Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
1,3- and 1,4-Benzdiyne equivalents for regioselective synthesis of polycyclic heterocycles†
Takashi
Ikawa
*,
Shigeaki
Masuda
,
Akira
Takagi
and
Shuji
Akai
*
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: ikawa@phs.osaka-u.ac.jp; akai@phs.osaka-u.ac.jp
First published on 18th April 2016
Abstract
We have devised a novel 1,3-benzdiyne equivalent, capable of quadruple functionalization by sequential benzyne generation and reaction with arynophiles. The key features of this method include the chemoselective generation of two triple bonds in a single benzene ring under fluoride-mediated mild conditions, and the regiocontrol of each benzyne reaction by the substituent next to the triple bond. This method produced various benzo-fused heteroaromatic compounds via reactions with arynophiles, such as furans, azides, and diazo compounds. A validation of the method is given in the convergent synthesis of the antipsychotic drug risperidone. A similar strategy has also been applied to a 1,4-benzdiyne equivalent to construct linearly benzo-fused heteroaromatics.
Introduction
The reactions of benzynes with arynophiles are widely utilized for introducing substituents to adjacent carbons of benzene rings.1 The direct installation of fused rings onto benzenes is an advantage specific to the benzyne reaction and is not possible through other methods. Furthermore, a variety of new arynophiles have been recently reported, enriching the diversity of the method.2The reactions of benzdiynes, possessing two triple bonds in a single benzene ring, and two arynophiles, would provide a few-step synthesis for the convergent preparation of multi-fused benzenes. However, benzdiynes are observed only under gas-phase conditions due to their extreme instability,3 and it would be impossible to react one with two different arynophiles for the synthesis of unsymmetrically fused benzene rings.
An alternative approach is to use benzdiyne equivalents, where two benzynes are generated sequentially in one pot to provide substituted acenes and polycyclic aromatic compounds. If we could control the regiochemistry of consecutive benzyne reactions, starting from benzdiyne equivalents, each with different arynophiles, we could produce a wide range of multi-ring fused unsymmetrical aromatic compounds.4 However, only a limited number of such reactions have been reported, and most of them require several steps for functional group transformations to generate the second benzyne.5,6 Therefore, the development of more sophisticated benzdiyne equivalents is needed to facilitate two-step sequential benzyne reactions. Crucial factors in the design of these benzdiyne equivalents include suitable functional groups which enable the generation of the second benzyne without further transformations, and a way to control the regiochemistry of each benzyne reaction. The work of Suzuki et al. involving their original 1,4-benzdiyne equivalent meets these requirements, which uses n-butyllithum to generate the benzynes.6b Very recently, Peña et al. have demonstrated that triple bonds were sequentially generated twice under fluoride-mediated mild conditions from 1,4-benzdiyne equivalents and reacted with two different arynophiles in both stepwise and one-pot manners.7 In contrast, there have been no reports of a suitable 1,3-benzdiyne equivalent.5,8,9
We have attempted sequential benzyne reactions starting from 1,3-benzdiyne equivalents 1, with various arynophiles (Scheme 1). This method was designed to afford unsymmetrically substituted polycyclic aromatic compounds 3, possessing consecutive fused-rings, as are often seen in material and pharmaceutical science.10 While compounds like 3 have been mainly synthesized via linear, multi-step routes, our approach is convergent and rapid, proceeding by the combination of 1 and two different arynophiles (I and II), and allows a rational design for the production of a library of compounds. We were particularly interested in its application to the synthesis of benzo-fused heterocycles for medicinal chemistry. Therefore, we planned reactions using heteroatomic 1,3-dipoles, such as azides, nitrones, diazo compounds, and nitrile oxides, as the arynophiles.
We aimed to develop a synthetic methodology in which (1) two benzynes (4 and 5) are chemoselectively generated in a stepwise manner without any additional functionalization steps, (2) each benzyne is generated under mild conditions using a fluoride, and (3) the two cycloaddition reactions of 4 and 5 with I and II proceed in a highly regioselective manner (Scheme 1). In this article, we report the preparation of a new 1,3-benzdiyne equivalent 1b [SiR3 = Si(t-Bu)Me2], and a method for the preparation of unsymmetrical, angular, and multi-ring fused heterocyclic compounds 3, which satisfies the above criteria. One significant advantage of this method is the high regioselectivity of both benzyne reactions, in which the first step is controlled by the traceless directing group, R3Si (ref. 11) of 4, and the second step by the cyclic systems12 of 5.
Results and discussion
We synthesized two 1,3-benzdiyne equivalents, 1a and 1b, which were treated with CsF in the presence of 2,4-dimethylfuran 6a. The reaction of 1a with 6a produced the undesired cycloaddition product 8via benzyne 7 (Scheme 2). However, the reaction of 1b afforded the desired cycloaddition product 10a (78% yield) through the Diels–Alder reaction of the expected benzyne 4a with 6a. An important observation is that the double cycloaddition product 3a was not detected by GC analysis of the crude reaction mixture after 30 min (see ESI†). This may be due to the lower reactivity of the Me2(t-Bu)Si group, even in the presence of excess CsF and 6a. The generation of the second benzyne 5a was achieved after long reaction time (19 h) under the same reaction conditions using CsF to give 3a in 90% isolated yield. | ||
| Scheme 2 Sequential benzyne generation from benzdiyne equivalents 1a and 1b followed by Diels–Alder reaction with furan 6a. | ||
We attempted to synthesize compounds 3b–f through stepwise benzyne cycloaddition reactions from 1b (Table 1). All reactions of 3-silylbenzyne 4a with arynophiles 6b–e provided cycloaddition products 10 with good regioselectivities due to synergetic effect of the neighboring Me2(t-Bu)Si (ref. 11f) and the distant triflyloxy (TfO) groups.13 Among them, unexpected proximal regioselectivity (proximal-10d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distal-10d = 78
distal-10d = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22) was observed in the reaction between 3-silylbenzyne 4a and nitrone 6d (entry 4-1), which was opposite to the previously reported reactions of 3-silylbenzynes with nitrones (for structural determinations, see ESI†).11f–h This result is probably due to the inductively electron-withdrawing effect of the TfO group at C4.13a The reaction of 4a with sydnone 6e to give distal-2H-indazole 10e selectively (entry 5-1) is particularly noteworthy, as the reactions of unsymmetrical benzynes such as 3-methoxybenzyne with sydnones have been reported to afford mixtures of regioisomers in 1
22) was observed in the reaction between 3-silylbenzyne 4a and nitrone 6d (entry 4-1), which was opposite to the previously reported reactions of 3-silylbenzynes with nitrones (for structural determinations, see ESI†).11f–h This result is probably due to the inductively electron-withdrawing effect of the TfO group at C4.13a The reaction of 4a with sydnone 6e to give distal-2H-indazole 10e selectively (entry 5-1) is particularly noteworthy, as the reactions of unsymmetrical benzynes such as 3-methoxybenzyne with sydnones have been reported to afford mixtures of regioisomers in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.2f The reaction of benzynes 5 with arynophiles 6b and 6f–g provided polycyclic compounds 3b–f. This is the first report of the generation and reaction of 4,5-benzotriazolyne 5b (entry 2-2), 6,7-benzisoxazolyne 5c (entry 3-2), 4,5-benzisoxazolinyne 5d (entry 4-2), and 6,7-2H-indazolyne 5e (entry 5-2). The regioselectivity of these reactions is higher than that of sterically similar 4,5-indolyne (see preliminary theoretical discussion of these regioselectivities in ESI†).12a,b The reactions of 5c with 6b and 5e with 6g provided distal-3d and proximal-3f exclusively (entries 3-2 and 5-2).
1 ratio.2f The reaction of benzynes 5 with arynophiles 6b and 6f–g provided polycyclic compounds 3b–f. This is the first report of the generation and reaction of 4,5-benzotriazolyne 5b (entry 2-2), 6,7-benzisoxazolyne 5c (entry 3-2), 4,5-benzisoxazolinyne 5d (entry 4-2), and 6,7-2H-indazolyne 5e (entry 5-2). The regioselectivity of these reactions is higher than that of sterically similar 4,5-indolyne (see preliminary theoretical discussion of these regioselectivities in ESI†).12a,b The reactions of 5c with 6b and 5e with 6g provided distal-3d and proximal-3f exclusively (entries 3-2 and 5-2).
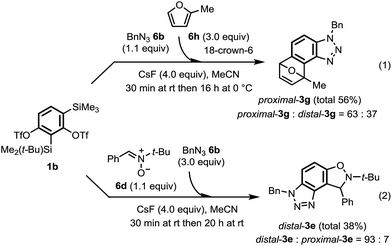
Next, one-pot sequential benzyne cycloadditions from 1b were demonstrated for the synthesis of angular tricyclic heterocycles 3 without isolating 10 (Scheme 3). After a mixture of 1b (1.0 equiv.), benzyl azide 6b (1.1 equiv.) and CsF (4.0 equiv.) in MeCN was stirred at room temperature for 30 min, 2-methylfuran 6h (3.0 equiv.) and 18-crown-6 (4.0 equiv.) were added and then the reaction mixture was stirred for 16 h at 0 °C (Scheme 3-1). Gratifyingly, proximal-3g was obtained as a main product (proximal-3g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distal-3g = 63
distal-3g = 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37, total 56%). The tricyclic compound, distal-3e was also synthesized as the predominant product (distal-3e
37, total 56%). The tricyclic compound, distal-3e was also synthesized as the predominant product (distal-3e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proximal-3e = 93
proximal-3e = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, total 38%) by a similar one-pot combination of arynophiles, nitrone 6d and 6b (Scheme 3-2). The yield and regioselectivity of these products were comparable to those obtained by the stepwise method (Table 1, entries 4-1 and 4-2).
7, total 38%) by a similar one-pot combination of arynophiles, nitrone 6d and 6b (Scheme 3-2). The yield and regioselectivity of these products were comparable to those obtained by the stepwise method (Table 1, entries 4-1 and 4-2).
We applied these findings to the convergent synthesis of the antipsychotic drug risperidone 14 (Scheme 4). The silylbenzyne 4a and a nitrile oxide 6i (ref. 2e) were simultaneously generated from a mixture of 1b and a chloro-oxime 11 and then reacted in situ to form distal-10f as a single regioisomer. The next reaction of 6,7-benzisoxazolyne 5f, generated by BnMe3NF (ref. 14 and 15) and a fluoride 6j, provided 3h with excellent regioselectivity. Finally, the synthesis was completed by the N-deprotection of 3h to give 12 and the alkylation with 13.16 This result suggests that 1b should be useful tool for the expeditious divergent synthesis of a wide variety of biologically active compounds and their derivatives by choosing different arynophiles 6 once 1b become easily available (for the first synthesis of 1b, see ESI†).
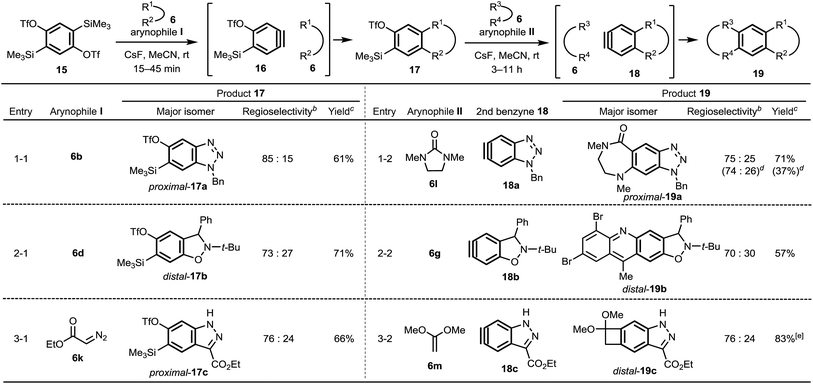
We also report the synthesis of linearly fused, unsymmetrical polycyclic aromatics 19 using 15 (ref. 17–19) as a 1,4-benzdiyne equivalent (Table 2). The first benzyne generation proceeds using CsF at room temperature in MeCN for a short time, under which conditions, generation of the second benzyne does not occur (see ESI†). The mono-cycloaddition products 17, obtained as a mixture of two regioisomers, were subjected to the second reaction with arynophiles II, without separation of the regioisomers,20 to afford the multicyclic compounds 19. Due to the dual effect of the TfO group13a and Me3Si group11e of 16, the all first benzyne reactions proceeded in a regioselective manner beyond expectation (Table 2, entries 1-1, 2-1 and 3-1), although these selectivities were only a little lower than those of the 1,3-benzdiyne equivalent 1b (see, Table 1). Interestingly, the second benzyne reactions also regioselectively provided cycloaddition products 19 probably because of the inductive effect of heteroatoms such as nitrogen and oxygen constructing heterocycles (entries 2-2 and 3-2). These results provide useful information for regioselectivity control of benzyne cycloadditions from distant positions. Importantly, the one-pot synthesis of a linear tricyclic compound 19a from 15, 6b and 6l was also successfully achieved (see entry 1-2).
| a Conditions: 15 or 17 (1.0 equiv.), arynophile 6 (3.0 equiv.), CsF (3.0 equiv.) in MeCN at rt. b A ratio of major and minor products was determined by 1H NMR. c Total isolated yield of distal-17 (or distal-19) and its regioisomer proximal-17 (or proximal-19). d One-pot reaction conditions: 15 (1.0 equiv.), 6b (1.1 equiv.), 6l (3.0 equiv.), CsF (4.0 equiv.) in MeCN (0.1 M) at rt for 30 min and then at rt for 14 h. The yield was calculated based on 15. e Isolated as a corresponding ketone 19c′ after the hydrolysis of acetal 19c. |
|---|
|
Conclusions
In conclusion, we have developed a novel synthetic route to multi-ring fused heterocycles by the combination of benzdiyne equivalents and arynophiles. In this study, the newly generated azole-fused benzynes were found to exhibit higher regioselectivities than those of sterically similar 4,5-indolyne.12b This method has facilitated the convergent synthesis of the antipsychotic risperidone. Therefore, we believe that this synthetic methodology will be invaluable to drug discovery. Work is ongoing into easier, scalable synthetic methods for these benzdiyne equivalents,19 analysis of the origin of the regioselectivity (see ESI†), and applications to medicinal chemistry.Acknowledgements
We thank Dr N. Kotoku of Osaka University for analyzing NMR data. This work was financially supported by the JSPS KAKENHI (grant numbers 23790017, 24390005 and 25460018) and Grant-in-Aid for JSPS (grant number 15J06024), the Platform for Drug Discovery, Informatics, and Structural Life Science from the MEXT and the Hoansha foundation.References
- For selected reviews on benzynes, see: (a) H. Pellissier and M. Santelli, Tetrahedron, 2003, 59, 701 CrossRef CAS; (b) A. M. Dyke, A. J. Hester and G. C. Lloyd-Jones, Synthesis, 2006, 4093 CAS; (c) R. Sanz, Org. Prep. Proced. Int., 2008, 40, 215 CrossRef CAS; (d) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC; (e) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (f) C. Wu and F. Shi, Asian J. Org. Chem., 2013, 2, 116 CrossRef CAS; (g) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2013, 11, 191 RSC; (h) S. Yoshida and T. Hosoya, Chem. Lett., 2015, 44, 1450 CrossRef. For recently published very important paper on benzyne chemistry (i) T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, Nature, 2012, 490, 208 CrossRef CAS PubMed.
- For selected papers on benzyne reactions with new arynophiles, see: (a) T. Jin and Y. Yamamoto, Angew. Chem., Int. Ed., 2007, 46, 3323 CrossRef CAS PubMed; (b) F. Shi, J. P. Waldo, Y. Chen and R. C. Larock, Org. Lett., 2008, 10, 2409 CrossRef CAS PubMed; (c) D. C. Rogness and R. C. Larock, J. Org. Chem., 2010, 75, 2289 CrossRef CAS PubMed; (d) C. Spiteri, S. Keeling and J. E. Moses, Org. Lett., 2010, 12, 3368 CrossRef CAS PubMed; (e) A. V. Dubrovskiy and R. C. Larock, Org. Lett., 2010, 12, 1180 CrossRef CAS PubMed; (f) Y. Fang, C. Wu, R. C. Larock and F. Shi, J. Org. Chem., 2011, 76, 8840 CrossRef CAS PubMed; (g) M. E. Hayes, H. Shinokubo and R. L. Danheiser, Org. Lett., 2005, 7, 3917 CrossRef CAS PubMed.
- For selected papers on benzdiynes, see: (a) T. Sato, H. Niino and A. Yabe, J. Am. Chem. Soc., 2003, 125, 11936 CrossRef CAS PubMed; (b) T. Sato and H. Niino, Aust. J. Chem., 2010, 63, 1048 CrossRef CAS.
- For a review on the synthesis of acenes using aromatic ynes including benzdiynes, see: (a) J. Li and Q. Zhang, Synlett, 2013, 24, 686 CrossRef CAS; (b) D. Pérez, D. Peña and E. Guitián, Eur. J. Org. Chem., 2013, 5981 CrossRef.
- For papers on the 1,3-benzdiyne equivalents that generate two triple bonds by a step-by-step manner, see: (a) T. Hamura, Y. Ibusuki, K. Sato, T. Matsumoto, Y. Osamura and K. Suzuki, Org. Lett., 2003, 5, 3551 CrossRef CAS PubMed; (b) Y.-L. Chen, J.-Q. Sun, X. Wei, W.-Y. Wong and A. W. M. Lee, J. Org. Chem., 2004, 69, 7190 CrossRef CAS PubMed; For papers on the 1,3,5-benztriyne equivalents, see: (c) T. Hamura, Y. Ibusuki, H. Uekusa, T. Matsumoto and K. Suzuki, J. Am. Chem. Soc., 2006, 128, 3534 CrossRef CAS PubMed; (d) T. Hamura, Y. Ibusuki, H. Uekusa, T. Matsumoto, J. S. Siegel, K. K. Baldridge and K. Suzuki, J. Am. Chem. Soc., 2006, 128, 10032 CrossRef CAS PubMed.
- For selected papers on 1,4-benzdiyne equivalents that generate two triple bonds by a step-by-step manner, see: (a) S.-H. Chan, C.-Y. Yick and H. N. C. Wong, Tetrahedron, 2002, 58, 9413 CrossRef CAS; (b) T. Hamura, T. Arisawa, T. Matsumoto and K. Suzuki, Angew. Chem., Int. Ed., 2006, 45, 6842 CrossRef CAS PubMed; (c) D. Chun, Y. Cheng and F. Wudl, Angew. Chem., Int. Ed., 2008, 47, 8380 CrossRef CAS PubMed; (d) K. Gondo and T. Kitamura, Adv. Synth. Catal., 2014, 356, 2107 CrossRef CAS; (e) K. Gondo, J. Oyamada and T. Kitamura, Heterocycles, 2015, 90, 681 CrossRef CAS . See also ref. 5b.
- (a) B. Schuler, S. Collazos, L. Gross, G. Meyer, D. Pérez, E. Guitián and D. Peña, Angew. Chem., Int. Ed., 2014, 53, 9004 CrossRef CAS PubMed; (b) D. Rodríguez-Lojo, D. Peña, D. Pérez and E. Guitián, Synlett, 2015, 26, 1633 CrossRef; (c) N. Pavliček, B. Schuler, S. Collazos, N. Moll, D. Pérez, E. Guitián, G. Meyer, D. Peña and L. Gross, Nat. Chem., 2015, 7, 623 CrossRef PubMed; For related example of 2,6-naphthalenediyne synthon, see: (d) D. Rodríguez-Lojo, D. Pérez, D. Peña and E. Guitián, Chem. Commun., 2015, 51, 5418 RSC.
- An activation with a strong acid, such as TfOH (ref. 5b) and BF3·OEt2,6d was necessary before the generation of each benzyne using a fluoride.
- For related work on iterative generation of benzynes, see: (a) C.-J. Frank Du and H. Hart, J. Org. Chem., 1987, 52, 4311 CrossRef; (b) H. Hart and T. Ghosh, Tetrahedron Lett., 1988, 29, 881 CrossRef CAS; (c) J. Shi, D. Qiu, J. Wang, H. Xu and Y. Li, J. Am. Chem. Soc., 2015, 137, 5670 CrossRef CAS PubMed.
- For selected papers on angular aromatic compounds, see; (a) S. Blättermann, L. Peters, P. A. Ottersbach, A. Bock, V. Konya, C. D. Weaver, A. Gonzalez, R. Schröder, R. Tyagi, P. Luschnig, J. Gäb, S. Hennen, T. Ulven, L. Pardo, K. Mohr, M. Gütschow, A. Heinemann and E. Kostenis, Nat. Chem. Biol., 2012, 8, 631 CrossRef PubMed; (b) C.-K. Ryu, J.-H. Nho, G. Jin, S. Y. Oh and S. J. Choi, Chem. Pharm. Bull., 2014, 62, 668 CrossRef CAS PubMed; (c) L. Nagarapu, H. R. Vulupala, R. Bantu, Y. Sajja and J. B. Nanubolu, Tetrahedron: Asymmetry, 2014, 25, 578 CrossRef CAS.
- For selected papers on silyl group directed nucleophilic additions of benzynes, see: (a) C. Heiss, F. Cottet and M. Schlosser, Eur. J. Org. Chem., 2005, 5236 CrossRef CAS; (b) V. Diemer, M. Begaud, F. R. Leroux and F. Colobert, Eur. J. Org. Chem., 2011, 341 CrossRef CAS; (c) T. Ikawa, T. Nishiyama, T. Shigeta, S. Mohri, S. Morita, S.-I. Takayanagi, Y. Terauchi, Y. Morikawa, A. Takagi, Y. Ishikawa, S. Fujii, Y. Kita and S. Akai, Angew. Chem., Int. Ed., 2011, 50, 5674 CrossRef CAS PubMed; (d) S. M. Bronner, J. L. Mackey, K. N. Houk and N. K. Garg, J. Am. Chem. Soc., 2012, 134, 13966 CrossRef CAS PubMed; (e) H. Yoshida, R. Yoshida and K. Takaki, Angew. Chem., Int. Ed., 2013, 52, 8629 CrossRef CAS PubMed; For silyl group directed [4 + 2] cycloadditions, see; (f) S. Akai, T. Ikawa, S.-I. Takayanagi, Y. Morikawa, S. Mohri, M. Tsubakiyama, M. Egi, Y. Wada and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 7673 CrossRef CAS PubMed; For silyl group directed (3 + 2) cycloadditions, see: (g) T. Matsumoto, T. Sohma, S. Hatazaki and K. Suzuki, Synlett, 1993, 843 CrossRef CAS; (h) M. Dai, Z. Wang and S. J. Danishefsky, Tetrahedron Lett., 2008, 49, 6613 CrossRef CAS PubMed; (i) T. Ikawa, A. Takagi, M. Goto, Y. Aoyama, Y. Ishikawa, Y. Itoh, S. Fujii, H. Tokiwa and S. Akai, J. Org. Chem., 2013, 78, 2965 CrossRef CAS PubMed.
- For selected fused five-membered ring directed benzyne reactions, see: (a) A. N. Garr, D. Luo, N. Brown, C. J. Cramer, K. R. Buszek and D. VanderVelde, Org. Lett., 2010, 12, 96 CrossRef CAS PubMed; (b) G.-Y. J. Im, S. M. Bronner, A. E. Goetz, R. S. Paton, P. H.-Y. Cheong, K. N. Houk and N. K. Garg, J. Am. Chem. Soc., 2010, 132, 17933 CrossRef CAS PubMed; For various fused ring directed benzyne reactions, see: (c) A. E. Goetz, S. M. Bronner, J. D. Cisneros, J. M. Melamed, R. S. Paton, K. N. Houk and N. K. Garg, Angew. Chem., Int. Ed., 2012, 51, 2758 CrossRef CAS PubMed.
- For unique 3- or 4-TfO group-directed benzyne reactions, see: (a) T. Ikawa, H. Kaneko, S. Masuda, E. Ishitsubo, H. Tokiwa and S. Akai, Org. Biomol. Chem., 2015, 13, 520 RSC; (b) S. Yoshida, K. Uchida, K. Igawa, K. Tomooka and T. Hosoya, Chem. Commun., 2014, 50, 15059 RSC . See also ref. 9c.
- The use of BnMe3NF as an anhydrous fluoride source was necessary both for the benzyne generation and for the nucleophilic addition of the fluoride because a significant amount of the phenol derivative was formed when Bu4NF·(t-BuOH)4 was used. The latter results are probably owing to the nucleophilic addition of contaminant water to generate benzyne 5f.15.
- For fluorination reactions of benzynes with fluorides, see; T. Ikawa, S. Masuda, T. Nishiyama, A. Takagi and S. Akai, Aust. J. Chem., 2014, 67, 475 CrossRef CAS.
- A. V. Dubrovskiy, P. Jain, F. Shi, G. H. Lushington, C. Santini, P. Porubsky and R. C. Larock, ACS Comb. Sci., 2013, 15, 193 CrossRef CAS PubMed.
- 2,4-Bis(trimethylsilyl)-1,3-bis(trifluoromethanesulfonyloxy)benzene 15 has been recognized as a one-step 1,4-benzdiyne equivalent that consecutively generates two triple bonds at the C1 and C4 positions of a single benzene to react with two equivalents of a single arynophile.4,18 Peña et al. have recently used 15 as a quasi-step-by-step benzdiyne equivalent for the synthesis of polyaromatic hydrocarbon (PAH), in which the second benzyne was not generated because of the precipitation of the mono-cycloaddition product7a–c.
- (a) H. M. Duong, M. Bendikov, D. Steiger, Q. Zhang, G. Sonmez, J. Yamada and F. Wudl, Org. Lett., 2003, 5, 4433 CrossRef CAS PubMed; (b) D. Rodríguez-Lojo, A. Cobas, D. Peña, D. Pérez and E. Guitián, Org. Lett., 2012, 14, 1363 CrossRef PubMed.
- We have developed an improved, 3-step synthesis of 15 with 70% overall yield, while the reported method required 4 steps and gave 13% overall yield.18a Recently, another improved method was reported which required 3 steps in 59% overall yield7c (see ESI†).
- Both regioisomers 17 should be transformed to the same benzynes 18.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc00798h |
| This journal is © The Royal Society of Chemistry 2016 |