Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oil-in-oil emulsions stabilised solely by solid particles†
Bernard P.
Binks
* and
Andrew T.
Tyowua
Department of Chemistry, University of Hull, Hull HU6 7RX, UK. E-mail: b.p.binks@hull.ac.uk
First published on 9th November 2015
Abstract
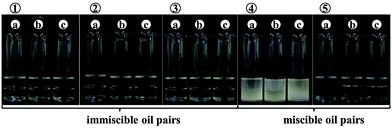
A brief review of the stabilisation of emulsions of two immiscible oils is given. We then describe the use of fumed silica particles coated with either hydrocarbon or fluorocarbon groups in acting as sole stabilisers of emulsions of various vegetable oils with linear silicone oils (PDMS) of different viscosity. Transitional phase inversion of emulsions, containing equal volumes of the two oils, from silicone-in-vegetable (S/V) to vegetable-in-silicone (V/S) occurs upon increasing the hydrophobicity of the particles. Close to inversion, emulsions are stable to coalescence and gravity-induced separation for at least one year. Increasing the viscosity of the silicone oil enables stable S/V emulsions to be prepared even with relatively hydrophilic particles. Predictions of emulsion type from calculated contact angles of a silica particle at the oil–oil interface are in agreement with experiment provided a small polar contribution to the surface energy of the oils is included. We also show that stable multiple emulsions of V/S/V can be prepared in a two-step procedure using two particle types of different hydrophobicity. At fixed particle concentration, catastrophic phase inversion of emulsions from V/S to S/V can be effected by increasing the volume fraction of vegetable oil. Finally, in the case of sunflower oil + 20 cS PDMS, the study is extended to particles other than silica which differ in chemical type, particle size and particle shape. Consistent with the above findings, we find that only sufficiently hydrophobic particles (clay, zinc oxide, silicone, calcium carbonate) can act as efficient V/S emulsion stabilisers.
Introduction
It has been well documented that a variety of solid particles can adsorb at fluid interfaces1 and hence stabilise emulsions and foams as well as enabling the preparation of novel materials like dry water,2–4 dry oil,5–7 liquid marbles8–11 and powdered emulsions.12 The fluid pairs include oil and water (Pickering emulsions),13 air and water (particle-stabilised aqueous foams),14 air and oil (particle-stabilised oil foams)15 and water and water (Pickering emulsions).16 This paper is concerned with mixtures of immiscible oils stabilised by particles, so-called oil-in-oil, o/o, emulsions. We refer specifically to immiscible liquids of low dielectric constant ε (both ≤3.2 at 25 °C), like vegetable oil + silicone oil. Although in the literature water-free emulsions have been termed non-aqueous or even oil-in-oil emulsions,17–20 in which the water component (ε = 80.1) has been replaced by other polar solvents like ethylene glycol (ε = 37.0), dimethyl formamide (ε = 36.7) or dimethyl sulphoxide (ε = 46.7), we will not be concerned with these here. However, we acknowledge the earlier studies on alcohol (ε = 24.5–32.7)–alkane (ε = 2.0) mixtures stabilised by particles yielding bijels or conventional droplet emulsions depending on the processing route in changing the temperature of the system.21 In addition, literature exists on the use of both hydrophobic and hydrophilic particles in stabilising immiscible polymer blends,22–25e.g. poly(isobutylene)-in-poly(dimethylsiloxane) blends which are very stable to coalescence have been prepared in the presence of fumed silica particles.22There are very few reports in the literature on oil-in-oil emulsions, where both oils have low dielectric constants, and the majority of these are patents. Table 1 contains a summary of those we could find in year order with an indication of which oil constitutes the dispersed/continuous phase, the type of emulsifier and the kind of application.26–40 The mutual solubilities of the oil pairs are not reported but from our own work we have established that certain combinations exhibit non-trivial mutual miscibility. It can be seen that o/o emulsions are of use in a wide variety of industries including cosmetics,31–33 personal care,26,28,31,34 electronics29,30,39 and pharmaceuticals.35,40 It is also worth adding that these emulsions are sometimes used as metalworking fluids which must exclude water and as chemical reaction vehicles involving reactants sensitive (even explosive) to the presence of traces of water.37 Nearly all of the examples given involve a silicone oil (low intermolecular forces) as either the droplet or continuous phase in combination with either mineral/paraffin oil or vegetable oil. Ten of the fifteen compositions contain molecular surfactant28,35,37,40 or polymer27,30,31,34,36,39 as the emulsifier, whilst in four of them the emulsions are stabilised by solid particles alone. The latter are all hydrophobic in nature (organo-clay,26 fluoro-silicone,32 fluoro-lauroyl taurate33 and wax38). In a couple of papers, Nonomura et al. determined the phase diagram of ternary mixtures of a silicone oil, perfluoropolymethylisopropyl ether (a fluorinated oil) and either spherical fluorinated silicone resin particles (diameter 4.5 μm)32 or plate-like fluorinated calcium lauroyl taurate particles (diameter 8 μm).33 Seven or eight different regions were identified in which, inter alia, powders, granules, dispersions and emulsions were formed. The emulsions were only of the kind silicone oil-in-fluorinated oil with droplets being shown to be coated by a dense layer of particles. No description of their sizes or bulk emulsion stability was given however. In the patent authored by Cauvin et al.,38 silicone oil-in-rapeseed oil emulsions were stabilised by wax crystals formed in situ by cooling the vegetable oil phase prior to emulsification. A triggered release of small antifoam particles of silica dispersed in the silicone oil droplets, achieved by heating the emulsion above the melting temperature of the wax particles coating the drops, allowed effective defoaming of surfactant-stabilised aqueous foams. Since the other reports are patents without scientific insight, it is thus clear that a detailed understanding of oil-in-oil emulsions is lacking and this is what we set out to achieve here.
| Dispersed phase | Continuous phase | Emulsifier | Application | Ref. |
|---|---|---|---|---|
| a PDMS – poly(dimethylsiloxane). b PMPS – poly(methylphenylsiloxane). c Although its dielectric constant is >3 it is included as an interesting oil. d PFPMIE – perfluoropolymethylisopropyl ether. e BA – butyl acrylate. f O/NPE – octyl/nonylpolyoxyethylene ether. | ||||
| Silicone oil (PDMS,a PMPSb) | Mineral oil | Hydrophobic bentonite particles | Lubricants on fibers | 26 |
| Silicone oil, >300 cS (polydialkylsiloxane) | Mineral oil | Ethylene–vinyl acetate copolymer | Foam inhibitor for lubricating oils | 27 |
| Triglyceride oil (soybean, rapeseed…) | Silicone oil, <500 cS (PDMS) | Silicone surfactant | Lubricant on fibers | 28 |
| Silicone oil, 2500 cS (PDMS) | Chlorinated paraffin oil, 340 cS | None (drops had short lifetimes) | Electro-rheological fluid | 29 |
| Chlorinated paraffin oil, 340 cS | Silicone oil, 2500 cS (PDMS) | None (drops had short lifetimes) | ||
| Silicone oil, 1000 cS (PDMS) | Thermotropic liquid crystalc | Cyanobiphenyl-PDMS oligomer | Electro-optical display devices | 30 |
| Thermotropic liquid crystal | Silicone oil, 1000 cS (PDMS) | Cyanobiphenyl-PDMS polymer | ||
| Mineral oil or vegetable oil | Silicone oil, <1000 cS | Elastomeric silicone polyether | Personal care and cosmetics | 31 |
| Silicone oil (PDMS) | PFPMIEd | Fluorinated silicone resin particles (sphere) | Cosmetics | 32 |
| Silicone oil (PDMS) | PFPMIE | Fluorinated Ca lauroyl taurate particles (plate) | Cosmetics | 33 |
| Mineral oil or animal or vegetable oil | Silicone oil (PDMS) | Block copolymer, e.g. p(BAe)-PDMS-p(BA) | Personal care | 34 |
| Castor oil | Silicone oil, <100 cS (PDMS) | Nonionic surfactant (O/NPEf) | Pharmaceutical formulations | 35 |
| Organic phosphate | Hydrocarbon | Diblock or triblock copolymer | Liquid toning systems | 36 |
| Hydrocarbon | Fluorocarbon | Fluorocarbon surfactant | Vehicles for chemical reactions | 37 |
| (Silica in) PDMS | Vegetable oil or PEO–PPO copolymer | Wax crystals | Antifoam | 38 |
| Organic phosphate or silicone oil | Paraffin oil or white mineral oil or cyclic silicone | Hydrophobic fumed silica particles + polymer co-stabiliser | Electro-optical modulating display devices | 39 |
| Castor oil | Silicone oil, 20 cS | Silicone surfactant | Pharmaceutical formulations | 40 |
| Silicone oil, 20 cS (PDMS) | Castor oil | Silicone surfactant | ||
In this paper, we explore systematically the behaviour of immiscible mixtures of vegetable oil and silicone oil in the presence of fumed silica particles, coated with either hydrocarbon groups or fluorocarbon chains. We modify a previously derived theory to calculate the contact angle a spherical particle adopts at the relevant oil–oil interface and compare the experimental findings of emulsion type with these calculations. Simple emulsions of both types and multiple oil-in-oil-in-oil emulsions can be prepared which are ultra-stable to coalescence. Evidence is given for both transitional and catastrophic phase inversion of emulsions. We also explore the possibility of stabilising such emulsions with other types of particle.
Experimental
Materials
Two types of fumed silica particles were used, dichlorodimethylsilane Si(CH3)2Cl2, DCDMS-coated particles and fluoro-coated particles both from Wacker Chemie, Burghausen. The silica particles have varying percentages of residual surface SiOH groups. Both are composed of aggregates of particles of primary diameter 20–30 nm and were obtained by surface chemical modification of hydrophilic fumed silica (100% SiOH, surface area 200 m2 g−1). The silanol content of hydrocarbon-coated particles is varied from 100 to 14% and that of fluoro-coated particles was either 75%, 59% or 50%. They were obtained by reacting hydrophilic silica particles with varying amounts of either DCDMS41 or tridecafluoro-1,1,2,2-tetrahydrooctyltrimethoxysilane, CF3(CF2)5(CH2)2Si(OCH3)3, TDF42 respectively as described earlier. Details of the other particle types used are given in Table S1 of the ESI.† Three triglyceride vegetable oils were selected whose main fatty acid chains derive from oleic (C18, one double bond), linoleic (C18, two double bonds) or erucic (C22, one double bond) acids. They are, respectively, olive oil (Sigma, density ρ = 0.91 g cm−3, ε = 3.1 at 25 °C), sunflower oil (Tesco, ρ = 0.92 g cm−3, ε = 3.2) and rapeseed oil (Fluka, ρ = 0.92 g cm−3, ε = 3.1) and were passed twice through a basic alumina column to remove polar impurities before use. The polydimethylsiloxane, PDMS, silicone oils of viscosity 20 (ρ = 0.95 g cm−3, ε = 2.7), 50 (ρ = 0.96 g cm−3, ε = 2.7) and 100 cS (ρ = 0.97 g cm−3, ε = 2.7) were purchased from Dow Corning and used as received.Methods
| Oil–oil interface | γ o1o2/±0.2 mN m−1 |
|---|---|
| Olive–20 cS PDMS | 1.0 |
| Olive–50 cS PDMS | 2.0 |
| Olive–100 cS PDMS | 2.8 |
| Sunflower–20 cS PDMS | 1.8 |
| Sunflower–50 cS PDMS | 2.7 |
| Sunflower–100 cS PDMS | 1.5 |
| Rapeseed–20 cS PDMS | 1.0 |
| Rapeseed–50 cS PDMS | 2.3 |
| Rapeseed–100 cS PDMS | 2.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min. In other experiments to determine the influence of initial particle location, particles were wetted first by the vegetable oil after which PDMS oil was added and the mixture homogenised as before. Multiple oil-in-oil-in-oil emulsions were prepared in two stages. A simple emulsion of vegetable oil-in-silicone oil was prepared as above stabilised by 14% SiOH particles. This emulsion was then either stirred gently at 500 rpm with a magnetic stirrer or mixed with high shear at 6000 rpm with the Ultra-Turrax homogeniser into a vegetable oil dispersion of 51% SiOH particles. The converse was also attempted; silicone oil-in-vegetable oil emulsion with 51% SiOH particles mixed into silicone oil dispersion containing 14% SiOH particles.
000 rpm for 2 min. In other experiments to determine the influence of initial particle location, particles were wetted first by the vegetable oil after which PDMS oil was added and the mixture homogenised as before. Multiple oil-in-oil-in-oil emulsions were prepared in two stages. A simple emulsion of vegetable oil-in-silicone oil was prepared as above stabilised by 14% SiOH particles. This emulsion was then either stirred gently at 500 rpm with a magnetic stirrer or mixed with high shear at 6000 rpm with the Ultra-Turrax homogeniser into a vegetable oil dispersion of 51% SiOH particles. The converse was also attempted; silicone oil-in-vegetable oil emulsion with 51% SiOH particles mixed into silicone oil dispersion containing 14% SiOH particles.
The drop test was used to confirm the continuous phase of the emulsions. Vegetable oil (PDMS oil) continuous emulsions disperse easily in vegetable oil (PDMS oil), but did not disperse easily in PDMS oil (vegetable oil). The stability of the emulsions to creaming or sedimentation and coalescence was assessed by noting the fraction of PDMS oil fPDMS or vegetable oil fv and the necessary oil phase, respectively, released in a month. The fv represents the fraction of sunflower oil (fsuno), olive oil (foo) or rapeseed oil (fro) released within a given period. The parameter fv (fPDMS) refers to the ratio of the volume of vegetable oil (PDMS oil) released to the initial volume of vegetable oil (PDMS oil) used. For optical microscopy, a drop of the silica particle-stabilised emulsions was diluted with 1–5 drops of its continuous phase and viewed on a dimple glass slide (Fisher Scientific). The emulsions were viewed using an Olympus BX-51 optical microscope fitted with a DP50 digital camera using Image-Pro Plus 5.1 software (Media Cybernetics). The optical micrographs were used to determine the droplet size of the emulsions by averaging the diameter of ≈300 emulsion droplets.
A small volume of emulsion was pipetted on to a cryo-SEM stub (diameter ∼10 mm) and plunged into liquid nitrogen slush at −210 °C. The frozen sample was transferred to the cryo-SEM preparation chamber (PP3010T, Quorum Technologies Ltd) where it was fractured and sputter-coated with platinum to a thickness of ∼2 nm. The sample was then transferred to a Zeiss EVO 60 scanning electron microscope chamber for viewing and imaging. Micrographs were taken at a voltage of 20 kV and a probe current of 70 pA.
All experiments were carried out at room temperature (22 ± 2 °C) unless stated otherwise.
Results and discussion
We first describe the stabilisation of emulsions of vegetable oil and silicone oil by DCDMS-coated fumed silica particles and compare the findings with those expected from a consideration of the calculated oil–oil-particle contact angle. This is followed by a description of the behaviour of emulsions stabilised by TDF-coated silica particles. Finally, the study is extended to the use of a variety of other particle types differing in size, shape and chemical composition.1. Simple and multiple emulsions stabilised by DCDMS-coated silica particles
 | ||
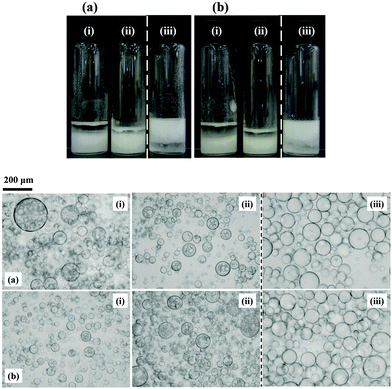
| Fig. 3 Corresponding optical micrographs of the emulsions shown in Fig. 2 at selected % SiOH (given) for (a) sunflower oil and (b) olive oil with DCDMS-coated particles. | ||
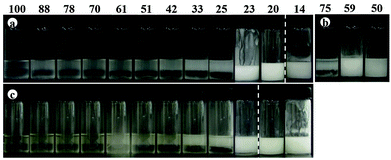
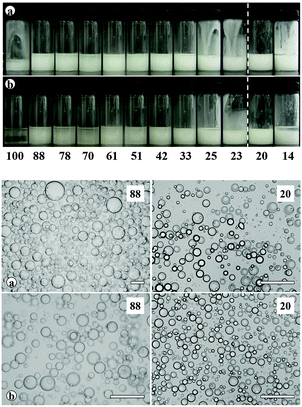
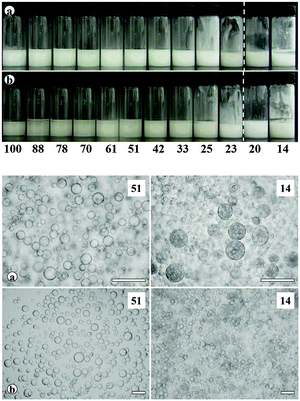
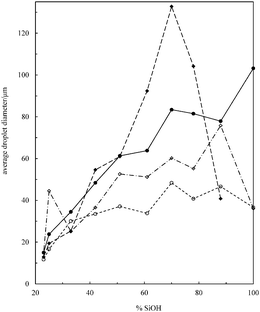
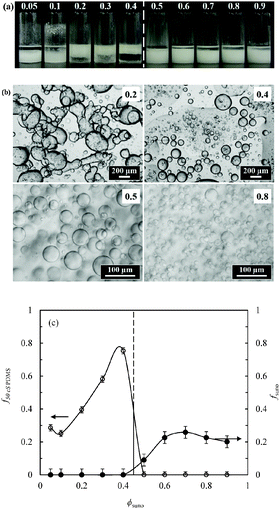
Transitional phase inversion is also observed for emulsions of sunflower or olive oil and 50 cS PDMS seen in Fig. 4. Phase inversion from S/V to V/S emulsions occurs for particles possessing between 23 and 20% SiOH. For both vegetable oils in this case, S/V emulsions stable to coalescence were obtained with particles containing between 88 and 23% SiOH on their surfaces. They released a small fraction of vegetable oil within a month through sedimentation however, with stability increasing towards phase inversion, Fig. S2 (ESI†). We note that an S/V emulsion stable to coalescence is formed with sunflower oil and particles containing 100% SiOH groups, i.e. even for inherently hydrophilic particles, although the corresponding emulsion is unstable to coalescence in the case of olive oil. Similarly, V/S emulsions stabilised by particles possessing 20% SiOH groups were stable to both coalescence and creaming. Also, a V/S emulsion stable to coalescence forms with the most hydrophobic particles (14% SiOH) and sunflower oil, although the corresponding emulsion with olive oil is an unstable multiple one (V/S/V). A similar trend was observed for the more viscous 100 cS PDMS oil systems, with emulsion phase inversion again occurring for particles possessing between 23 and 20% SiOH on their surfaces, Fig. 5 and Fig. S2 (ESI†). The relationship between the average droplet diameter of the S/V emulsions and the % SiOH on particle surfaces is shown in Fig. 6. Although there are a few exceptions, the droplet size decreases as the % SiOH decreases approaching phase inversion in line with the increase in emulsion stability. Silicone oil droplets are in general smaller for the higher viscosity PDMS oil.
The formation of stable emulsions with fumed silica particles having a relatively high percentage of SiOH groups, e.g. 88–70%, was not anticipated based on existing literature (Table 1). The stabilisation of emulsions in the presence of these particles might be due to the tendency of the particles to behave more hydrophobically in the oils due to a reduction in the effective SiOH content on the particles surfaces as a result of gel formation via hydrogen bonds between silanol groups.45 In order to verify this, oil dispersions were prepared in both types of oil for completely hydrophilic (100% SiOH) and the most hydrophobic (14% SiOH) fumed silica particles. Photographs of the glass vials containing the oil dispersions are shown in Fig. S3 (ESI†). The PDMS oil dispersions containing 2 wt% of 100% SiOH fumed silica were fluid, but those containing 5 wt% of the particles gelled (a). The viscosity of the gels was seen visually to increase with the viscosity of the PDMS oil. On the contrary, the vegetable oil dispersions were fluid at both particle concentrations (b). For the 14% SiOH fumed silica particles, no gel was obtained with either the PDMS oils or the vegetable oils up to 5 wt% of the particles (c). These findings indicate that the silica particles are interacting more (via hydrogen bonding) in the PDMS oils than in the vegetable oils. Microscopic oil droplets can be trapped in an oil-particle gel contributing to the overall stability the emulsions. This is different in water where solvation of particle surfaces prevents the formation of hydrogen bonds between particles such that particles behave hydrophilically.45
For some particle-stabilised emulsions incorporating water, the initial location of the particles in oil or water dictates the type of the final emulsion, argued in terms of the hysteresis of contact angle at the three-phase line.46,47 As such, the effect of the initial location of the fumed silica particles in the oil phases was also investigated here for selected particles. DCDMS-coated fumed silica particles possessing either 51% or 14% SiOH were used with the oils. Because the oils wet these particles spontaneously, the required amount (0.2 g) of particles was placed on the surface of either vegetable oil or PDMS oil (5 cm3). The particles were wetted completely by the oils and entered in them before the second oil phase was added. Photographs and the corresponding optical micrographs of the simple oil-in-oil emulsions prepared are given in Fig. S4 (ESI†). For all the vegetable oil–PDMS oil combinations studied, the emulsion type is independent of the initial location of the particles. Their ensuing stability and average drop size are also very similar and not affected by which oil contacts the particles first.
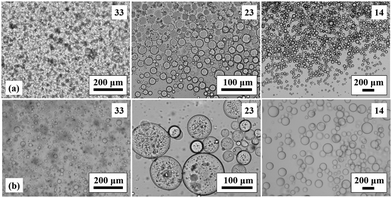
As in the case of surfactant-stabilised emulsions where two surfactants of different hydrophile–lipophile balance number are required for the stabilisation of multiple emulsions,48 two particle types of different wettability are normally required for the stabilisation of multiple Pickering emulsions.49,50 Kinetically stable multiple emulsions of both types (oil-in-water-in-oil and water-in-oil-in-water) have been prepared using a mixture of hydrophilic and hydrophobic silica particles (for inner drops and outer globules) in a two stage emulsification method.49 In this study, we have seen that particles possessing relatively high percentages of SiOH groups prefer to stabilise vegetable oil-continuous emulsions whilst those with 20 or 14% SiOH prefer to stabilise silicone oil-continuous emulsions. Using a two stage emulsification method exploiting this variation of emulsion type with particle type, oil-in-oil-in-oil multiple emulsions were prepared stabilised by a mixture of 51 and 14% SiOH particles. The concentration of these particles in the multiple emulsions was approx. 1 wt%. A V/S emulsion stabilised by 14% SiOH particles was stirred at 500 rpm with a vegetable oil dispersion of 51% SiOH particles resulting in a V/S/V multiple emulsion stable to coalescence for up to one year. If however high shear was used (6000 rpm via Ultra-Turrax homogeniser) only a simple S/V emulsion formed as a result of catastrophic phase inversion of the precursor V/S emulsion. A photo' of the glass vials containing the V/S/V emulsions prepared using low shear and their corresponding optical micrographs are shown in Fig. 7. The V/S/V multiple emulsions contain different volume fractions of the initial V/S emulsions. On the contrary, in attempts to prepare S/V/S emulsions, gently stirring S/V emulsions stabilised by 2 wt% of 51% SiOH silica into a silicone oil dispersion of 14% SiOH silica only produced unstable V/S emulsions (Fig. 7).
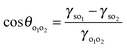 | (1) |
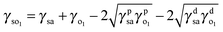 | (2) |
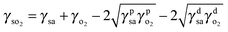 | (3) |
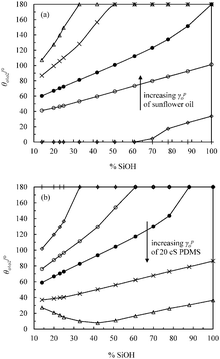
In order to mimic the surface of the most hydrophilic (100% SiOH) and most hydrophobic (14% SiOH) particles, we prepared completely hydrophilic glass slides and glass slides coated with the highest density of DCDMS groups respectively, and measured the contact angles of the individual oils in air and of a drop of PDMS oil under vegetable oil (θo1o2) on these substrates, Table 4. All oils wet the hydrophilic substrate in air as expected (θo < 5°). For hydrophilic slides, θo1o2 is around 150° implying the substrate is more wet by vegetable oil. For hydrophobic slides, the advancing θo1o2 is around 70° and the more wetting oil is now PDMS. In line with these measurements for every oil–oil combination investigated, the preferred emulsion type (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is S/V for relatively hydrophilic particles and V/S or S/V/S for more hydrophobic ones (last column in Table 4). The findings are consistent with the principle put forward by Finkle et al.54 for oil–water-particle systems which stated that the liquid wetting the particles less becomes the dispersed phase. This is the first time this principle has been verified in oil–oil-particle systems.
1) is S/V for relatively hydrophilic particles and V/S or S/V/S for more hydrophobic ones (last column in Table 4). The findings are consistent with the principle put forward by Finkle et al.54 for oil–water-particle systems which stated that the liquid wetting the particles less becomes the dispersed phase. This is the first time this principle has been verified in oil–oil-particle systems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures stabilised by 100% SiOH or 14% SiOH silica particles respectively
1 mixtures stabilised by 100% SiOH or 14% SiOH silica particles respectively
| Glass surface | Fluid pair | Contact angle/° | Emulsion type |
|---|---|---|---|
| a Contact angle is through water. | |||
| Hydrophilic | Water–air | <5a | |
| Sunflower oil–air | <5 | ||
| Olive oil–air | <5 | ||
| 20 cS PDMS–air | <5 | ||
| 50 cS PDMS–air | <5 | ||
| 100 cS PDMS–air | <5 | ||
| Sunflower oil–water | 52, 32a | Not studied | |
| Olive oil–water | 43, 19a | Not studied | |
| Sunflower oil–20 cS PDMS | 150, 150 | Unstable S/V | |
| Sunflower oil–50 cS PDMS | 148, 160 | Stable S/V | |
| Sunflower oil–100 cS PDMS | 152, 158 | Stable S/V | |
| Olive oil–20 cS PDMS | 148, 151 | Unstable S/V | |
| Olive oil–50 cS PDMS | 148, 150 | Unstable S/V | |
| Olive oil–100 cS PDMS | 152, 154 | Stable S/V | |
| Hydrophobic | Water–air | 107, 110a | |
| Sunflower oil–air | 52, 35 | ||
| Olive oil–air | 58, 34 | ||
| 20 cS PDMS–air | <5 | ||
| 50 cS PDMS–air | <5 | ||
| 100 cS PDMS–air | <5 | ||
| Sunflower oil–water | 168, 171a | Not studied | |
| Olive oil–water | 167, 169a | Not studied | |
| Sunflower oil–20 cS PDMS | 67, 44 | Stable V/S | |
| Sunflower oil–50 cS PDMS | 72, 34 | Stable V/S | |
| Sunflower oil–100 cS PDMS | 81, 42 | Unstable S/V/S multiple emulsion | |
| Olive oil–20 cS PDMS | 70, 36 | Stable V/S | |
| Olive oil–50 cS PDMS | 82, 32 | Unstable S/V/S multiple emulsion | |
| Olive oil–100 cS PDMS | 71, 45 | Stable V/S | |
2. Emulsions stabilised by TDF-coated silica particles
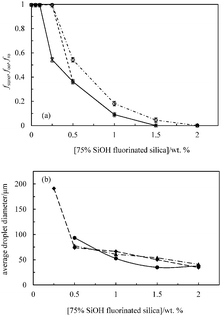 | ||
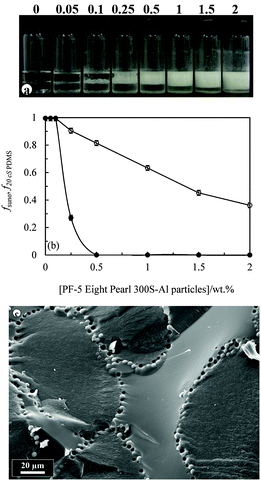
| Fig. 10 (a) Fraction of sunflower oil fsuno (○) or olive oil foo (⋄) or rapeseed oil fro (×) released from 50 cS PDMS-in-sunflower oil, 50 cS PDMS-in-olive oil or 50 cS PDMS-in-rapeseed oil emulsions, respectively, shown in Fig. 9versus concentration of 75% SiOH fluorinated silica particles. (b) Average droplet diameter after 1 month of PDMS drops in sunflower oil (●), olive oil (▲) or rapeseed oil (◆) emulsions versus particle concentration. The standard deviation decreases from ∼40 μm at 0.25 wt% to ∼10 μm at 2 wt%. | ||
3. Emulsions stabilised by other types of particle
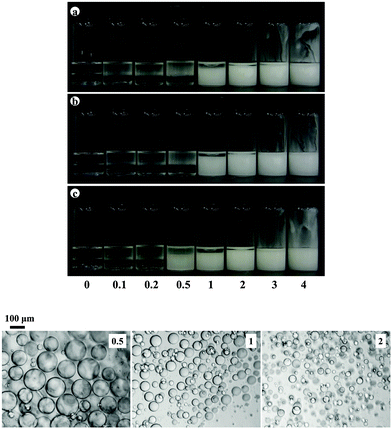
In order to ascertain how general the findings obtained using fumed silica particles are, we have studied emulsions stabilised by a wide range of different particle types, shape and size for the mixture sunflower oil–PDMS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The majority of the particles are hydrophobic imparted by either long hydrocarbon or fluorocarbon chains. The PF-5 Eight Pearl 300S-Al powder comprises partially fluorinated sericite platelet particles ca. a few μm long and several nm thick.7 The influence of particle concentration on the appearance of emulsions containing 20 cS PDMS is shown in Fig. 12(a). Preferred emulsions are V/S and their stability to both creaming and coalescence increases with particle concentration, Fig. 12(b). Similar trends in behaviour are witnessed with the same particles and both olive oil–PDMS emulsions (Fig. S7 and S8, ESI†) and rapeseed oil–PDMS emulsions (Fig. S9 and S10, ESI†), where it can be seen that around 1 wt% of particles is required for emulsions stable to coalescence.
1). The majority of the particles are hydrophobic imparted by either long hydrocarbon or fluorocarbon chains. The PF-5 Eight Pearl 300S-Al powder comprises partially fluorinated sericite platelet particles ca. a few μm long and several nm thick.7 The influence of particle concentration on the appearance of emulsions containing 20 cS PDMS is shown in Fig. 12(a). Preferred emulsions are V/S and their stability to both creaming and coalescence increases with particle concentration, Fig. 12(b). Similar trends in behaviour are witnessed with the same particles and both olive oil–PDMS emulsions (Fig. S7 and S8, ESI†) and rapeseed oil–PDMS emulsions (Fig. S9 and S10, ESI†), where it can be seen that around 1 wt% of particles is required for emulsions stable to coalescence.
Emulsions comprising equal volumes of sunflower oil and 20 cS PDMS oil were also prepared in the presence of 1 wt% of either (i) hydrophilic rod-shaped CaCO3 particles (25 μm, Whiscal A) or platelet bentonite particles (>100 μm, PFX-10 Kunipia F) or (ii) hydrophobic spherical silicone particles (4.5 μm, PF-5 Tospearl 145A), platelet sericite particles (few μm, PF-10 FSE Al), spherical ZnO particles (few μm, PFX-10 ZnO (TP)), platelet bentonite particles (2–30 μm, Bentone 34), spherical CaCO3 particles (0.1 μm, Calofort SV) and irregular shaped PTFE particles (1–10 μm, Zonyl MP1400). As seen in Fig. S11 (ESI†), the hydrophilic particles stabilised S/V emulsions temporarily but these exhibited complete phase separation within several days as expected. Hydrophobic particles however stabilised V/S emulsions which, apart from those containing Bentone 34, were stable to coalescence for at least one year. The cryo-SEM image in Fig. 12(c) is that of a V/S emulsion stabilised by spherical particles of fluorinated silicone resin. The two oil phases are distinguished from each other by their different texture upon freezing; vegetable oil is relatively rough compared with silicone oil which is smooth. Particles are entirely at the interface of the droplets and relatively close-packed. The appearance of spherical voids on interfaces where particles were originally located suggests these particles are dislodged from the interface during either the freezing or fracturing of the emulsion. This brief study extends what we discovered with silica particles to a wider range of systems and reinforces our earlier findings that hydrophobic particles of different size and shape are effective stabilisers of oil-in-oil emulsions.
Conclusions
By systematically varying the inherent hydrophobicity of fumed silica particles in mixtures of immiscible vegetable oil and silicone oil, we find that emulsions are S/V for relatively hydrophilic particles but V/S for very hydrophobic particles. Close to the inversion condition, emulsions are stable to coalescence and gravity-induced separation and of minimum drop size. Predictions of emulsion type based on calculated contact angles of a particle at the oil–oil interface are in good agreement with experiment providing a small polar contribution is included in the surface tension of the oils. By utilising two particle types of different hydrophobicity, we prepare a multiple V/S/V emulsion in which more hydrophobic particles coat inner droplets and more hydrophilic particles coat outer globules. Importantly, catastrophic phase inversion from V/S to S/V emulsions can be effected by increasing the volume fraction of vegetable oil, demonstrating that both emulsion types can be stabilised with one and the same particles. For a range of hydrophobic particles of different chemical type, size and shape, we confirm that they can also be efficient stabilisers of V/S emulsions.Acknowledgements
We are grateful to the Tertiary Education Trust Fund of Nigeria and the University of Hull for a studentship to ATT and Mr A. Sinclair for carrying out SEM analysis.References
- Colloidal Particles at Liquid Interfaces, ed. B. P. Binks and T. S. Horozov, Cambridge University Press, Cambridge, 2006 Search PubMed.
- B. P. Binks and R. Murakami, Nat. Mater., 2006, 5, 865–869 CrossRef CAS PubMed.
- L. Forny, I. Pezron, K. Saleh, P. Guigon and L. Komunjer, Powder Technol., 2007, 171, 15–24 CrossRef CAS.
- B. O. Carter, W. Wang, D. J. Adams and A. I. Cooper, Langmuir, 2010, 26, 3186–3193 CrossRef CAS PubMed.
- R. Murakami and A. Bismarck, Adv. Funct. Mater., 2010, 20, 732–737 CrossRef CAS.
- B. P. Binks, T. Sekine and A. T. Tyowua, Soft Matter, 2014, 10, 578–589 RSC.
- B. P. Binks, S. K. Johnston, T. Sekine and A. T. Tyowua, ACS Appl. Mater. Interfaces, 2015, 7, 14328–14337 CAS.
- E. Bormashenko, Curr. Opin. Colloid Interface Sci., 2011, 16, 266–271 CrossRef CAS.
- A. Hashmi, A. Strauss and J. Xu, Langmuir, 2012, 28, 10324–10328 CrossRef CAS PubMed.
- G. McHale and M. I. Newton, Soft Matter, 2015, 11, 2530–2546 RSC.
- N. M. Oliveira, C. R. Correia, R. L. Reis and J. F. Mano, Adv. Healthcare Mater., 2015, 4, 264–270 CrossRef CAS PubMed.
- R. Murakami, H. Moriyama, M. Yamamoto, B. P. Binks and A. Rocher, Adv. Mater., 2012, 24, 767–771 CrossRef CAS.
- J. Tang, P. J. Quinlan and K. C. Tam, Soft Matter, 2015, 11, 3512–3529 RSC.
- M. V. Tzoumaki, D. Karefyllakis, T. Moschakis, C. G. Billaderis and E. Scholten, Soft Matter, 2015, 11, 6245–6253 RSC.
- B. P. Binks, A. Rocher and M. Kirkland, Soft Matter, 2011, 7, 1800–1808 RSC.
- B. T. Nguyen, T. Nicolai and L. Benyahia, Langmuir, 2013, 29, 10658–10664 CrossRef CAS PubMed.
- R. V. Petersen and R. D. Hamill, J. Soc. Cosmet. Chem., 1968, 19, 627–640 CAS.
- A. Imhof and D. J. Pine, J. Colloid Interface Sci., 1997, 192, 368–374 CrossRef CAS.
- A. K. F. Dyab and A. M. Atta, RSC Adv., 2013, 3, 25662–25665 RSC.
- B. P. Binks, P. D. I. Fletcher, M. A. Thompson and R. P. Elliott, Langmuir, 2013, 29, 5723–5733 CrossRef CAS PubMed.
- P. S. Clegg, E. M. Herzig, A. B. Schofield, S. U. Egelhaaf, T. S. Horozov, B. P. Binks, M. E. Cates and W. C. K. Poon, Langmuir, 2007, 23, 5984–5994 CrossRef CAS PubMed.
- J. Vermant, G. Cioccolo, K. Golapan Nair and P. Moldenaers, Rheol. Acta, 2004, 43, 529–538 CrossRef CAS.
- P. Thareja and S. Velankar, Rheol. Acta, 2007, 46, 405–412 CrossRef CAS.
- L. Elias, F. Fenouillot, J. C. Majeste and P. Cassagnau, Polymer, 2007, 48, 6029–6040 CrossRef CAS.
- L. Elias, F. Fenouillot, J. C. Majesté, P. Alcouffe and P. Cassagnau, Polymer, 2008, 49, 4378–4385 CrossRef CAS.
- R. D. Vartanian, Stable mineral oil-silicone oil compositions, US Pat., 3445385, American Cyanamid Company, 1969 Search PubMed.
- A. Guillaume and F. Sagi, Homogeneous dispersions of diorganopolysiloxane compositions in mineral oils, US Pat., 4115343, Rhone-Poulenc Industries, 1978 Search PubMed.
- G. H. Greene, J. C. Phillips and J. F. Stults, Non-aqueous emulsion of silicone oil and stearine, WO1991010771 A1, Karlshamns AB, 1991 Search PubMed.
- X.-D. Pan and G. H. McKinley, J. Colloid Interface Sci., 1997, 195, 101–113 CrossRef CAS.
- J. C. Loudet, H. Richard, G. Sigaud and P. Poulin, Langmuir, 2000, 16, 6724–6730 CrossRef CAS.
- Z. Lin, W. J. Schulz Jr. and J. M. Smith, Oil-in-oil and three-phase emulsions, US Pat., 6238657 B1, Dow Corning Corporation, 2001 Search PubMed.
- Y. Nonomura, T. Sugawara, A. Kashimoto, K. Fukuda, H. Hotta and K. Tsujii, Langmuir, 2002, 18, 10163–10167 CrossRef CAS.
- Y. Nonomura, K. Fukuda, S. Komura and K. Tsujii, Langmuir, 2003, 19, 10152–10156 CrossRef CAS.
- H. Lannibois-Drean, J.-M. Ricca, M. Destarac and P. Olier, Oil-in-oil emulsions comprising a silicone, dispersions and use of said emulsions, WO03/000396 A1, Rhodia Chimie, 2003 Search PubMed.
- V. Jaitely, T. Sakthivel, G. Magee and A. T. Florence, J. Drug Delivery Sci. Technol., 2004, 14, 113–117 CrossRef CAS.
- M. Nair, T. K. Jones and M. C. Brick, Oil-in-oil emulsions, US Pat., 2007/0189998 A1, Eastman Kodak Company, 2007 Search PubMed.
- C. Holtze, R. E. Guerra, J. Agresti, D. A. Weitz, K. Ahn, J. B. Hutchison, A. Griffiths, A. El Harrak, O. J. Miller, J.-C. Baret, V. Taly, M. Ryckelynck and C. Merten, Fluorocarbon emulsion stabilizing surfactants, WO2008/021123 A1, Harvard College, 2008 Search PubMed.
- S. Cauvin, A. Colson, T. Dimitrova and J.-P. Lecomte, WO2010072711 A1, Dow Corning Corporation, 2010.
- T. K. Jones and M. Nair, Oil-in-oil dispersions stabilised by solid particles and methods of making the same, US Pat., 8323392 B2, Eastman Kodak Company, 2012 Search PubMed.
- S. Verma and J. S. Dangi, Ind. J. Novel Drug Del., 2012, 4, 223–226 Search PubMed.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 8622–8631 CrossRef CAS.
- B. P. Binks and A. T. Tyowua, Soft Matter, 2013, 9, 834–845 RSC.
- C. Huh and S. G. Mason, Colloid Polym. Sci., 1975, 253, 566–580 CAS.
- B. P. Binks, P. D. I. Fletcher, B. L. Holt, P. Beaussoubre and K. Wong, Phys. Chem. Chem. Phys., 2010, 12, 11954–11966 RSC.
- B. P. Binks, J. Philip and J. A. Rodrigues, Langmuir, 2005, 21, 3296–3302 CrossRef CAS PubMed.
- B. P. Binks and S. O. Lumsdon, Phys. Chem. Chem. Phys., 2000, 2, 2959–2967 RSC.
- B. P. Binks and J. A. Rodrigues, Langmuir, 2003, 19, 4905–4915 CrossRef CAS.
- M.-F. Ficheux, L. Bonakdar, F. Leal-Calderon and J. Bibette, Langmuir, 1998, 14, 2702–2706 CrossRef CAS.
- H. Barthel, B. P. Binks, A. Dyab and P. Fletcher, Multiple Emulsions, US Pat., 2003/0175317 A1, Wacker-Chemie GmbH, 2003 Search PubMed.
- S. Zou, C. Wang, Q. Gao and Z. Tong, J. Dispersion Sci. Technol., 2013, 34, 173–181 CrossRef CAS.
- B. P. Binks and J. H. Clint, Langmuir, 2002, 18, 1270–1273 CrossRef CAS.
- D. K. Owens and R. C. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741–1747 CrossRef CAS.
- M.-C. Michalski, S. Desobry, M.-N. Pons and J. Hardy, J. Am. Oil Chem. Soc., 1998, 75, 447–454 CrossRef CAS.
- P. Finkle, H. D. Draper and J. H. Hildebrand, J. Am. Chem. Soc., 1923, 45, 2780–2788 CrossRef CAS.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 2539–2547 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sm02438b |
| This journal is © The Royal Society of Chemistry 2016 |