Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust hybrid elastomer/metal-oxide superhydrophobic surfaces†
S.
Hoshian
*,
V.
Jokinen
and
S.
Franssila
*
Aalto University, School of Chemical Technology Department of Material Science and Engineering, FI-02150 Espoo, Finland. E-mail: Sasha.hoshian@aalto.fi; Sami.franssila@aalto.fi
First published on 1st July 2016
Abstract
We introduce a new type of hybrid material: a nanostructured elastomer covered by a hard photoactive metal-oxide thin film resembling the exoskeleton of insects. It has extreme water repellency and fast self-recovery after damage. A new fabrication method for replicating high aspect ratio, hierarchical re-entrant aluminum structures into polydimethylsiloxane (PDMS) is presented. The method is based on a protective titania layer deposited by atomic layer deposition (ALD) on the aluminum template. The ALD titania transfers to the elastomeric scaffold via sacrificial release etching. The sacrificial release method allows for high aspect ratio, even 100 μm deep and successful release of overhanging structures, unlike conventional peeling. The ALD titania conformally covers the 3D multihierarchical structures of the template and protects the polymer during the release etch. Afterwards it prevents the high aspect ratio nanostructures from elasticity based collapse. The resulting nanostructured hybrid PDMS/titania replicas display robust superhydrophobicity without any further fluoro-coating or modification. Their mechanical and thermal robustness results from a thick nanostructured elastomeric layer which is conformally covered by ceramic titania instead of a monolayer hydrophobic coating. We have demonstrated the durability of these replicas against mechanical abrasion, knife scratches, rubbing, bending, peel tape test, high temperature annealing, UV exposure, water jet impingement and long term underwater storage. Though the material loses its superhydrophobicity in oxygen plasma exposure, a fast recovery from superhydrophilic to superhydrophobic can be achieved after 20 min UV irradiation. UV-assisted recovery is correlated with the high photoactivity of ALD titania film. This novel hybrid material will be applicable to the large area superhydrophobic surfaces in practical outdoor applications.
Introduction
Fabricating thick high aspect ratio nanostructured layers using replication methods is difficult due to challenges related to the release process and the collapse of the slender structures during or after the release.1 An additional challenge for release emerges if the structures to be replicated are overhanging, or re-entrant, which means that simple pulling or peeling is likely to tear the overhangs apart.Superhydrophobic surfaces are one application that could greatly benefit from deep layers of interconnected overhanging structures. A variety of robust superhydrophobic surfaces have been proposed by scientists over the last decade in attempts to enable practical applications.2–15 Typically superhydrophobic surfaces are obtained by micro and nanostructuring followed by a low surface energy or hydrophobic coating. However, the coating is often very thin, even monolayer, which is easily damaged mechanically or degraded chemically. Additionally, unlike superhydrophobic surfaces in nature such as plant leaves, artificial surfaces mostly do not have the ability to refresh themselves after damage. The lack of self-healing properties could severely hinder the practical usage of superhydrophobic materials.2,3
If overhanging structures are utilized, then there is no need to use a coating. This is achieved by re-entrant T-shape microstructures.5,16–19 We recently reported superhydrophobicity of silicon without any coatings5 using metal assisted chemical etching to produce collapsed silicon nanowires. Even though they are chemically and thermally stable, they cannot withstand mechanical wear such as knife scratches or rubbing with a finger. The recently reported double-T silicon structures display superhydrophobicity and oleophobicity without coatings.18 They share, however, all the drawbacks of silicon-based surfaces, the major one being mechanical fragility. Additionally, silicon microstructuring is limited by wafer size. So developing a new large area and facile method to produce robust and self-recoverable non-fragile superhydrophobic materials without low surface-energy coatings is desired.
An alternative to the coating approach is micro/nanostructuring of bulk hydrophobic polymers. Polydimethylsiloxane (PDMS) is a popular candidate because it is easily processable and its native water contact angle (WCA) is 105 ± 2°. However, so far the structuring of the PDMS surface has mostly resulted in a sticky Wenzel state,20–22 and in order to achieve real superhydrophobicity a hydrophobic coating was still needed to keep droplets in the Cassie state.11,23–32
Various direct write and replication methods have been employed to micro/nanostructure bulk hydrophobic materials. Replication methods such as casting a polymer solution or melt on a template,33,34 injection moulding,35–37 hot embossing,38,39 and sacrificial etching7,40,41 have been utilized. Photolithography and femtosecond laser ablation have been used for structuring of the master, but both are time consuming and expensive. It needs to be noted that the superhydrophobicity of a template (master) does not ensure the superhydrophobicity of the replicated surface.42
Here we introduce a new method to precisely replicate a thick layer (100 μm) of re-entrant hierarchical nanostructures into polymers with the help of a protective ALD titania film. The replica is released by sacrificial etching of the aluminum template. The resulting material is a hybrid exoskeleton-like elastomer covered with a hard and photoactive metal-oxide (titania) which provides a combination of comprehensive robustness and fast self-healing properties. To our knowledge this is the first report that UV exposure assists the fast recovery from superhydrophilic back to the superhydrophobic state.
Results and discussion
Fabrication process
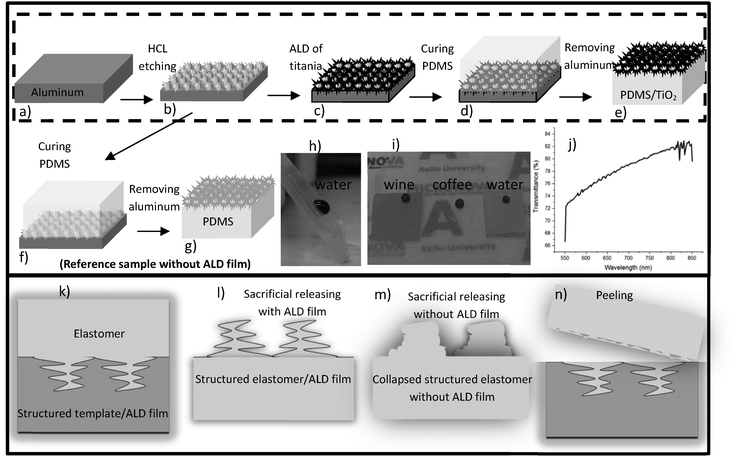
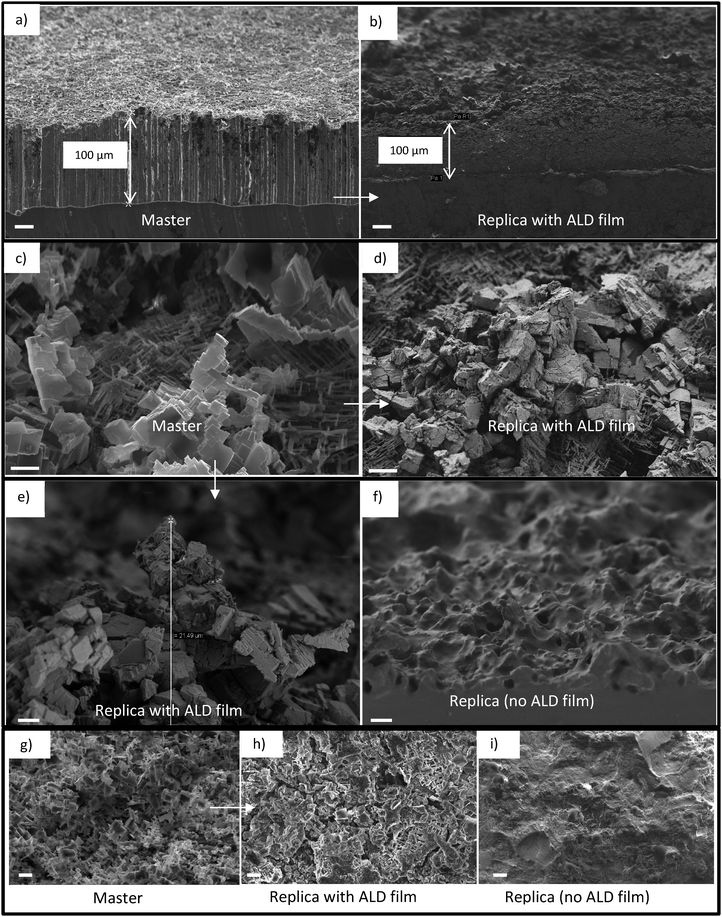
The non-lithography fabrication process is explained in Fig. 1 and the full fabrication details are given in the supporting information. The first step is to select the master (Fig. 1a and b). We focused primarily on HCl etched aluminum wafers although other templates were successfully replicated as well. The HCl etched aluminum wafers provided an extremely challenging starting point for replication. The micro and nanostructured layer consists of a layer of interconnected micron sized cavities which can have considerable thickness (up to 100 μm) and high aspect ratios. Furthermore, the depth and size of the aluminum nanostructure were possible to tune with the HCl concentration and etch time (Fig. S1, ESI†). A 100 μm deep hierarchical re-entrant aluminum structure obtained by etching in 1 M HCl is shown in Fig. 2a, c and g. The template material in our nanoreplication process should be chosen so that, (i) it tolerates ALD thin film deposition; and (ii) its selective etching is possible to ensure the removal of the substrate without attacking the ALD film.The next step (Fig. 1c) is the deposition of a protective film by ALD. A highly conformal coating process such as ALD is needed to cover the deep and complex nanostructures of the template without clogging. Other coating methods such as wet, spray or spin coatings are not as conformal as ALD to fully cover the 3D multihierarchical structures. The advantage of using an ALD protective layer is threefold: (i) it guarantees accurate replication of nanostructures due to its conformality, (ii) it protects the polymer nanostructures during the release etch, and (iii) it is non-sticky and prevents the polymer structure from collapsing onto itself.43 We found that a 10–25 nm titania ALD film is thick enough to reliably protect the polymer during the release etch without clogging the nanocavities of the template. Compared to a previously suggested method of a polymer film coated nanostructures,40 the ALD process allows much more control over the thickness and step coverage, and thus more accurate replication. In addition, we recently reported the high photoactivity of ALD titania that can be used for the fast recovery of the surface from superhydrophilic to the superhydrophobic state.44 In fact, the combination of the hard ALD titania film and elastomeric nanostructures is the key to the superior robustness of the final surface, while the photoactivity of titania enables its fast recovery.
Following the ALD coating, a curable polymer is cast on top of the master (Fig. 1d). We chose to study the widely used PDMS. Uncured PDMS with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer to crosslinking agent ratio was poured on top of the master, after which a vacuum desiccator was used to remove air bubbles and ensure the penetration of the polymer into the master. The polymer was then thermally cured.
1 monomer to crosslinking agent ratio was poured on top of the master, after which a vacuum desiccator was used to remove air bubbles and ensure the penetration of the polymer into the master. The polymer was then thermally cured.
The final step is the release of the replica from the master (Fig. 1e). A simple peeling process was not possible due to the high aspect ratio and overhanging structures. Instead, we utilized sacrificial etching of the aluminum wafer to avoid damage from mechanical peeling (Fig. 1k–n). The release etch was done in HCl which has adequate selectivity between aluminum and titania. X-ray photo-electron spectroscopy (XPS) confirmed the existence of titania on the PDMS surface after transfer (Fig. S2, ESI†) which shows that the etching stopped at the titania layer and the etchants did not come into contact with the polymer. In the present work, the ALD film was let to remain on the structured elastomer, but in some applications it might be beneficial to remove it after release. Fig. 2b, d, e and h show successful replications with the ALD film, while Fig. 2f and i present the corresponding control experiments without the ALD film. It is clearly visible that the ALD-protected structures have much sharper features which bear close similarity to the masters, Fig. 2c and g. In contrast, the features of the control experiments are much more rounded because the nanoscale structures are not accurately replicated. Two factors explain the success of the process with ALD: PDMS is known for its strong tendency for making elastic contact with materials, including itself, and the hard ALD titania film protects the nanostructures from this. Additionally, nanostructured PDMS can be damaged chemically during the HCl release etch, by e.g. absorbing the etchant and ALD titania can protect them.
Contact angle measurements with 2 μl water droplets (Table 1 average of 5 measurements) show the contact angles of advancing CA 163 ± 1° and receding CA 161 ± 1° with extremely small contact angle hysteresis (2°) for PDMS/25 nm titania. This surface also repelled 2 μl wine droplets (14% ethanol) with a sliding angle of 15° and hot coffee with a sliding angle of 8°, but not low surface tension oils. Fig. 1i shows wine, coffee and colored water droplets on PDMS/titania samples with different thicknesses of titania left to right 15, 10 and 25 nm respectively. In contrast, PDMS replicas without ALD film (Fig. 1f–h) display a contact angle of advancing CA = 136 ± 1°, receding CA = 100 ± 1°, and 36° hysteresis (Wenzel state with sliding angles >90°). We have also studied the effect of ALD titania thicknesses on the optical properties of the final hybrid material. By tuning the ALD titania thickness we could tune the transparency of the final material. Transmittance measurement for the PDMS/10 nm titania sample is presented in Fig. 1j showing 70–80% transparency in the visible wavelengths. By increasing the thickness of titania to 25 nm, the material became non-transparent to visible light.
| ALD TiO2/PDMS replica | PDMS replica | |||
|---|---|---|---|---|
| Contact angle (CA) | Advancing | Receding | Advancing | Receding |
| After fabrication | 163 ± 1° | 161 ± 1° | 136 ± 1° | 100 ± 1° |
| 50 cycles of abrasion | 157 ± 1° | 155 ± 1° | 110 ± 2° | 85 ± 2° |
| Rubbing 10× | 161 ± 1° | 158 ± 1° | 63 ± 1° | 44 ± 1° |
| Knife cuts 10× | 157 ± 2° | 153 ± 2° | 60 ± 2° | 36 ± 2° |
| Bending 100× | 163 ± 1° | 161 ± 1° | 125 ± 1° | 98 ± 1° |
| Scotch test 10× | 162 ± 1° | 161 ± 2° | 126 ± 1° | 73 ± 2° |
| Annealing 1 h 300 °C | 163 ± 2° | 160 ± 2° | 128 ± 2° | 100 ± 2° |
| UV exposure 1 h | 163 ± 1° | 161 ± 2° | 135 ± 2° | 97 ± 2° |
| Wine CA | 152 ± 2° | 150 ± 1° | ∼0° | ∼0° |
| Hot coffee CA | 155 ± 2° | 152 ± 1° | 112 ± 1° | 92 ± 2° |
| Water CA after 90 days in water | 157 ± 1° | 152 ± 2° | ∼0° | ∼0° |
Robustness of superhydrophobicity
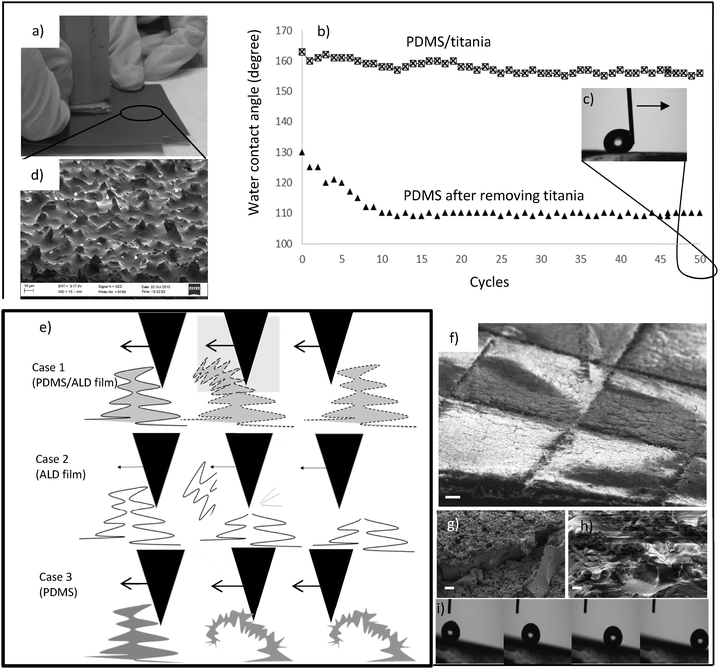
To study the durability of superhydrophobicity of the PDMS/titania surfaces the following tests were conducted, (1) sand paper abrasion, (2) knife scratches, (3) bending, (4) rubbing, (5) tape peeling, (6) water drop from height, (7) water jet, (8) annealing, (9) UV exposure, (10) immersion in harsh acids and (11) long term under water storage. The advancing and receding contact angles were measured after each test (Table 1). Fig. S3 (ESI†) shows that the hybrid PDMS/titania samples maintain the water repellent properties. Test details for robustness studies are given in the ESI.†Mechanical robustness has been highly problematic due to weakness of micro- and nanostructures that are necessary for water repellency. We clarified nanostructure survival during mechanical wear by a sandpaper abrasion test (Fig. 3a–d) that has been recently suggested as a standard robustness test for superhydrophobic surfaces.45 It was carried out as follows: the PDMS/titania sample was placed face down to the sandpaper with a 100 g weight (1.5 cm2 contact area) on top and it was dragged for 10 cm (Video S1, ESI†). Water advancing and receding contact angle measurements (advancing CA = 157 ± 1°, receding CA = 155 ± 1°) revealed a robust superhydrophobic surface even after 50 cycles of abrasion. Furthermore, when a water droplet was guided by a needle across the entire abraded surface, no pinning was observed (Video S2, ESI†). Mechanical tolerance of the surface was also studied by using 10 parallel knife cuts (Fig. S3a and Video S3, ESI†). After the cutting test, superhydrophobicity remained quite the same (advancing CA = 157 ± 2°, receding CA = 153 ± 2°) and also the rolling droplet test on the 7° inclined sample (Fig. 3) confirms the durability.
We suggest that the surface robustness is achieved by a combination of thick flexible hierarchical layers (>100 μm) covered with hard metal-oxide titania. This PDMS/titania surface resembles the exoskeleton of insects:46 consisting of a rubber-like protein covered by a hard exocuticle. Nanostructures bend during mechanical contact and the elastomeric part allows recovery, while the ALD film prevents PDMS self-adhesiveness. ALD film may break down into small pieces due to mechanical stress in the abrasion test, but the elastomeric scaffold keeps these pieces in place as the ALD film is strongly adhered to the elastomer (case 1 in Fig. 3e). The pure ALD nanostructure is likely to break under mechanical stress (case 2 Fig. 3e), while the purely elastomeric structure would stick to itself due to self-adhesiveness (case 3 Fig. 3e). The SEM micrograph of the PDMS/titania sample after 10 knife scratches and 50 cycles of abrasion are shown in Fig. 3f and g respectively, while Fig. 3i presents the sequence photos of a water droplet in the rolling state on the 7° inclined sample after knife scratches.
To confirm the necessity of ALD titania film for high robustness, we removed the titania film from the sample that we used for the abrasion study and repeated the same abrasion study on the structured polymer. Please note that this sample is not similar to the replicated PDMS sample without ALD film in Table 1. Titania film was removed using potassium hydroxide (KOH) wet etching at 60 °C followed by water rinsing and drying in an oven for 10 min at 50 °C (Fig. 3h). The water contact angle after titania removal was 130°. After 10 cycles of abrasion, the contact angle dropped to 110° (close to the native contact angle of PDMS) and remained around it after next abrasion cycles (Fig. 3b). This result confirms that the hard ALD titania film can protect the elastomer nanostructures from mechanical damage.
To study the effect of bending and deformation of the substrate on the superhydrophobicity of the surface we conduct 100 times bending concave and convex of the PDMS/titania sample (Fig. S3b and Video S4, ESI†) and then measured the advancing and receding water contact angles (advancing CA = 163 ± 1°, receding CA = 161 ± 1°). Even harsh deformation of the sample had no effect on the water repellency of the surface. Such flexibility of superhydrophobic surfaces is needed in many applications for example in the tubing and piping industry.
Adhesion of the bulk material to thin films is another challenge for the durability of superhydrophobic surfaces. We investigated the adhesion strength of PDMS to titania by the peel tape test on a cross-hatched sample (Fig. S3c, ESI†). The cross-hatched cut is a standardized damage to the surface before the tape peel test to make a more extensive study on the adhesion.47 Tape (3M Scotch Brand Tape, core series 4-1000) was then placed on the damaged area and subsequently removed. After 10 peeling cycles the advancing and receding contact angle measurements (advancing CA = 162 ± 1°, receding CA = 161 ± 2°) show that the surface remained superhydrophobic in the rolling state. We are convinced that the good adhesion of titania film to the PDMS substrate is ensured by the multiscale re-entrant structures that are hooked to the ALD film and keep it in place (Fig. 2d).
To show the robustness of our samples against water pressure we study the effect of a water jet on the PDMS/titania surface (Fig. S3d and Video S5, ESI†). Although the water jet impingement is harsh, the surface remained superhydrophobic without any noticeable water penetration. We assume that the main factor ensuring the robustness against the water jet is the nanoscale porous structure in which one needs very high Laplace pressure to remove the nanoscale air pockets trapped in the nano overhangs. Another way to study the influence of the water pressure was to conduct the water free fall test (Fig. S3e and Video S6, ESI†). In it, water droplets were let to drop from 3 meter height to the surface. Here, the dynamic pressure corresponding to the impact velocity “v” is PDYN = ρv2/2 where ρ = 1000 kg m−3 is the density of water and “v” is equal to (2gh) 1/2 where “g” is the gravitational acceleration. The droplets had a speed of 7.6 m s−1, and thus the dynamic pressure applied reached 29 kPa. Even this pressure could not break the superhydrophobicity of our sample surfaces that clearly showed a highly robust super-repellency.
Handling the superhydrophobic surfaces with bare human hands is a typical contamination mechanism. We studied the influence of the finger prints by rubbing the PDMS/titania surface while pushing it with a bare finger (Fig. S3f and Video S7, ESI†). However, we observed no reduction of water contact angles (advancing CA = 161 ± 1°, receding CA = 158 ± 1°) after 10 bare finger rubbing tests. The bare finger rubbed sample was used for the dirt removal test in order to study the human finger print on the self-cleaning properties of the surface. We used methylene blue powder as dirt. A water droplet was placed on the dirty sample on an inclined surface (7° angle) (Video S8, ESI†) to remove the dirt while rolling. No pinning of the droplet was observed on the surface meaning there was no contamination during the bare finger rubbing test. For comparison, we repeated the same test on a PDMS replica without ALD titania. The water contact angle dropped from 130° to 60° (Table 1) due to contamination on the PDMS replica without titania. Thus, it can be concluded that the ALD titania layer protects the PDMS from the contamination of the finger marks.
For anti-biofouling and drag reduction applications, e.g., in the marine industry, long term storage under water is crucial.5 We stored the PDMS/titania sample under DI water (Fig. S3h, ESI†) for 90 days and measured the water contact angle of its surface every 10 days. The contact angle decreased from 163° to 158° in 20 days and it stabilized to this value for the remaining 70 days. There was a minor but noticeable change in the optical contrast of the plastron after 20 days and also this property remained without changing for the remaining 70 days (Fig. S3i and j, ESI†). We assume that the change in the optical contrast at the solid–liquid interface is partially due to the escape of the trapped air pockets (plastron) under structures. However, the sample was fully dry when it was taken out of the water after 90 days. So we rationalize that the nano-sized air pockets remained in the structure though the micro-sized air pockets escaped from the solid–liquid interface. Thus, the observed transition is not from Cassie to Wenzel as we have reported elsewhere,44 but it occurs between micro and nano-Cassie states.48 The micro-sized air pockets exhibit higher mobility compared to nano-pockets due to the inverse relation of Laplace pressure with their sizes.48 Therefore, the long term durability of superhydrophobicity under water is obtained with the successful ALD-assisted replication of both micro and nano overhangs. As a reference, when the PDMS replica sample without titania film was stored in DI water for 90 days, it was fully wet with the contact angle close to zero.
The chemical stability of the samples was studied by investigating their resistance against high temperature annealing, UV exposure and 6 hour-immersion in different harsh acidic and basic solutions at room temperature, including HF (30 M), HCl (12 M), and KOH (12 M), for 6 hours. Contact angle measurements (Table 1) show that 1 hour annealing at 300 °C (advancing CA = 163 ± 2°, receding CA = 160 ± 2°), 1 hour UV exposure (advancing CA = 163 ± 1°, receding CA = 161 ± 2°) and 6 hours in HF or HCl solution had no effect on the superhydrophobicity of the samples. Both HF and HCl immersion caused a colour change of the PDMS so that it turned matte, but its contact angles remained rolling superhydrophobic (after 6 h-immersion in HF solution advancing CA = 161 ± 2°, receding CA = 160 ± 1° and for HCl solution advancing CA = 163 ± 1°, receding CA = 161 ± 1°). However, KOH etched the titania film and therefore caused a clear reduction of the contact angle (advancing CA = 138 ± 1°, receding CA = 130 ± 1°). SEM images in Fig. S4a–c (ESI†) respectively show how titania remains intact after 6 hours of immersion in HF and HCl solution but it etches in KOH solution.
Self-healing
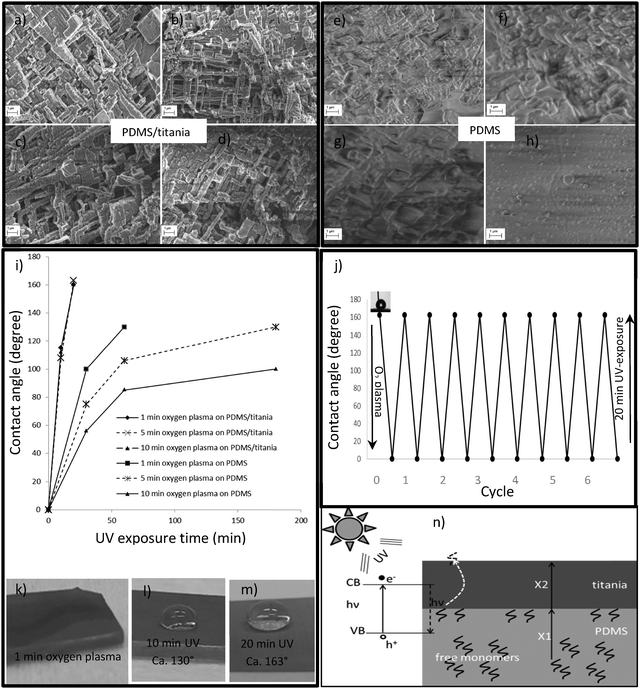
The PDMS/titania hybrid material also exhibited self-healing properties. Oxygen plasma (1000 W) was first used to turn the surface to superhydrophilic with the water contact angle close to zero. One minute oxygen plasma was enough to turn the surface to superhydrophilic. We studied the recovery of the surface after 1, 5 and 10 minutes of oxygen plasma exposure, but the increased length had no noticeable effect either on the recovery time or on the mechanical deformation of the nanostructures. Recovery to the superhydrophobic state was observed (i) at room temperature, (ii) at a mildly elevated temperature (50 °C), and also (iii) after UV exposure (ECE 2000 modular DYMAX, wavelength 365 nm, 105 mW cm−2). The recovery to rolling state superhydrophobicity took place at room temperature in 10 hours and it was speeded up when using elevated temperature (2 hour annealing at 50 °C) or 20 min of UV exposure. SEM images of PDMS/titania nanostructures (Fig. 4a–d) revealed that the plasma treatment did not cause any damage to the surfaces as the ALD titania film protected them. Contact angle measurements for PDMS/titania samples (1, 5 and 10 min oxygen plasma treated) in Fig. 4i show the recovery of the contact angle zero back to its initial superhydrophobic values (advancing CA = 163 ± 1°, receding CA = 161 ± 2°) after 20 min of UV exposure, independent of plasma duration. Fig. 4j demonstrates the robust self-healing of the PDMS/titania sample after 10 cycles of oxygen plasma treatment (1 min) and 20 min UV exposure. This is confirmed by the optical images of the water droplet on the PDMS/titania sample after 1 min of oxygen plasma, 10 and 20 min of UV exposure, as shown in Fig. 4k–m, respectively.On the other hand, the SEM images of the PDMS reference samples (Fig. 4e–h) revealed increasing damage of the surface with longer plasma exposure. Furthermore, also the recovery time increases after longer oxygen plasma treatment (Fig. 4i). For example, when the sample has been in oxygen plasma for 1 min, a 1 hour annealing at 50 °C or a 1 hour UV exposure is needed to recover it from the superhydrophilic to the hydrophobic state with the contact angle of 130°. After 10 min of plasma treatment, the recovery time increases to 3 hours of annealing at 50 °C or 3 hours of UV exposure, and even then it is not fully recovered as the water contact angle remains at 100°. These results confirm that UV exposure is not better than mild annealing in the recovery of pure PDMS samples, though it was much more effective with the titania coated samples. Thus, the photoactivity of the titania film provides faster recovery from the superhydrophilic to the initial state when applying UV exposure.
Usually, superhydrophobic surfaces with low surface-energy coatings suffer from UV exposure as it induces the decomposition of the coating.5 But when considering our PDMS/titania samples, UV light promotes recovery to the superhydrophobic rolling state. The mechanism of self-healing can be explained by the migration of the free PDMS monomers to the surface: they can easily diffuse to the surface via the open nanostructures and then turn the surface back to superhydrophobic.49–51 We suggest that in UV light, the highly photoactive ALD titania facilitates those free monomers which are closer to the titania–PDMS interface to recover the surface. UV exposure can excite electrons from the valence to the conductive band of titania and produce electron–hole pairs. These pairs can release the same energy after recombination and this energy can manipulate the free monomers at the PDMS–titania interface. When these free monomers have diffused through the titania thin film (25 nm) they will recover the surface. In this case the required diffusion length is much shorter than in the bulk PDMS (range of millimeters). The diffusion length is given by 〈x2〉 = αiDt where “D” is the diffusion coefficient and “t” is the diffusion time and “αi” is the numerical constant which depends on dimensionality. It means that shorter diffusion length decreases dramatically the diffusion time. Fig. 4n shows a speculated schematic of the titania–PDMS interface and how the UV exposure can reduce the diffusion length of free monomers from millimeters (X1 in bulk PDMS) to nanometers (X2 in titania thin film).
Conclusions
We introduce a novel method to produce hybrid elastomer/metal-oxide nanostructured materials for robust and self-healing, water repelling surfaces. In this work we studied the PDMS/titania replicated from nanostructured aluminum. There are earlier reports using titania and elastomer combinations to produce superhydrophobic surfaces.52–54 Titania is usually used in them as nanoparticles embedded in the elastomer surfaces, but we used a thin film of titania to cover the structured elastomer as an exoskeleton. Thus, the titania cover would act as a hard protection layer against mechanical, thermal, chemical and even plasma attack.Our novel material indeed remained superhydrophobic after various types of mechanical and chemical exposure including knife scratches, abrasion, bending, handling by a bare finger, tape peeling, water jet, simulated rain drop, high temperature annealing, UV exposure as well as immersion in HF and HCl solutions. The material was superhydrophobic even after 90 day-storing under water.
We explain the high robustness of these surfaces by the thick elastomeric nano overhangs conformally covered with hard titania ALD films. Superhydrophobicity arises from their geometrical structure and bulk hydrophobic material without any further fluoro-coatings. The self-healing of the studied titania/PDMS samples after exposure to oxygen plasma was observed at room temperature in 10 hours and at 50 °C in 2 hours, while the most effective way was to use UV light, with which just 20 min treatment was enough to recover the surface from the superhydrophilic to the superhydrophobic rolling state. Further investigation is needed to fully understand the mechanism of this UV-assisted self-recovery, but we believe that the highly photoactive ALD titania can facilitate the migration of free monomers on the PDMS–titania interface and thus enhance the recovery process. A combination of robust superhydrophobicity and fast self-recovery can be key factors when considering these materials in real world applications such as anti-icing surfaces.55–57
Though we concentrated in this study only on the titania/PDMS combination and aluminum templates, it is noteworthy that this method can be modified easily with the needs of the final application. A large variety of other template materials could be used for the replication process, including aluminum, copper, steel, silicon and even biological samples such as crystal nanocellulose papers and butterfly wings. This replication method produces hybrid polymer/metal-oxide structures. In addition, one can choose whether the metal oxide film is left with the structure or removed afterwards to obtain a fully polymeric surface. Different combinations of polymers with available ALD films can result in novel hybrid materials with interesting new mechanical and optical properties.
Acknowledgements
This research was supported by the Academy of Finland (PROWET project number 263538 and 266820). Wafer processing took place at Aalto Nanofab cleanroom. The authors thank Sara Pourjamal for her help with optical measurements and Outi Söderberg for her general comments on the manuscript.Notes and references
- C. N. LaFratta, L. Li and J. T. Fourkas, Soft-lithographic replication of 3D microstructures with closed loops, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8589 CrossRef CAS PubMed.
- X. Yao, Y. Song and L. Jiang, Applications of bio-inspired special wettable surfaces, Adv. Mater., 2011, 23, 719 CrossRef CAS PubMed.
- Y. Tian, B. Su and L. Jiang, Interfacial material system exhibiting superwettability, Adv. Mater., 2014, 26, 6872 CrossRef CAS PubMed.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Mechanically durable superhydrophobic surfaces, Adv. Mater., 2011, 23, 673 CrossRef CAS PubMed.
- S. Hoshian, V. Jokinen, V. Somerkivi, A. R. Lokanathan and S. Franssila, Robust superhydrophobic silicon without a low surface-energy hydrophobic coating, ACS Appl. Mater. Interfaces, 2015, 7, 941 CAS.
- X. Zhu, Z. Zhang, X. Men, J. Yang, K. Wang, X. Xu, X. Zhou and Q. Xue, Robust superhydrophobic surfaces with mechanical durability and easy repairability, J. Mater. Chem., 2011, 21, 15793 RSC.
- C. Su, Y. Xu, F. Gong, F. Wang and C. Li, The abrasion resistance of a superhydrophobic surface comprised of polyurethane elastomer, Soft Matter, 2010, 6, 6068 RSC.
- G. Azimi, R. Dhiman, H. M. Kwon, A. T. Paxson and K. K. Varanasi, Hydrophobicity of rare-earth oxide ceramics, Nat. Mater., 2013, 12, 315 CrossRef CAS PubMed.
- H. Mertaniemi, V. Jokinen, L. Sainiemi, S. Franssila, A. Marmur, O. Ikkala and R. H. A. Ras, Superhydrophobic tracks for low-friction, guided transport of water droplets, Adv. Mater., 2011, 23, 2911 CrossRef CAS PubMed.
- X. Deng, L. Mammen, Y. Zhao, P. Lellig, K. Mullen, C. Li, H. J. Butt and D. Vollmer, Transparent, thermally stable and mechanically robust superhydrophobic surfaces made from porous silica capsules, Adv. Mater., 2011, 23, 2962 CrossRef CAS PubMed.
- Q. Ke, W. Fu, H. Jin, L. Zhang, T. Tang and J. Zhang, Fabrication of mechanically robust superhydrophobic surfaces based on silica micro-nanoparticles and polydimethylsiloxane, Surf. Coat. Technol., 2011, 205, 4910 CrossRef CAS.
- H. Jin, X. Tian, O. Ikkala and R. H. A. Ras, Preservation of superhydrophobic and superoleophobic properties upon wear damage, ACS Appl. Mater. Interfaces, 2013, 5, 485 CAS.
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Robust self-cleaning surfaces that function when exposed to either air or oil, Science, 2015, 347, 1132 CrossRef CAS PubMed.
- R. C. Remsing, E. Xi, S. Vembanur, S. Sharma, P. G. Debenedetti, S. Garde and A. J. Patel, Pathways to dewetting in hydrophobic confinement, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8181 CrossRef CAS PubMed.
- P. Papadopoulos, L. Mammen, X. Deng, D. Vollmer and H. J. Butt, How superhydrophobicity breaks down, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3254 CrossRef CAS PubMed.
- L. Cao, T. P. Price, M. Weiss and D. Gao, Super water- and oil-repellent surfaces on intrinsically hydrophilic and oleophilic porous silicon films, Langmuir, 2008, 24, 1640 CrossRef CAS PubMed.
- J. Wang, F. Liu, H. Chen and D. Chen, Superhydrophobic behavior achieved from hydrophilic surfaces, Appl. Phys. Lett., 2009, 95, 084104 CrossRef.
- T. L. Liu and C. J. Kim, Repellent surfaces, Turning a surface superrepellent even to completely wetting liquids, Science, 2014, 346, 1096 CrossRef CAS PubMed.
- A. Tuteja, W. Choi, J. M. Mabry, G. H. McKinley and R. E. Cohen, Robust omniphobic surfaces, Proc. Natl. Acad. Sci. U. S. A, 2008, 105(47), 18200 CrossRef CAS PubMed.
- M. Cheng, M. Song, H. Dong and F. Shi, Surface adhesive forces: a metric describing the drag-reducing effect of superhydrophobic coatings, Small, 2015, 11, 1665 CrossRef CAS PubMed.
- M. M. Stanton, R. E. Ducker, J. C. MacDonald, C. R. Lambert and W. G. McGimpsey, Super-hydrophobic, highly adhesive, polydimethylsiloxane (PDMS) surfaces, J. Colloid Interface Sci., 2012, 367, 502 CrossRef CAS PubMed.
- S. M. Lee, H. S. Lee, D. S. Kim and T. H. Kwon, Fabrication of hydrophobic films replicated from plant leaves in nature, Surf. Coat. Technol., 2006, 201, 553 CrossRef CAS.
- Z. He, M. Ma, X. Lan, F. Chen, K. Wang, H. Deng, Q. Zhang and Q. Fu, Fabrication of a transparent superamphiphobic coating with improved stability, Soft Matter, 2011, 7, 6435 RSC.
- S. M. Kang, S. M. Kim, H. M. Kim, M. K. Kwak, D. H. Tahk and K. Y. Suh, Robust superomniphobic surfaces with mushroom-like micropillar arrays, Soft Matter, 2012, 8, 8563 RSC.
- E. J. Park, Y. K. Cho, D. H. Kim, M. G. Jeong, Y. H. Kim and Y. D. Kim, Hydrophobic polydimethylsiloxane (PDMS) coating of mesoporous silica and its use as a preconcentrating agent of gas analytes, Langmuir, 2014, 30, 10256 CrossRef CAS PubMed.
- M. Im, H. Im, J. H. Lee, J. B. Yoon and Y. K. Choi, A robust superhydrophobic and superoleophobic surface with inverse-trapezoidal microstructures on a large transparent flexible substrate, Soft Matter, 2010, 6, 1401 RSC.
- A. Tropmann, L. Tanguy, P. Koltay, R. Zengerle and L. Riegger, Completely superhydrophobic PDMS surfaces for microfluidics, Langmuir, 2012, 28, 8292 CrossRef CAS PubMed.
- X. Zhao, L. Li, B. Li, J. Zhang and A. Wang, Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages, J. Mater. Chem. A, 2014, 2, 18281 CAS.
- Z. He, M. Meng, X. Xu, J. Wang, F. Chen, H. Deng, K. Wang, Q. Zhang and Q. Fu, Fabrication of superhydrophobic coating via a facile and versatile method based on nanoparticle aggregates, Appl. Surf. Sci., 2012, 258, 2544 CrossRef CAS.
- J. Yong, F. Chen, Q. Yang, D. Zhang, G. Du, J. Si, F. Yun and X. Hou, Femtosecond laser weaving superhydrophobic patterned PDMS surfaces with tunable adhesion, J. Phys. Chem. C, 2013, 117, 24907 CAS.
- Z. Pan, H. Shahsavan, W. Zhang, F. K. Yang and B. Zhao, Superhydro-oleophobic bio-inspired polydimethylsiloxane micropillared surface via FDTS coating/blending approaches, Appl. Surf. Sci., 2015, 324, 612 CrossRef CAS.
- B. Cortese, S. DAmone, M. Manca, I. Viola, R. Cingolani and G. Gigli, Superhydrophobicity due to the hierarchical scale roughness of PDMS surfaces, Langmuir, 2008, 24, 2712 CrossRef CAS PubMed.
- L. Feng, S. Li, H. Li, J. Zhai, Y. Song, L. Jiang and D. Zhu, Super-hydrophobic surface of aligned polyacrylonitrile nanofibers, Angew. Chem., Int. Ed., 2002, 41, 1221 CrossRef CAS.
- Y. Lee, S. H. Park, K. B. Kim and J. K. Lee, Fabrication of hierarchical structures on a polymer surface to mimic natural superhydrophobic surfaces, Adv. Mater., 2007, 19, 2330 CrossRef CAS.
- W. Michaeli, M. Schongart, F. Klaiber and S. Beckemper, Production of superhydrophobic surfaces using a one-step variothermal injection moulding process, Micro Nanosyst., 2011, 3, 222 CrossRef CAS.
- C. Hopmann, C. Behmenburg, U. Recht and K. Zeuner, Injection molding of superhydrophobic liquid silicone rubber surfaces, Silicon, 2013, 6, 35 CrossRef.
- M. Yamaguchi, S. Sasaki, S. Suzuki and Y. Nakayama, Injection-molded plastic plate with hydrophobic surface by nanoperiodic structure applied in uniaxial direction, J. Adhes. Sci. Technol., 2015, 29, 24 CrossRef CAS.
- V. Jokinen, P. Suvanto, A. R. Garapaty, J. Lyytinen, J. Koskinen and S. Franssila, Durable superhydrophobicity in embossed CYTOP fluoropolymer micro and nanostructures, Colloids Surf., A, 2013, 434, 207 CrossRef CAS.
- M. Röhrig, M. Schneider, G. Etienne, F. Oulhadj, F. Pfannes, A. Kolew, M. Worgull and H. Hölscher, Hot pulling and embossing of hierarchical nano- and micro-structures, J. Micromech. Microeng., 2013, 23, 105014 CrossRef.
- X. Liu, W. Wu, X. Wang, Z. Luo, Y. Liang and F. Zhou, A replication strategy for complex micro/nanostructures with superhydrophobicity and superoleophobicity and high contrast adhesion, Soft Matter, 2009, 5, 3097 RSC.
- Y. Yoon, D. Lee and J. B. Lee, Fabrication of optically transparent PDMS artificial lotus leaf film using underexposed and underbaked photoresist mold, J. Microelectromech. Syst., 2013, 22, 1073 CrossRef CAS.
- L. Sainiemi, V. Jokinen, A. Shah, M. Shpak, S. Aura, P. Suvanto and S. Franssila, Non-reflecting silicon and polymer surfaces by plasma etching and replication, Adv. Mater., 2011, 23, 122 CrossRef CAS PubMed.
- W. Zhou, Y. Huang, E. Menard, N. R. Aluru, J. A. Rojers and A. G. Alleyne, Mechanism for stamp collapse in soft lithography, Appl. Phys. Lett., 2005, 87(25), 251925 CrossRef.
- S. Hoshian, V. Jokinen, K. Hjort, R. H. A. Ras and S. Franssila, Amplified and localized photoswitching of TiO2 by micro- and nanostructuring, ACS Appl. Mater. Interfaces, 2015, 7, 15593 CAS.
- X. Tian, T. Verho and R. H. A. Ras, Moving superhydrophobic surfaces toward real-world applications, Science, 2016, 352(6282), 142 CrossRef CAS PubMed.
- T. Weis-Fogh, A Rubber-like protein in insect cuticle, J. Exp. Biol., 1960, 37, 889 CAS.
- A. Milionis, E. Loth and I. S. Bayer, Recent advances in the mechanical durability of superhydrophobic materials, Adv. Colloid Interface Sci., 2016, 229, 57 CrossRef CAS PubMed.
- T. Verho, J. T. Korhonen, L. Sainiemi, V. Jokinen, C. Bower, K. Franze, S. Franssila, P. Andrew, O. Ikkala and R. H. A. Ras, Reversible switching between superhydrophobic states on a hierarchically structured surface, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10210 CrossRef CAS PubMed.
- X. Yin, Z. Liu, D. Wang, X. Pei, B. Yu and F. Zhou, Bioinspired self-healing organic materials: chemical mechanisms and fabrications, Journal of Bionic Engineering, 2015, 12, 1 CrossRef.
- X. Wang, X. Liu, F. Zhou and W. Liu, Self-healing superamphiphobicity, Chem. Commun., 2011, 47, 2324 RSC.
- J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H. Wu, O. J. Schueller and G. M. Whitesides, Fabrication of microfluidic systems in poly(dimethylsiloxane), Electrophoresis, 2000, 21, 27 CrossRef CAS PubMed.
- K. Nakata, H. Kimura, M. Sakai, T. Ochiai, H. Sakai, T. Murakami, M. Abe and A. Fujishima, UV/Thermal Driven Rewritable Wettability Patterns on TiO2-PDMS Composite Films, ACS Appl. Mater. Interfaces, 2010, 2(9), 2485 CAS.
- V. A. Ganesh, S. S. Dinachali, A. S. Nair and S. Ramakrishna, Robust Superamphiphobic Film from Electrospun TiO2 nanostructures, ACS Appl. Mater. Interfaces, 2013, 5(5), 1527 CAS.
- K. Nakata, K. Udagawa, T. Ochiai, H. Sakai, T. Murakami, M. Abe and A. Fujishima, Rapid Erasing of Wettability Patterns Based on TiO2-PDMS Composite Films, Mater. Chem. Phys., 2011, 126(3), 484 CrossRef CAS.
- J. Liu, H. Guo, B. Zhang, S. Qiao, M. Shao, X. Zhang, X. Feng, Q. Li, Y. Song, L. Jiang and J. Wang, Guided Self-Propelled Leaping of Droplets on a Mico-Anisotropic Superhydrophobic Surfaces, Angew. Chem., Int. Ed., 2016, 55, 4265 CrossRef CAS PubMed.
- Q. Zhang, M. He, J. Chen, J. Wang, Y. Song and L. Jiang, Anti-Icing Surfaces based on Enhanced Self-Propelled Jumping of Condensed Water Microdroplets, Chem. Commun., 2013, 49, 4516 RSC.
- J. Lv, Y. Song, L. Jiang and J. Wang, Bio-Inspired Strategies for Anti-Icing, ACS Nano, 2014, 8(4), 3152 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sm01095d |
| This journal is © The Royal Society of Chemistry 2016 |