Tuffin and D. Wilkes, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 022503 CrossRef CAS PubMed.
- S. M. Salili, C. Kim, S. Sprunt, J. T. Gleeson, O. Parri and A. Jakli, RSC Adv., 2014, 4, 57419–57423 RSC.
- J. W. Emsley, G. R. Luckhurst and G. N. Shilstone, Mol. Phys., 1984, 53, 1023–1028 CrossRef CAS.
- J. W. Emsley, G. R. Luckhurst, G. N. Shilstone and I. Sage, Mol. Cryst. Liq. Cryst., 1984, 102, 223–233 CrossRef CAS.
- R. J. Mandle, E. J. Davis, C. T. Archbold, S. J. Cowling and J. W. Goodby, J. Mater. Chem. C, 2014, 2, 556–566 RSC.
- A. Hoffmann, A. G. Vanakaras, A. Kohlmeier, G. H. Mehl and D. J. Photinos, Soft Matter, 2015, 11, 850–855 RSC.
- E. Gorecka, M. Salamonczyk, A. Zep, D. Pociecha, C. Welch, Z. Ahmed and G. H. Mehl, Liq. Cryst., 2015, 42, 1–7 CrossRef CAS.
- C. J. Yun, M. R. Vengatesan, J. K. Vij and J. K. Song, Appl. Phys. Lett., 2015, 106, 173102 CrossRef.
- C. T. Archbold, E. J. Davis, R. J. Mandle, S. J. Cowling and J. W. Goodby, Soft Matter, 2015, 11, 7547–7557 RSC.
- A. A. Dawood, M. C. Grossel, G. R. Luckhurst, R. M. Richardson, B. A. Timimi, N. J. Wells and Y. Z. Yousif, Liq. Cryst., 2016, 43, 2–12 CrossRef CAS.
- L. Beguin, J. W. Emsley, M. Lelli, A. Lesage, G. R. Luckhurst, B. A. Timimi and H. Zimmermann, J. Phys. Chem. B, 2012, 116, 7940–7951 CrossRef CAS PubMed.
- V. P. Panov, M. C. M. Varney, I. I. Smalyukh, J. K. Vij, M. G. Tamba and G. H. Mehl, Mol. Cryst. Liq. Cryst., 2015, 611, 180–185 CrossRef CAS.
- C. S. P. Tripathi, P. Losada-Perez, C. Glorieux, A. Kohlmeier, M. G. Tamba, G. H. Mehl and J. Leys, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 041707 CrossRef PubMed.
- V. P. Panov, M. Nagaraj, J. K. Vij, Y. P. Panarin, A. Kohlmeier, M. G. Tamba, R. A. Lewis and G. H. Mehl, Phys. Rev. Lett., 2010, 105, 167801 CrossRef CAS PubMed.
- R. J. Mandle, E. J. Davis, C. T. Archbold, C. C. A. Voll, J. L. Andrews, S. J. Cowling and J. W. Goodby, Chem. – Eur. J., 2015, 21, 8158–8167 CrossRef CAS PubMed.
- R. J. Mandle and J. W. Goodby, ChemPhysChem, 2016, 17, 967–970 CrossRef CAS PubMed.
- R. J. Mandle and J. W. Goodby, Soft Matter, 2016, 12, 1436–1443 RSC.
- R. J. Mandle, C. C. A. Voll, D. J. Lewis and J. W. Goodby, Liq. Cryst., 2016, 43, 13–21 CrossRef CAS.
- D. A. Paterson, J. Xiang, G. Singh, R. Walker, D. M. Agra-Kooijman, A. Martinez-Felipe, M. Gan, J. M. D. Storey, S. Kumar, O. D. Lavrentovich and C. T. Imrie, J. Am. Chem. Soc., 2016, 138, 5283–5289 CrossRef CAS PubMed.
- E. Ramou, Z. Ahmed, C. Welch, P. K. Karahaliou and G. H. Mehl, Soft Matter, 2016, 12, 888–899 RSC.
- R. J. Mandle, E. J. Davis, S. A. Lobato, C. C. A. Vol, S. J. Cowling and J. W. Goodby, Phys. Chem. Chem. Phys., 2014, 16, 6907–6915 RSC.
- R. J. Mandle, E. J. Davis, C. C. A. Voll, C. T. Archbold, J. W. Goodby and S. J. Cowling, Liq. Cryst., 2015, 42, 688–703 CAS.
- V. P. Panov, R. Balachandran, M. Nagaraj, J. K. Vij, M. G. Tamba, A. Kohlmeier and G. H. Mehl, Appl. Phys. Lett., 2011, 99, 261903 CrossRef.
- M. Mathews, R. S. Zola, D.-k. Yang and Q. Li, J. Mater. Chem., 2011, 21, 2098–2103 RSC.
- J. Xiang, Y. Li, Q. Li, D. A. Paterson, J. M. D. Storey, C. T. Imrie and O. D. Lavrentovich, Adv. Mater., 2015, 27, 3014–3018 CrossRef CAS PubMed.
- Y. Wang, Z.-g. Zheng, H. K. Bisoyi, K. G. Gutierrez-Cuevas, L. Wang, R. S. Zola and Q. Li, Mater. Horiz., 2016, 3, 442–446 RSC.
- H. K. Bisoyi and Q. Li, Acc. Chem. Res., 2014, 47, 3184–3195 CrossRef CAS PubMed.
- L. Wang and Q. Li, Adv. Funct. Mater., 2016, 26, 10–28 CrossRef CAS.
- Z.-g. Zheng, Y. Li, H. K. Bisoyi, L. Wang, T. J. Bunning and Q. Li, Nature, 2016, 531, 352–356 CrossRef CAS PubMed.
- M. Sepelj, A. Lesac, U. Baumeister, S. Diele, D. W. Bruce and Z. Hamersak, Chem. Mater., 2006, 18, 2050–2058 CrossRef CAS.
- M. Sepelj, A. Lesac, U. Baumeister, S. Diele, H. L. Nguyen and D. W. Bruce, J. Mater. Chem., 2007, 17, 1154–1165 RSC.
- D. A. Paterson, A. Martinez-Felipe, S. M. Jansze, A. T. M. Marcelis, J. M. D. Storey and C. T. Imrie, Liq. Cryst., 2015, 42, 928–939 CAS.
- Z. Ahmed, C. Welch and G. H. Mehl, RSC Adv., 2015, 5, 93513–93521 RSC.
- R. J. Mandle and J. W. Goodby, RSC Adv., 2016, 6, 34885–34893 RSC.
- A. Zep, S. Aya, K. Aihara, K. Ema, D. Pociecha, K. Madrak, P. Bernatowicz, H. Takezoe and E. Gorecka, J. Mater. Chem. C, 2013, 1, 46–49 RSC.
- S. M. Jansze, A. Martinez-Felipe, J. M. D. Storey, A. T. M. Marcelis and C. T. Imrie, Angew. Chem., Int. Ed., 2015, 54, 643–646 CAS.
- Z. B. Lu, P. A. Henderson, B. J. A. Paterson and C. T. Imrie, Liq. Cryst., 2014, 41, 471–483 CrossRef CAS.
- M. G. Tamba, S. M. Salili, C. Zhang, A. Jakli, G. H. Mehl, R. Stannarius and A. Eremin, RSC Adv., 2015, 5, 11207–11211 RSC.
- N. Sebastian, M. G. Tamba, R. Stannarius, M. R. de la Fuente, M. Salamonczyk, G. Cukrov, J. Gleeson, S. Sprunt, A. Jakli, C. Welch, Z. Ahmed, G. H. Mehl and A. Eremin, Phys. Chem. Chem. Phys., 2016 10.1039/c6cp03899a.
- P. A. Henderson, O. Niemeyer and C. T. Imrie, Liq. Cryst., 2001, 28, 463–472 CrossRef CAS.
- P. A. Henderson and C. T. Imrie, Liq. Cryst., 2011, 38, 1407–1414 CrossRef CAS.
- T. Ivsic, M. Vinkovic, U. Baumeister, A. Mikleusevic and A. Lesac, Soft Matter, 2015, 11, 6716 RSC.
- T. Ivsic, M. Vinkovic, U. Baumeister, A. Mikleusevic and A. Lesac, RSC Adv., 2016, 6, 5000–5007 RSC.
- K. J. Toyne, in Thermotropic Liquid Crystals, ed. G. W. Gray, Wiley, 1987, vol. 1, pp. 22–63 Search PubMed.
- R. J. Mandle and J. W. Goodby, Chemistry, 2016, 22, 9366–9374 CrossRef CAS PubMed.
- J. W. Goodby, E. J. Davis, R. J. Mandle and S. J. Cowling, Isr. J. Chem., 2012, 52, 863–880 CrossRef CAS.
- M. Cestari, E. Frezza, A. Ferrarini and G. R. Luckhurst, J. Mater. Chem., 2011, 21, 12303–12308 RSC.
- S. M. Morris, M. J. Clarke, A. E. Blatch and H. J. Coles, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 041701 CrossRef CAS PubMed.
- K. L. Atkinson, S. M. Morris, M. M. Qasim, F. Castles, D. J. Gardiner, P. J. W. Hands, S. S. Choi, W. S. Kim and H. J. Coles, Phys. Chem. Chem. Phys., 2012, 14, 16377–16385 RSC.
- M. Stocchero, A. Ferrarini, G. J. Moro, D. A. Dunmur and G. R. Luckhurst, J. Chem. Phys., 2004, 121, 8079–8097 CrossRef CAS PubMed.
- K. L. Atkinson, S. M. Morris, F. Castles, M. M. Qasim, D. J. Gardiner and H. J. Coles, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 85, 012701 CrossRef PubMed.
- H. J. Coles, M. J. Clarke, S. M. Morris, B. J. Broughton and A. E. Blatch, J. Appl. Phys., 2006, 99, 034104 CrossRef.
- M. Sepelj, U. Baumeister, T. Ivsic and A. Lesac, J. Phys. Chem. B, 2013, 117, 8918–8929 CrossRef CAS PubMed.
- R. Mandle and J. W. Goodby, presented in part at the BLCS conference, Edinburgh, UK, 21/3/2016, 2016.
- C. T. Imrie, D. A. Paterson, M. Gao, Y.-K. Kim, A. Jamali, K. Finley, D. O. Lopez, S. Diez-Berart, J. Salud, G. R. Luckhurst, O. D. Lavrentovich, J. M. D. Storey, M. R. de la Fuente, A. Ferrarini, C. Greco, H. Zimmermann, B. Timimi and B. Robles-Hernandez, Soft Matter, 2016 10.1039/C6SM00537C.
- F. R. Simpson and R. J. Mandle, unpublished results, 2016.
- M. W. Schroder, S. Diele, G. Pelzl, U. Dunemann, H. Kresse and W. Weissflog, J. Mater. Chem., 2003, 13, 1877–1882 RSC.
- V. Gortz, C. Southern, N. W. Roberts, H. F. Gleeson and J. W. Goodby, Soft Matter, 2009, 5, 463–471 RSC.
- M. G. Tamba, U. Baumeister, G. Pelzl and W. Weissflog, Ferroelectrics, 2014, 468, 52–76 CrossRef CAS.
- H. J. Coles and M. N. Pivnenko, Nature, 2005, 436, 997–1000 CrossRef CAS PubMed.
- P. Keller, Mol. Cryst. Liq. Cryst., 1985, 123, 247–256 CrossRef CAS.
- C. T. Imrie and G. R. Luckhurst, J. Mater. Chem., 1998, 8, 1339–1343 RSC.
- J. Andersch, S. Diele, D. Lose and C. Tschierske, Liq. Cryst., 1996, 21, 103–113 CrossRef CAS.
- W. Kreuder, H. Ringsdorf, O. Herrmannschonherr and J. H. Wendorff, Angew. Chem., Int. Ed. Engl., 1987, 26, 1249–1252 CrossRef.
- A. C. Griffin, S. L. Sullivan and W. E. Hughes, Liq. Cryst., 1989, 4, 677–684 CrossRef CAS.
- C. T. Imrie, D. Stewart, C. Remy, D. W. Christie, I. W. Hamley and R. Harding, J. Mater. Chem., 1999, 9, 2321–2325 RSC.
- C. V. Yelamaggad, S. A. Nagamani, U. S. Hiremath, D. S. S. Rao and S. K. Prasad, Liq. Cryst., 2002, 29, 231–236 CrossRef CAS.
- P. A. Henderson and C. T. Imrie, Macromolecules, 2005, 38, 3307–3311 CrossRef CAS.
- C. V. Yelamaggad, A. S. Achalkumar, D. S. S. Rao and S. K. Prasad, Org. Lett., 2007, 9, 2641–2644 CrossRef CAS PubMed.
- A. Yoshizawa, J. Mater. Chem., 2008, 18, 2877–2889 RSC.
- A. Martinez-Felipe and C. T. Imrie, J. Mol. Struct., 2015, 1100, 429–437 CrossRef CAS.
- P. Davidson, D. Petermann and A. M. Levelut, J. Phys. II, 1995, 5, 113–131 CrossRef CAS.
- T. T. Mills, G. E. S. Toombes, S. Tristram-Nagle, D. M. Smilgies, G. W. Feigenson and J. F. Nagle, Biophys. J., 2008, 95, 669–681 CrossRef CAS PubMed.
- Y. Wang, H. G. Yoon, H. K. Bisoyi, S. Kumar and Q. Li, J. Mater. Chem., 2012, 22, 20363–20367 RSC.
- M. R. J. Simpson F., 2016, unpublished results.
- G. Ungar, V. Percec and M. Zuber, Macromolecules, 1992, 25, 75–80 CrossRef CAS.
- C. V. Yelamaggad, V. P. Tamilenthi, D. S. S. Rao, G. G. Nair and S. K. Prasad, J. Mater. Chem., 2009, 19, 2906–2908 RSC.
- R. Prabhu and C. V. Yelamaggad, J. Phys. Chem. B, 2015, 119, 11935–11952 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |