Open Access Article
Open Access ArticleDirected peptide amphiphile assembly using aqueous liquid crystal templates in magnetic fields†
Pim
van der Asdonk‡
 ab,
Masoumeh
Keshavarz‡
abc,
Peter C. M.
Christianen
bc and
Paul H. J.
Kouwer
*ab
ab,
Masoumeh
Keshavarz‡
abc,
Peter C. M.
Christianen
bc and
Paul H. J.
Kouwer
*ab
aDepartment of Molecular Materials, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. E-mail: p.kouwer@science.ru.nl
bInstitute for Molecules and Materials, Radboud University, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands
cHigh Field Magnet Laboratory (HFML - EMFL), Radboud University, Toernooiveld 7, 6525 ED Nijmegen, The Netherlands
First published on 13th June 2016
Abstract
An alignment technique based on the combination of magnetic fields and a liquid crystal (LC) template uses the advantages of both approaches: the magnetic fields offer non-contact methods that apply to all sample sizes and shapes, whilst the LC templates offer high susceptibilities. The combination introduces a route to control the spatial organization of materials with low intrinsic susceptibilities. We demonstrate that we can unidirectionally align one such material, peptide amphiphiles in water, on a centimeter scale at a tenfold lower magnetic field by using a lyotropic chromonic liquid crystal as a template. We can transform the aligned supramolecular assemblies into optically active π-conjugated polymers after photopolymerization. Lastly, by reducing the magnetic field strength needed for addressing these assemblies, we are able to create more complex structures by initiating self-assembly of our supramolecular materials under competing alignment forces between the magnetically induced alignment of the assemblies (with a positive diamagnetic anisotropy) and the elastic force dominated alignment of the template (with a negative diamagnetic anisotropy), which is directed orthogonally. Although the approach is still in its infancy and many critical parameters need optimization, we believe that it is a very promising technique to create tailor-made complex structures of (aqueous) functional soft matter.
Introduction
Self-assembly in aqueous solutions is an excellent approach to fabricate functional materials that can interface with biological and biochemical processes, for instance within sensing devices.1–3 Controlling the macroscopic organization (such as micropatterns and alignment) of such aqueous supramolecular materials across multiple length scales is essential for device performance,4–7 but remains a major challenge to date. Over the years, many techniques have been developed to control the spatial organization of aqueous self-assembling materials, including photolithography,8,9 soft lithography,9,10 electrospinning,11–13 electric fields14–17 and magnetic fields.18–20 All these techniques have demonstrated their use in specific situations, but they also suffer from limitations that are often directly associated to the organization of aqueous soft materials. For instance, such materials frequently display a low susceptibility and incompatibility to strong electric fields. Also complex pattern formation of these structures over multiple length scales on a wide range of surfaces is very challenging.We recently explored liquid crystal templating as a tool to direct the spatial organization of supramolecular materials in water.21 This approach offers a number of unique advantages: (i) LC templating does not depend on delicate molecular interactions and is therefore suitable to organize a wide range of soft materials; (ii) the liquid crystal (LC) template itself is highly susceptible to external stimuli and is readily manipulated to generate complex patterns; (iii) after alignment, organization and optional post-modification, the LC template can be removed, leaving only the aligned functional material on the substrate. LC templating has been applied successfully to align organic22–31 and, to a lesser extent, aqueous functional materials32–37 Mostly, surface interactions were used to control the alignment of aqueous soft matter, although one example of carbon nanotube alignment in a magnetic field aligned lyotropic liquid crystal was published.32
Here, we use the advantages of LC templating and combine them with the unique features of magnetic field alignment, which is an intrinsic contact-free alignment technique (no specific directing surfaces needed). We demonstrate that this approach allows for the formation of complex patterns by cleverly exploiting the differences in sign and strength of the diamagnetic anisotropies of the LC template and the dispersed soft material.
To demonstrate this concept, we aligned and patterned peptide amphiphiles (PA) in a lyotropic chromonic liquid crystal (LCLC) template in a magnetic field. In an aqueous LCLC template solution, PA self-assembles into nanometer-wide fibers and progressively bundles into hierarchical micron to millimeter-sized structures. We show that we need a tenfold lower magnetic field to align these amphiphilic bundles over a centimeter range in the presence of the magnetically responsive LCLC template. Post-modification provides an easy method to introduce functionality and dimensional stability to the structures; the latter allows for removal of the template by washing. The reduction of the magnetic field alignment threshold opens up a window for the creation of more complex structures at high magnetic fields. Here, we anticipate a competition between the magnetically induced alignment of the PA assemblies (with a positive diamagnetic anisotropy) and the elastic force dominated alignment of the template (with a negative diamagnetic anisotropy), which is directed orthogonally.
Materials and methods
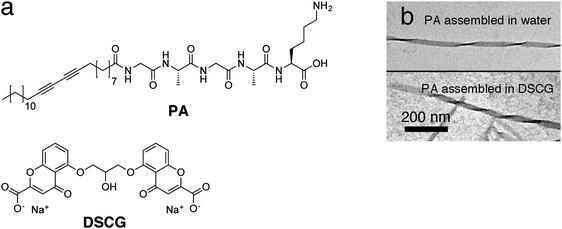
The peptide amphiphile that we use are self-assembling supramolecular materials which have shown a lot of promise in both biomedical engineering and sensing applications.38–45 Its synthesis and characterization has been reported before.21,45 The amphiphile consists of a 25 carbon hydrophobic tail and a GAGAK hydrophilic head section (Fig. 1a). In both water and in nematic DSCG at room temperature, PA self-assembles due to hydrophobic–hydrophilic interactions, forming long 1D beta-sheet fibers (Fig. 1b). In water, these beta-sheets form isotropic assemblies at larger length scales.21 Furthermore, PA was functionalized with a diacetylene-moiety, which allows us to crosslink these materials by a topological photopolymerization step, resulting in a greatly improved mechanical stability and a strong chromatic response due to the π-conjugated backbones. For device applications, such as in tissue engineering38,39,41,42 and molecular electro-optics40 controlled long range ordering of these materials is essential. Macroscopic alignment was realized with shear flow,38,40,46 electrospinning13 and a high magnetic field approach,18,19 whereas multi-length scale control was accomplished using soft lithography47 and recently with an LCLC template on photopatterned substrates.21 The high magnetic field experiments showed that, at sufficient field strengths, PA assemblies align with their long axis parallel to the magnetic fields (with the hydrogen bonds parallel and the alkyl chains perpendicular to the field).18,48 The magnetic alignment of such materials depends on the anisotropy of the diamagnetic susceptibility and the strength of the applied field. We calculated a value of Δχ = Δχ∥ − Δχ⊥ ≈ −611 × 10−12 m3 mol−1 for the molar diamagnetic susceptibility for PA (see ESI†). The number is negative (i.e.PA will align with the molecular axis perpendicular to the field, but 1D assemblies will align parallel to the field) and small, which confirms that even for large assemblies high magnetic fields are necessary to obtain alignment.We chose disodium cromoglycate (DSCG) as a lyotropic chromonic liquid crystal (LCLC) template to direct the organization of PA in magnetic fields. DSCG is a rigid plank-like molecule that consists of an aromatic core with water-solubilizing groups on the periphery (Fig. 1a). In water, the molecules stack face-to-face in columnar aggregates due to π–π stacking and hydrophobic interactions, and at certain concentrations and temperatures, these aggregates form nematic and smectic phases.49 Recently, nematic DSCG solutions have been used to template the organization of motile bacteria35–37 and the alignment of carbon nanotubes34 and peptide amphiphiles.21
The molar diamagnetic susceptibility of DSCG is positive and much larger. We calculated a value of Δχ = χ∥ − χ⊥ ≈ 1226 × 10−12 m3 mol−1. Now the molecules will order with their long axis parallel to the field and the 1D assemblies will order in a plane perpendicular to it. As the concentration DSCG is much higher than the concentration PA, one expects that DSCG can be aligned at much lower field strengths. Indeed, experimentally was found that magnetic fields as low as 0.7 T50,51 are sufficient to align a nematic solution of DSCG. By placing DSCG solutions in a confined space (such as in a glass cell of a several microns spacing), the stacks are forced to align in-plane and perpendicular to the magnetic field vector. The ability of DSCG to act as an aqueous anisotropic solvent which can direct other dispersed materials, while also having relatively high magnetic susceptibilities, makes this particular template an excellent material to control the structural organization of PAs, but potentially a much wider range of aqueous functional soft materials using magnetic fields.
Results and discussion
Unidirectional aligned arrays of π-conjugated peptide amphiphiles
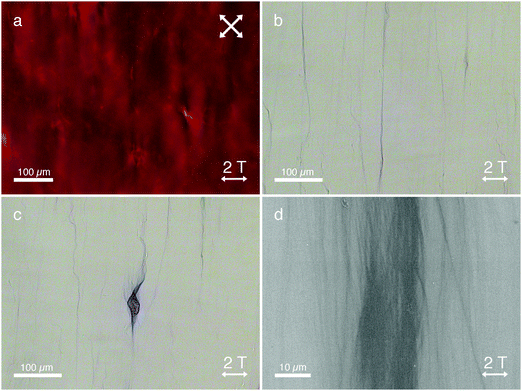
To create unidirectionally aligned arrays of dichromic PAs, we filled glass cells (23 micron spacing) with a mixture of PA (0.026 wt%) and DSCG (13.7 wt%) in milli-Q. The glass cells were sealed with epoxy glue to prevent water evaporation and after 1 hour of drying, the cells were loaded in the magnetic field setup. The temperature was increased to 80 °C after which a magnetic field (B = 2 T) was turned on. With the magnetic field on, the sample was cooled to room temperature over a period of 40–60 minutes. Subsequently, the magnet field was switched off and the sample was removed. Polarized optical microscopy (POM) was used to investigate the assembly and alignment of PA and DSCG.Fig. 2 shows microscopy images of PA and DSCG after cooling to room temperature in the presence of a 2 T magnetic field. DSCG forms a unidirectionally aligned monodomain over centimeter dimensions (Fig. 2a). The DSCG stacks are aligned in-plane and perpendicular to the direction of the magnetic field, as confirmed by analyzing the optical appearance after inserting a quarter wave plate between analyzer and sample in the POM stage (Fig. S1, ESI†). Over the same centimeter length scales, OM reveals extended fibrous bundles aligned (Fig. 2b), which are macroscopically aligned self-assembled PA fibers, oriented parallel to the DSCG template and thus perpendicular to the magnetic field direction. Furthermore, in many locations, spindle-like structures are observed (Fig. 2c) from which aligned fibrous bundles emerge. We believe that these spindles are nucleation sites of the initial PA fibers formation from which (aligned) fiber growth was initiated later in the cooling process.
After assembly in the magnetic field, a photopolymerization step (initiated by leaving the glass cells uncovered on a lab table for several hours or by a 10 minute illumination with a conventional UV-lamp) crosslinked the aligned PA bundles, which consequently became bright blue in color due to the π-conjugated backbone structure (Fig. 2b and c). At this stage, the cells were opened and DSCG was washed away, leaving mechanically stable PA bundles (as a result of the photopolymerization process) organized unidirectionally on the substrate. Scanning electron microscopy (SEM, Fig. 2d) shows massive arrays of fibrous PA bundles unidirectionally aligned perpendicular to the magnetic field. Control experiments demonstrated the role of the liquid crystalline template. Without DSCG present, we found no macroscopic fiber alignment for either the samples grown in the absence (Fig. S2, ESI†) or in the presence (Fig. S3, ESI†) of the magnetic field.
Mixtures of PA in DSCG in the absence of a magnetic field showed random in-plane LCLC alignment, resulting the formation of disordered fibers (Fig. S4, ESI†). We found that the applied magnetic field has no observable influence on the observed PA bundle morphology; the microscopy images of mixtures of PA and DSCG assembled in the absence of a magnetic field (Fig. S5, ESI†) show similar PA assembly morphologies, compared to when PA is assembled in a DSCG template with a magnetic field applied (Fig. 2).
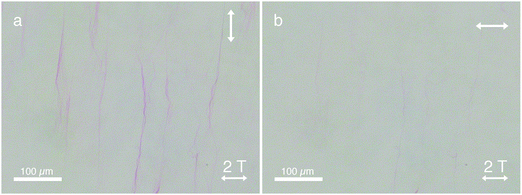
Due to the macroscopic unidirectional alignment of the π-conjugated backbones of these amphiphilic polydiacetylenes, strong linear dichroism is induced (Fig. 3). Linearly polarized light parallel to the backbone of the PA assemblies is strongly absorbed (Fig. 3a), whereas the absorption of linearly polarized light oriented perpendicular to the assemblies is drastically reduced (Fig. 3b). Additionally, the polymerized assemblies are very stable in demanding conditions (Fig. S6, ESI†), even at temperatures as high as 90 °C.21
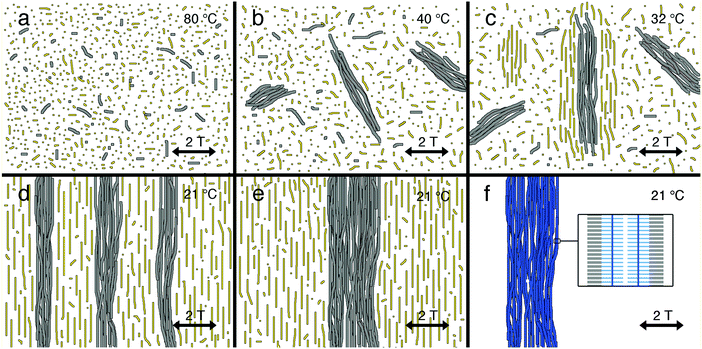
Fig. 4 schematically displays the templating mechanism responsible for macroscopic PA alignment. At high temperatures (>80 °C) both PA (grey, Fig. S7, ESI†) and DSCG49 (yellow) are (mostly) molecularly dissolved (Fig. 4a). Cooling to 50 °C initiates self-assembly of PA fibers, which are not responsive to the 2 T magnetic field thus orient isotropically (Fig. 4b). Simultaneously during cooling, the self-assembled DSCG stacks have grown long enough to form nematic LCLC domains wherein the stacks align perpendicular to the magnetic field (Fig. 4c) as a result of their negative diamagnetic anisotropy. The presence of PA bundles may facilitate LCLC formation (Fig. S8, ESI†) since we observed a slight increase (from 32.7 to 33.7 °C) in the clearing temperature of DSCG (13.4 wt%) with PA present. Whilst the LC domains expand into a single monodomain, reorienting shear forces and elastic mediated forces direct the alignment of PA nanofibers parallel to the LCLC template (Fig. 4d). Meanwhile during the cooling process, depletion interactions with the LCLC solvent forces these nanometer-wide fibers to form much thicker bundles (Fig. 4e and Fig. S9, ESI†). After photopolymerization, the PA assemblies are very stable due to the crosslinked diacetylene cores which are oriented parallel to the fiber's long axis (indicated by the vertical dark blue line in the inset in Fig. 4f). Due to the increased stability, the template can be readily removed. In addition, the PA bundles show strong linear polarized absorption characteristics because of the presence of a transition dipole moment parallel to the π-conjugated polydiacetylene PA backbones.
Hierarchically patterned π-conjugated peptide amphiphiles
Besides utilizing a template to realize macroscopic unidirectional peptide amphiphile orientation, we used the orthogonal diamagnetic anisotropies of DSCG and PA to create complex hierarchical PA structures in high magnetic fields. At these high fields, we anticipated a competition between the two PA alignment forces: the LCLC templates that directs the orientation perpendicular to the magnetic field and the (high) magnetic field itself, which forces the PA stacks to organize parallel to the field.Sealed glass cells containing PA (0.026 wt%) and DSCG (13.7 wt% in milli-Q) were placed in a high magnetic field setup. The glass cells were heated to 80 °C and a 20 T magnetic field was applied. After cooling the cells to room temperature, the magnetic field was switched off and the sample was studied with (polarized) optical microscopy (Fig. 5).
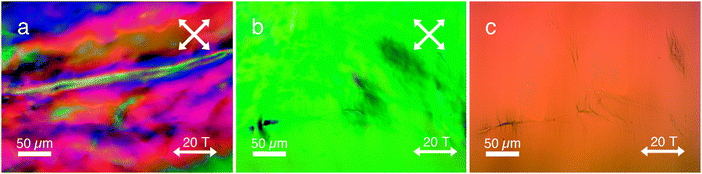
Surprisingly, we observed complex LC director fields (Fig. 5a) as well as areas of large single domains in the range of several millimeters (Fig. 5b). In contrast, when DSCG was aligned in the absence of PA (Fig. S10, ESI†) in a high magnetic field, a perfectly aligned monodomain was obtained. Upon closer inspection with optical microscopy (Fig. 5c), we observed two distinct organizations of fibrous aggregates present everywhere in the sample: (i) fibers aligned parallel to the direction of the applied magnetic field, sometimes in conjunction with spindle-like structures (aligned perpendicular to the field); and (ii) fibers aligned perpendicular to the field in both bundle- and spindle-like formations, identical to the assemblies found after applying a 2 T magnetic field sweep (Fig. 2).
After a photopolymerization step, the orthogonally aligned PA assemblies turned blue in color (Fig. 5c), analogous to the assemblies in Fig. 2. After the cross-linking step, the glass cells were opened and the LCLC was washed away. We used SEM (Fig. 6) to investigate the hierarchical structures with the two distinct organizations at higher magnifications in order to unravel its morphology. Fig. 6a–c shows aligned fibrous bundles oriented parallel to the field, in conjunction with perpendicularly aligned spindle-like aggregates. In some locations (Fig. 6d), only spindle-like aggregates were found (oriented perpendicular to the magnetic field) without any conjoined bundles that were aligned parallel to the field. These structures are also visible with optical microscopy (Fig. 5c, top right corner) and are identical to the assemblies found after a 2 T magnetic field sweep (Fig. 2).
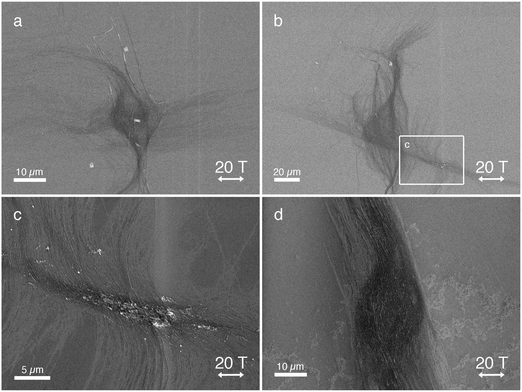 | ||
| Fig. 6 SEM images of PA (0.026 wt%) in a nematic DSCG template (13.7 wt% in milli-Q) after alignment in a 20 T magnetic field. SEM Images (a and b) show orthogonally aligned PA assemblies with fibers oriented both perpendicular and parallel to the magnetic field. Image (c) shows unraveled fibers (perpendicular aligned to the field) from the thicker unidirectionally aligned bundle (parallel to the field). Image (d) shows a unidirectionally aligned spindle similar to a 2 T aligned PA bundle (Fig. 2c). | ||
We postulate that these two distinct PA fiber orientations actually are the result of competition in alignment introduced by the positive diamagnetic susceptibility of PA and perpendicular alignment along the LCLC template induced by its negative diamagnetic susceptibility.52
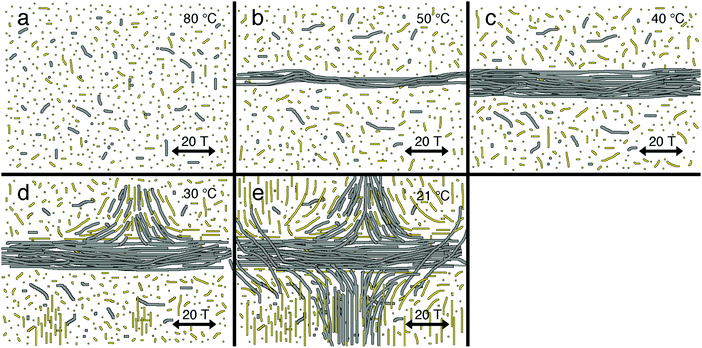
Fig. 7 schematically shows the prime processes when cooling the solution from the molecularly dissolved state at 80 °C (Fig. 7a) in the presence of a high (20 T) magnetic field. Again at approximately 50 °C, PA fiber (grey) formation commences which now is directed by the strong magnetic field (Fig. 7b), yielding bundles parallel to the field. Due to the time and temperature-dependent depletion interactions and the ongoing PA assembly, these fibers continue to grow in dimensions (Fig. 7c). Simultaneously during cooling, small nematic DSCG domains form and grow to eventually form large nematic domains throughout the sample. Newly formed and still dissolved PA fibers may now follow the elastic forces induced by the nematic DSCG phase and orient perpendicular to the fibers that are already present before. They may give rise to new bundles that are entirely aligned perpendicular to the field (Fig. 6d) or associate tangentially to the existing parallel bundles (Fig. 6a–c and 7d).
The elastic forces are not high enough to reorient large PA bundles, which maintain their parallel orientation. One should consider, however, that phase formation in DSCG sets in at a lower temperature than PA assembly. The fact that we see significant amounts of perpendicularly oriented PA fibers suggests that the elastic forces are stronger than the magnetic forces (at 20 T). This is supported by the observation of tiny fibers emerging from the large parallel aligned bundles that unravel and predominantly bend in the direction of the nematic LCLC template (Fig. 6c). These assemblies then act as a nucleation point for further PA assembly and bundling until room temperature is reached (Fig. 7e).
When conducting the same experiment at a lower magnetic field of 15 T, PA neither formed assemblies that aligned parallel to the field nor orthogonal bundled aggregates. Instead, only homogeneously aligned structures as in Fig. 2 and Fig. 6d (perpendicular to the magnetic field direction and parallel to the DSCG stacks) were observed (Fig. S11a and b, ESI†). We found similar PA aggregates (again perpendicular to the field) when PA was assembled at lower concentrations (0.013 wt%) in a DSCG template at 20 T (Fig. S11c, ESI†). Apparently, at these lower concentrations the assembly of PA is dominated by the LCLC template despite the presence of the high magnetic field. On the other hand, at increased PA concentrations (0.039 wt%), the orthogonal structures similar to the ones in Fig. 5c and 6a, b were observed (Fig. S11d, ESI†). The higher PA concentrations result in the formation of larger assemblies which in turn have an increased diamagnetic susceptibility and hence an increased responsiveness to the magnetic field.48 This is further supported by control experiments which show that 0.1 wt% PA (without DSCG present) assembled at 20 T form macroscopic aligned bundles along the magnetic field direction, while at 0.015 wt% PA (without DSCG present) at 20 T no macroscopic alignment is observed (Fig. S12, ESI†).
Overall, the structure formation of these supramolecular materials in LCLC templates and magnetic fields depends on the relative strengths of the two opposing contributions, which both can be tuned independently. The shear and elastic forces of the template can be tailored by the particular LCLC used, as well as the temperature and concentration of the template. The diamagnetic susceptibility of PA can be tuned by its concentration and also the temperature. In addition, the magnetic field itself is an important parameter as is the (temperature, time and concentration dependent) depletion effect. A more detailed understanding of all of these factors will be the starting point to control and utilize the complex structures that are available through these methods.
Conclusion
In this work, we show that we can create unidirectional aligned arrays of functional supramolecular materials on a centimeter scale by combining LCLC templates with 2 T magnetic fields. After photopolymerization, these materials show strong linear polarized absorption characteristics due to the macroscopic aligned π-conjugated backbones. Their increased mechanical strength also allows for the removal of the LCLC template by a simple washing step, leaving the optically active amphiphiles unidirectionally aligned on the substrate. With this approach it is possible to align these materials at 10 times lower magnetic field strengths than previously reported.18This approach has the great advantage that it can be applied to macroscopically align a wide range of soft matter, since the alignment procedure solely relies on orientational shear and elastic forces and lacks the requirement for delicate molecular interactions that require fine tuning. Since we are also able to drastically lower the requirements for magnetic field alignment with this approach, we envisage the use of small commercially available permanent magnets, for instance based on neodymium, integrated within tabletop setups for applying this technique to a huge number of aqueous functional soft materials.
Additionally, the reduction of the field required for alignment opens up the opportunity to create more complex structures at much higher fields. We created orthogonally aligned PA assemblies by exploiting the opposite diamagnetic anisotropies of our LC template and self-assembled amphiphilic materials. Such self-assembled off-equilibrium structures are impossible to create on such a small length scales using other conventional alignment techniques. We believe, therefore, that this approach is very promising to create tailor-made complex structures of (aqueous) functional soft matter highly beneficial for device applications in many diverse areas. A better control of the critical parameters that we set out in the manuscript will be necessary to achieve this.
Acknowledgements
We would like to thank both Britta Ramakers and Dennis Löwik for the synthesis of PA and the TechnoCentrum for the fabrication of the cell construction apparatus. This work is supported by NanoNextNL (PvdA, PHJK, grant 07A.07 & MK, grant 07A.06), a micro and nanotechnology consortium of the Government of the Netherlands and 130 partners. Part of this work is supported by EuroMagNET II under the EU Contract No. 228043. We acknowledge the support of the HFML-RU/FOM, member of the European Magnetic Field Laboratory (EMFL).References
- M. Gerard, A. Chaubey and B. D. Malhotra, Biosens. Bioelectron., 2002, 17, 345–359 CrossRef CAS PubMed.
- N. K. Guimard, N. Gomez and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876–921 CrossRef CAS.
- S. Cosnier and M. Holzinger, Chem. Soc. Rev., 2011, 40, 2146–2156 RSC.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS PubMed.
- L. C. Palmer and S. I. Stupp, Acc. Chem. Res., 2008, 41, 1674–1684 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- B. Su, Y. Wu and L. Jiang, Chem. Soc. Rev., 2012, 41, 7832–7856 RSC.
- Z. Nie and E. Kumacheva, Nat. Mater., 2008, 7, 277–290 CrossRef CAS PubMed.
- H. M. Saavedra, T. J. Mullen, P. Zhang, D. C. Dewey, S. A. Claridge and P. S. Weiss, Rep. Prog. Phys., 2010, 73, 036501 CrossRef.
- B. D. Gates, Q. Xu, M. Stewart, D. Ryan, C. G. Willson and G. M. Whitesides, Chem. Rev., 2005, 105, 1171–1196 CrossRef CAS PubMed.
- D. Li, Y. Wang and Y. Xia, Adv. Mater., 2004, 16, 361–366 CrossRef CAS.
- D. Yang, B. Lu, Y. Zhao and X. Jiang, Adv. Mater., 2007, 19, 3702–3706 CrossRef CAS.
- A. S. Tayi, E. T. Pashuck, C. J. Newcomb, M. T. McClendon and S. I. Stupp, Biomacromolecules, 2014, 15, 1323–1327 CrossRef CAS PubMed.
- M. Wang, L. Du, X. Wu, S. Xiong and P. K. Chu, ACS Nano, 2011, 5, 4448–4454 CrossRef CAS PubMed.
- L. Sardone, V. Palermo, E. Devaux, D. Credgington, M. de Loos, G. Marletta, F. Cacialli, J. van Esch and P. Samorì, Adv. Mater., 2006, 18, 1276–1280 CrossRef CAS.
- Y. Shoji, M. Yoshio, T. Yasuda, M. Funahashi and T. Kato, J. Mater. Chem., 2009, 20, 173–179 RSC.
- M. Yoshio, Y. Shoji, Y. Tochigi, Y. Nishikawa and T. Kato, J. Am. Chem. Soc., 2009, 131, 6763–6767 CrossRef CAS PubMed.
- D. W. P. M. Löwik, I. O. Shklyarevskiy, L. Ruizendaal, P. C. M. Christianen, J. C. Maan and J. C. M. van Hest, Adv. Mater., 2007, 19, 1191–1195 CrossRef.
- M. van den Heuvel, A. M. Prenen, J. C. Gielen, P. C. M. Christianen, D. J. Broer, D. W. P. M. Löwik and J. C. M. van Hest, J. Am. Chem. Soc., 2009, 131, 15014–15017 CrossRef CAS PubMed.
- M. Wallace, A. Z. Cardoso, W. J. Frith, J. A. Iggo and D. J. Adams, Chem. – Eur. J., 2014, 20, 16484–16487 CrossRef CAS PubMed.
- P. van der Asdonk, H. C. Hendrikse, M. F.-C. Romera, D. Voerman, B. E. I. Ramakers, D. W. P. M. Löwik, R. P. Sijbesma and P. H. J. Kouwer, Adv. Funct. Mater., 2016, 26, 2609–2616 CrossRef CAS.
- T. Kato, Y. Hirai, S. Nakaso and M. Moriyama, Chem. Soc. Rev., 2007, 36, 1857–2128 RSC.
- Y. Hirai, S. S. Babu, V. K. Praveen, T. Yasuda, A. Ajayaghosh and T. Kato, Adv. Mater., 2009, 21, 4029–4033 CrossRef CAS.
- K. Akagi, G. Piao, S. Kaneko, K. Sakamaki, H. Shirakawa and M. Kyotani, Science, 1998, 282, 1683–1686 CrossRef CAS PubMed.
- M. Goh, S. Matsushita and K. Akagi, Chem. Soc. Rev., 2010, 39, 2466–2476 RSC.
- M. D. Lynch and D. L. Patrick, Nano Lett., 2002, 2, 1197–1201 CrossRef CAS.
- I. Dierking, G. Scalia, P. Morales and D. LeClere, Adv. Mater., 2004, 16, 865–869 CrossRef CAS.
- O. Catanescu and L.-C. Chien, Adv. Mater., 2005, 17, 305–309 CrossRef CAS.
- S. Moynihan, P. Lovera, D. O'Carroll, D. Iacopino and G. Redmond, Adv. Mater., 2008, 20, 2497–2502 CrossRef CAS.
- X. Tong, D. Han, D. Fortin and Y. Zhao, Adv. Funct. Mater., 2012, 23, 204–208 CrossRef.
- J.-W. Chen, C.-C. Huang and C.-Y. Chao, ACS Appl. Mater. Interfaces, 2014, 6, 6757–6764 CAS.
- J. Lagerwall, G. Scalia, M. Haluska, U. Dettlaff-Weglikowska, S. Roth and F. Giesselmann, Adv. Mater., 2007, 19, 359–364 CrossRef CAS.
- M. Hara, S. Nagano, N. Kawatsuki and T. Seki, J. Mater. Chem., 2008, 18, 3259 RSC.
- N. Ould-Moussa, C. Blanc, C. Zamora-Ledezma, O. D. Lavrentovich, I. I. Smalyukh, M. F. Islam, A. G. Yodh, M. Maugey, P. Poulin, E. Anglaret and M. Nobili, Liq. Cryst., 2013, 40, 1628–1635 CrossRef CAS.
- P. C. Mushenheim, R. R. Trivedi, H. H. Tuson, D. B. Weibel and N. L. Abbott, Soft Matter, 2013, 10, 88 RSC.
- S. Zhou, A. Sokolov, O. D. Lavrentovich and I. S. Aranson, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1265–1270 CrossRef CAS PubMed.
- P. C. Mushenheim, R. R. Trivedi, D. B. Weibel and N. L. Abbott, Biophys. J., 2014, 107, 255–265 CrossRef CAS PubMed.
- S. Zhang, M. A. Greenfield, A. Mata, L. C. Palmer, R. Bitton, J. R. Mantei, C. Aparicio, M. O. de la Cruz and S. I. Stupp, Nat. Mater., 2010, 9, 594–601 CrossRef CAS PubMed.
- J. B. Matson and S. I. Stupp, Chem. Commun., 2012, 48, 26–33 RSC.
- S. R. Diegelmann, N. Hartman, N. Markovic and J. D. Tovar, J. Am. Chem. Soc., 2012, 134, 2028–2031 CrossRef CAS PubMed.
- M. T. McClendon and S. I. Stupp, Biomaterials, 2012, 33, 5713–5722 CrossRef CAS PubMed.
- E. J. Berns, S. Sur, L. Pan, J. E. Goldberger, S. Suresh, S. Zhang, J. A. Kessler and S. I. Stupp, Biomaterials, 2014, 35, 185–195 CrossRef CAS PubMed.
- X. Chen, G. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2012, 41, 4610–4621 RSC.
- B. E. I. Ramakers, M. van den Heuvel, N. Tsichlis i Spithas, R. P. Brinkhuis, J. C. M. van Hest and D. W. P. M. Löwik, Langmuir, 2012, 28, 2049–2055 CrossRef CAS PubMed.
- B. E. I. Ramakers, S. A. Bode, A. R. Killaars, J. C. M. van Hest and D. W. P. M. Löwik, J. Mater. Chem. B, 2015, 3, 2954–2961 RSC.
- B. D. Wall, S. R. Diegelmann, S. Zhang, T. J. Dawidczyk, W. L. Wilson, H. E. Katz, H.-Q. Mao and J. D. Tovar, Adv. Mater., 2011, 23, 5009–5014 CrossRef CAS PubMed.
- A. M. Hung and S. I. Stupp, Nano Lett., 2007, 7, 1165–1171 CrossRef CAS PubMed.
- G. Maret and K. Dransfeld, Strong and Ultrastrong Magnetic Fields and Their Applications, Springer, Berlin, Heidelberg, 1985, vol. 57, pp. 143–204 Search PubMed.
- J. Lydon, J. Mater. Chem., 2010, 20, 10071–10099 RSC.
- V. G. Nazarenko, O. P. Boiko, H. S. Park, O. M. Brodyn, M. M. Omelchenko, L. Tortora, Y. A. Nastishin and O. D. Lavrentovich, Phys. Rev. Lett., 2010, 105, 017801 CrossRef CAS PubMed.
- T. Ostapenko, Y. A. Nastishin, P. J. Collings, S. N. Sprunt, O. D. Lavrentovich and J. T. Gleeson, Soft Matter, 2013, 9, 9487–9498 RSC.
- We noticed that a part of the PA aggregates bundles are not completely aligned parallel or perpendicular to the magnet field direction (Fig. 5c and Fig. 6a, b), which we can attribute to the orthogonal alignment forces acting on the organization of bundled PA fibers (positive diamagnetic susceptibility versus LCLC template shear and elastic forces) which are in the same order of magnitude. A similar effect was described before in ref. 26.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sm00652c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |