Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering β-sheets employing N-methylated heterochiral amino acids†
Dipan
Ghosh‡
,
Priyanka
Lahiri‡
,
Hitesh
Verma
,
Somnath
Mukherjee
and
Jayanta
Chatterjee
*
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560012, India. E-mail: jayanta@mbu.iisc.ernet.in
First published on 21st April 2016
Abstract
There is a lack of functional group diversity in the reverse turn motifs nucleating a β-sheet conformation in designed peptides, proteins and foldamers. The majority of these sequences consist of D-Pro–L-Pro, D-Pro–Gly or Asn–Gly as the turn inducing motif restricting their biological application and physicochemical modulation. In this report, for the first time we elucidate that N-methylation of heterochiral amino acids in linear peptides nucleates β-sheet conformation without the necessity of having a ring or covalent constraint at the reverse turn. Our results show that D-Pro can be conveniently substituted by any other N-methylated D-amino acid followed by an N-methylated L-amino acid or sarcosine to adopt a βII′ turn inducing the β-sheet folding. Furthermore, we reveal that a single amino acid either at the i + 1 or i + 2 site of the reverse turn can modulate the right-handed twist, which eventually dictates the extent of the foldedness of the β-hairpin.
Introduction
The design of β-hairpin forming peptides is of great importance in developing chemical tools that can perturb protein–protein/protein–peptide interactions in a chemical biology context1 and in developing metabolically stable, well-folded synthetic proteins from a protein engineering perspective.2 Extensive efforts to understand the design principles underlying the formation of reverse turns leading to the formation of β-hairpin peptides and β-sheets have established mainly two dipeptide motifs, D-Pro–Xaa (Xaa is any L-amino acid, although the preferred ones are L-Pro and Gly)3 and Asn–Gly, as the most favored β-hairpin nucleators in linear peptides.4 However, this severely limits the biological applications of β-hairpins, as the functionalization of these turn-inducing sequences either compromises their nucleating efficacy5 or involves tedious chemical synthesis,6 resulting in gross neglect towards the development of β-hairpins as chemical tools in contrast to α-helices.1b Thus, to expand the repertoire of reverse turn-inducing motifs we focused on employing N-methylated amino acids despite their inherent conformational flexibility.7N-Methylation has been thoroughly investigated in cyclic peptides but its conformational impact on linear peptides is not well understood. We were thus keen to study the influence of N-methylation on the turn-inducing residues in linear β-hairpin peptides as there is no such report and the few known facts about the N-methylation of the turn residues are conflicting.8 In the present study, we have thoroughly investigated the influence of N-methylation on a varied selection of heterochiral residues in linear peptides using circular dichroism and NMR based structure calculations. We show that N-methylation indeed nucleates β-hairpin conformation irrespective of the amino acids present at the i + 1 and i + 2 positions of the β-turn. Furthermore, most of these hairpins are conformationally homogeneous9 in the NMR time scale in apolar and polar environments in spite of having consecutive N-methylated heterochiral amino acids. This indicates the wide applicability of the newly identified turn inducing motif towards peptide/protein engineering studies in lipophilic (e.g. membrane) and hydrophilic (e.g. cytosol) environments.10
Results and discussion
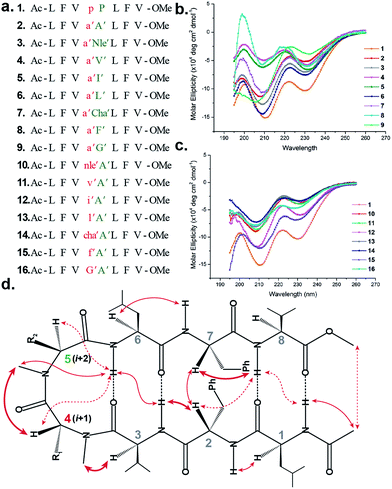
To initially study the influence of N-methylation on β-hairpin induction in apolar conditions, we selected the model octapeptide 1,11 and substituted the D-Pro (i + 1) and L-Pro (i + 2) at the reverse turn with various N-methylated amino acids (Fig. 1a). We chose to N-methylate both the i + 1 and i + 2 residues to minimize the conformational entropy about the reverse turn.12The hydrophobic amino acids: Ala, Val, Ile, Leu and Phe were chosen as substituents since they display the least propensity to occur at the β-turn region, according to Chou–Fasman's analysis of β-turns in protein structures.13 Besides, these residues also have a minimal stabilizing contribution from the side chain functionality on the turn conformation in the synthesized peptides. We also chose two unnatural amino acids: norleucine (Nle) which acts as a methionine isostere (methionine also has a low propensity to occur at the turn region) and a ring constrained leucine analog, cyclohexylalanine (Cha). All of the compounds were synthesized on a solid support and the N-methylation of the amino acids was performed on-resin using an optimized protocol.14 In order to determine whether any of our peptides undergo self-association under the conditions used for spectroscopic evaluation, we examined proton NMR chemical shift data for each peptide as a function of concentration. For the hydrophobic peptides, 1–16, the chemical shifts measured at the concentration employed for 2D NMR studies (1–3 mM) were indistinguishable from the chemical shifts measured after 100-fold dilution. For the hydrophilic peptides, 17–21, the chemical shifts measured at the concentration employed for 2D NMR studies were indistinguishable from the chemical shifts measured after ∼20-fold dilution.
The initial qualitative assessment of their potential to form β-hairpins was done using far UV (190–260 nm) CD measurements in methanol. The CD spectra of all of the i + 2 and i + 1 (Fig. 1) variants resembled the CD spectrum of 1, showing the characteristic minima at 210 and 232 nm due to the anomalous behavior arising from the Phe2–Phe7 stacking interaction.4b,15 This clearly suggested that the replacement of the i + 1 and i + 2 residues in 1 with N-methylated amino acids was not detrimental to the overall topology of the molecule. However, as several compounds showed varying CD intensities with a single amino acid substitution, to understand the underlying cause we calculated their average solution conformation using restraints derived from ROESY. In the following discussion, the D-residues will be denoted in lower case and N-methylation with a prime symbol (′).
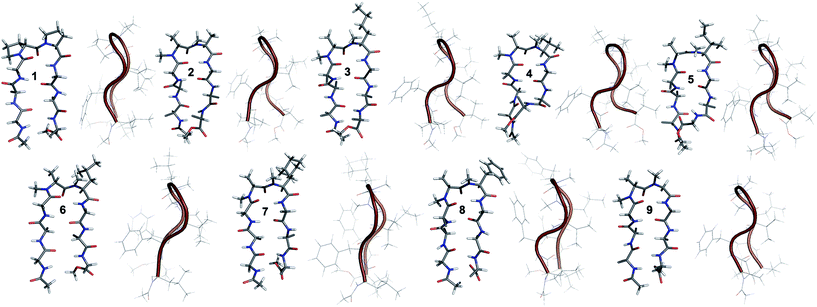
The end caps in 1–16 were carefully designed, so that if these were well folded, besides the characteristic NOEs (Fig. 1d) one would also expect the NOE between the end caps (NHAc and OMe) in the absence of strand fraying. The 1H NMR of 1 (in CDCl3) showed well dispersed signals in the Hα and HN region with 3JHN–Hα > 8 Hz suggestive of a well-defined and extended conformation (Table S1†). The characteristic long-range NOEs Phe2Hα–Phe7Hα and NHAc–OMe; strand NOEs Leu1Hα–Phe2HN, Phe2Hα–Val3HN, Leu6Hα–Phe7HN and Phe7Hα–Val8HN along with the turn region NOEs proHα–Pro5Hδ1/2 and Pro5Hδ1–Leu6HN with the solvent shielded Leu1, Val3, Leu6 and Val8HN (as determined by DMSO titration) (Table S2†) suggest the formation of a β-hairpin conformation. The solution structure of 1 (Fig. 2) revealed the formation of a β-hairpin with a centrally located βII′ turn (Table 1).
| i + 1 | i + 2 | |||
|---|---|---|---|---|
| Φ (°) | Ψ (°) | Φ (°) | Ψ (°) | |
| 1 | 50 ± 14 | −125 ± 13 | −77 ± 8 | 43 ± 24 |
| 2 | 51 ± 11 | −122 ± 15 | −106 ± 14 | 25 ± 12 |
| 3 | 63 ± 7 | −139 ± 6 | −91 ± 8 | 23 ± 12 |
| 4 | 63 ± 8 | −133 ± 8 | −122 ± 9 | 48 ± 16 |
| 5 | 74 ± 9 | −126 ± 15 | −113 ± 12 | 37 ± 13 |
| 6 | 56 ± 11 | −131 ± 9 | −95 ± 8 | 14 ± 10 |
| 7 | 66 ± 11 | −137 ± 10 | −99 ± 10 | 35 ± 19 |
| 8 | 74 ± 8 | −129 ± 7 | −99 ± 9 | 20 ± 10 |
| 9 | 65 ± 7 | −127 ± 6 | −89 ± 10 | 15 ± 8 |
In 2, the pP motif is substituted by the a′A′ motif and has a relatively less restricted Φ(i+1 and i+2) at the reverse turn due to the lack of any ring constraint. Furthermore, the tertiary amide bonds have a low rotational barrier and thus 2 can co-exist in cis/trans form.16 However, we were surprised to note that 2 displayed a single conformation in CDCl3 with the absence of any (ith)Hα–(i + 1st)Hα NOE confirming the presence of an all-trans conformer.17,18 It was interesting to note that most of the 3JHN–Hα values in 2 were higher than the corresponding values in 1. The NOEs ala′Hα–Ala′5NMe, ala′Hα–Leu6HN and Ala′5NMe–Leu6HN along with solvent shielded Leu6HN clearly define a βII′ turn.18 The long-range NOEs Leu1HN–Val8HN, Phe2Hα–Phe7Hα, Val3HN–Leu6HN and NHAc–OMe additionally suggest the antiparallel strand registry in the molecule. The conformation of 2 (Fig. 2) revealed that the Φ(i+1) and Ψ(i+1) are quite close to the ideal βII′ turn (Table 1). Whereas, peptide 2a with an A′A′ motif showed the presence of a cis-peptide bond between the 4th and 5th residue18 and the absence of any characteristic long-range NOEs defining the β-hairpin.
Compound 3, with the long un-branched amino acid norleucine, displays all of the characteristic NOEs (Fig. 1d) with a comparable solvent accessibility of the HNs as observed in 2. The conformation of 3 is quite close to 2 (backbone RMSD of 0.20 Å),18 with some variation in the dihedrals about the turn-motif. Compounds 4 and 5 both have an N-methylated β-branched amino acid at the i + 2 position and display a similar CD profile although with strikingly low intensity. However, we could assign the characteristic NOEs in both of the compounds, and the high 3JHN–Hα suggested the presence of a hairpin conformation. It was interesting to note that in these compounds the Leu1HN and Leu6HN are comparatively less solvent shielded than in 1, 2 and 3 (Table S2†). In these β-branched analogs, the i + 1/i + 2 and i + 2/i + 3 amide planes are forced away from each other due to the steric repulsion between Val′5/Ile′5 Cγ and their respective N-methyl group resulting in higher values for Φ(i+2) and Ψ(i+2) (Table 1). The conformations of 4 and 5 are quite similar with a backbone RMSD of 0.45 Å and a stark right handed twist (Fig. 2).
Introduction of a γ-branched residue at the i + 2 position (6), results in less deviation from the optimal Φ and Ψ values (Table 1) in the turn region in comparison to the β-branched analogs. This is also evident from the CD spectrum of 6 showing a higher intensity than 4 and 5. The ring constrained leucine isostere cyclohexylalanine substituted analog 7 and its aromatic congener 8, with an N-methylated phenylalanine (although 8 shows an anomalous behavior in CD), display a similar solution conformation to 6, suggesting the compatibility of the appended side chains at the i + 2 site.
Finally, we introduced an N-methylated glycine (sarcosine) (as it is achiral and lacks side chain) at the i + 2 position to assess its suitability in nucleating the β-hairpin. In spite of the absence of any side chain constraint, 9 exhibited conformational homogeneity in CDCl3 with all of the characteristic NOEs. To our surprise, the dihedral angles about the turn region of 9 (Table 1) in its solution conformation are quite similar to a designed β-hairpin peptide with a central pro–Gly turn motif in its crystal form.19 However, the solvent-shielded nature of the amide protons in 9 followed the trend that was observed for 5, suggesting a flexible nature for both of the compounds. Compound 2 with the heterochiral N-methylated alanine in the turn region can be classified into both the i + 1 and i + 2 libraries. This gave us the clue that an N-methylated D-amino acid could potentially replace proline to form a stable β-hairpin structure. However, to assess the suitability of various branched and un-branched amino acids at the i + 1 position we chose to substitute this site with all of the aforementioned amino acids but with reversed chirality.
10 shows the entire signature NOEs of the reverse turn (nle′Hα–Ala′5NMe, nle′Hα–Leu6HN and Ala′5NMe–Leu6HN) and displays the presence of a well-folded conformation (Leu1HN–Val8HN, Phe2Hα–Phe7Hα, Val3HN–Leu6HN and NHAc–OMe). The solvent-shielded Leu1HN, Val3HN, Leu6HN and Val8HN along with the 3JHN–Hα > 8 Hz implies a putative β-hairpin structure. The structure of 10 (Fig. 3) is comparable to 2 and the norleucine swapped analog 3 with a backbone RMSD of 0.56 Å and 0.50 Å respectively.18
The β-branched i + 1 analog 11 showed a CD spectrum comparable to 10 (Fig. 1c) suggesting a structural similarity between these two analogs unlike in the corresponding i + 2 analogs. The characteristic NOEs and the high 3JHN–Hα revealed the structural integrity of the molecule. The Φ(i+1) in 11 (Table 2) showed a significant increase that could be attributed to the steric interaction between the val′Cγ and val′NMe, which subsequently results in the reduced right handed twist of the hairpin unlike in 4. A fairly good agreement between the solution structures of 11 and 10 (backbone RMSD of 0.39 Å) indicates the differential behavior of valine at the i + 1 and i + 2 sites.
| i + 1 | i + 2 | |||
|---|---|---|---|---|
| Φ (°) | Ψ (°) | Φ (°) | Ψ (°) | |
| 10 | 73 ± 7 | −121 ± 8 | −105 ± 10 | 17 ± 10 |
| 11 | 86 ± 10 | −118 ± 7 | −106 ± 8 | 26 ± 9 |
| 12 | 93 ± 12 | −112 ± 13 | −107 ± 11 | 40 ± 17 |
| 13 | 67 ± 7 | −131 ± 6 | −90 ± 9 | 14 ± 7 |
| 14 | 69 ± 7 | −135 ± 6 | −88 ± 7 | 14 ± 7 |
| 15 | 65 ± 8 | −126 ± 6 | −93 ± 9 | 15 ± 8 |
| 16 | 75 ± 10 | −104 ± 7 | −128 ± 8 | 6 ± 5 |
Unlike 5, compound 12 with the Ile′ shows a CD spectrum with a high intensity. The explanation was beautifully provided by the solution structure of 12, where the Φ(i+1) and Ψ(i+2) deviate the most from the ideal geometry amongst all of the i + 1 analogs. The increased Φ(i+1) is a result of the strong steric interaction between the ile′Cγ1 and ile′NMe (Fig. 3) that eventually leads to a heavily restricted conformation about Ile′ as suggested by the multiple intra- and interresidue NOEs. This conformational restriction creates further steric clash between Ile′Cγ2 and Ala′5NMe resulting in a higher Ψ(i+2) value. This restriction is analogous to the ring constraint of Pro in 1. A combination of these effects result in a flattened conformation in 12 that is comparable to 1 (backbone RMSD of 0.64 Å).
The CD spectrum of the γ-branched analog 13 is less intense than the CD spectrum of the corresponding i + 2 analog 6. This observation is strikingly opposite to the trend observed for the β-branched analogs 5 and 12. The solution structures of these compounds revealed the presence of a considerable right-handed twist in 13 as compared to 6, validating the CD spectrum. The basis of this twist in 13 is the steric interaction between the isopropyl and the N-methyl group of leu′ resulting in a slightly higher value of Φ(i+1) in 13 than in 6. On the contrary, the steric repulsion between the isopropyl and the N-methyl group of leu′ in 6 is considerably less due to the greater torsion angle of C(NMe)–N–Cα–Cβ(i+2) than C(NMe)–N–Cα–Cβ(i+1) (due to the opposite stereochemistry at the i + 1 and i + 2 site) resulting in the flattened hairpin in 6.
Surprisingly, the CD spectrum of 14 showed a lower intensity in comparison to all other i + 1 analogs. Nevertheless, we could identify the characteristic NOEs, and the solution conformation of 14 is almost identical to that of 13 (backbone RMSD of 0.24 Å) displaying the right handed twist. On the other hand the aromatic i + 1 γ-branched analog 15 shows the most intense CD spectrum amongst all of the i + 1 congeners indicating the occurrence of a flatter hairpin. However, the conformation of 15 and the solvent exposure of amide protons show a striking similarity with the corresponding i + 2 analog 8 (backbone RMSD of 0.26 Å), which shows a less intense CD spectrum. This re-emphasizes the anomalous behavior of the electronic CD for peptides with aromatic residues.20 Furthermore, we did not observe any aggregation at the concentrations used for NMR and CD measurements,18 therefore the extent of twist in these peptides arises mainly due to the substitution pattern at the reverse turn.
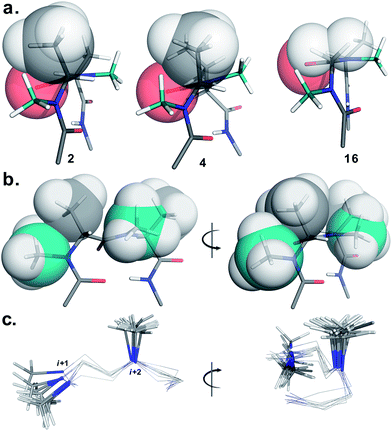
To determine the importance of the i + 1 side chain on the induction of the βII′ turn and the subsequent folding of the hairpin, we synthesized compound 16 with sarcosine at the i + 1 position. Although the CD spectrum of 16 is comparable to other i + 1 analogs, it has considerably less intensity than the CD spectrum of 9. This observation directly correlates with the flexibility of 16 in CDCl3,18 although the major conformer displays all of the characteristic signatures (NOEs, 3JHN–Hα and solvent shielded HN) of a β-hairpin. It is interesting to note that in spite of the absence of any side chains at the i + 1 site, there is a clear indication of a βII′ turn as suggested by the following NOEs: Gly′Hα1–Ala′5NMe, Gly′Hα1–Leu6HN, Ala′5NMe–Leu6HN and a shorter distance for Ala′5NMe–Ala′5Hβ than Ala′5NMe–Ala′5Hα. However, there is a substantial increase in the Φ(i+1) and Φ(i+2) values. A close assessment of the structure revealed the separation of i + 1/i + 2 and i + 2/i + 3 amide planes to a greater extent in 16 than all of the other analogs including the most sterically demanding i + 2 β-branched analogs, 4 and 5. This is mainly due to the absence of i + 1 Cβ in 16, which restricts the inward rotation of the i + 1/i + 2 amide plane in the other analogs due to the van der Waals repulsion with the i + 1 CO group (Fig. 4a) (note the greater torsion angle Cβ–Cα–C–O(i+1) in 2 and 4 in comparison to Hα(Pro−R)–Cα–C–O(i+1) in 16).
The tolerance of various N-methylated chiral or achiral amino acids at the turn region clearly indicates that the steric interactions in the reverse turn are crucial in nucleating the β-hairpin that is subsequently stabilized by the intramolecular hydrogen bonding.21 The relative orientations of the i + 1 N-methyl, i + 1 Cβ, i + 2 N-methyl and i + 2 Cβ along with the i + 1 CO are critical in dictating the stability of the β-turn (Fig. 4b). Any relaxation in the van der Waals repulsion about these residues destabilizes the global conformation of the hairpin. This is clearly evident from 16, where the absence of i + 1 Cβ destabilizes the global conformation. The torsion angles C(NMe)–N–Cα–Cβ(i+1) and C(NMe)–N–Cα–Cβ(i+2) also play a critical role in stabilizing the reverse turn as observed in the γ-branched analogs 6 and 13. Finally, the conformational flexibility of the N-methyl group at the i + 1 position (Fig. 4c) allows for a better tolerance of bulkier substituents (e.g. β-branched amino acids) at the i + 1 site than at i + 2. This leads to the differential behavior of certain substituents at these two sites (e.g.4 and 11).
Next, to assess the suitability of the designed reverse turn motif in the context of protein engineering, we studied several hydrophilic peptides with a common strand sequence22 but with varied turn inducing motifs. We were keen to understand the behavior of our turn inducing motif in aqueous conditions, as only a few reverse turn motifs have found utility in protein engineering.23 We initially synthesized 17, with D-Ala–L-Ala as the turn inducing motif, since in small cyclic peptides heterochirality is enough to induce the formation of a βII′ turn.1a
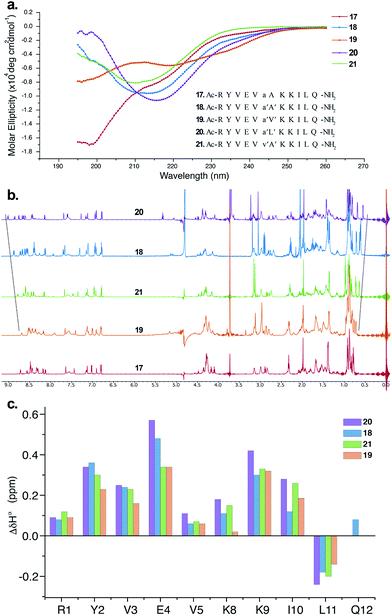
The CD spectrum of 17 (Fig. 5a) showed the signature of a random coil, suggesting that heterochirality in a linear peptide is not sufficient to induce a hairpin formation. To emphasize the role of N-methylation in inducing the β-hairpin formation, we synthesized 18, with N-methylation at D-Ala and L-Ala. The CD spectrum of 18 showed a broad minima centred around 215 nm that is characteristic of a β-sheet structure (Fig. 5a). Further, to show the compatibility of different amino acid side chains at the reverse turn of the β-hairpin, we synthesized 19, 20 and 21. It was gratifying to note that the CD spectrum for these analogs followed a similar pattern as observed in the hydrophobic peptides, with the i + 2 γ-branched amino acid (20) showing the maximum and the β-branched analog (19) showing the minimum intensity. It was also encouraging to observe the differential behavior of the i + 2 and i + 1 β-branched analogs 19 and 21, respectively, that followed the trend as noted for the hydrophobic peptides.
To obtain additional insights into the folding behavior of these compounds, we performed NMR spectroscopy in acetate buffer (pH 3.8). The 1H spectrum of 17 showed clear indications of an unfolded structure, with the absence of any upfield shifted methyl groups24 and overlapping resonance for the β-methyl groups (∼1.4 ppm) of alanine (Fig. 5b). On the contrary, all of the N-methylated analogs show the presence of two upfield shifted methyl groups that progressively increase in the order 19 < 21 < 18 < 20. This order also correlated with the enhanced dispersion of chemical shifts in the amide region of these compounds.
The secondary chemical shifts of these compounds (obtained from the respective unfolded controls, where the N-methylated D-residue was substituted with an N-methylated L-residue)18 also follows the order 19 < 21 ≈ 18 < 20, suggesting the increased foldedness of the β-sheet from left to right.25a The results from the NMR analyses corroborated the different intensities observed in the CD spectrum. Together these results strongly indicate the subtle modulation of the β-sheet folded conformation via a single substitution at the i + 1 or i + 2 site, which would find enormous utility in protein engineering.
Finally, to validate the broad scope of our design strategy in foldamer design, we calculated the solution structure of 18 at 25 °C. The conformation of 18 (Fig. 6a) was well-defined by several inter- and intraresidue NOEs. The reverse turn was specified by ala′Hβ–ala′NMe, ala′Hα–Ala′7NMe, ala′Hα–Lys8HN, and Ala′7NMe–Lys8HN NOEs, while Tyr2Hα–Leu11Hα, Tyr2Hα–Gln12HN, and Val5HN–Lys8HN NOEs confirmed the strand registry.18 The structure of 18 was calculated utilizing the distances derived from the NOEs, which showed the presence of a β-hairpin conformation with a central βII′ turn. It was gratifying to see that 18 displayed significant structural similarity with two different β-sheet peptides25 in aqueous conditions (Fig. 6b and c).
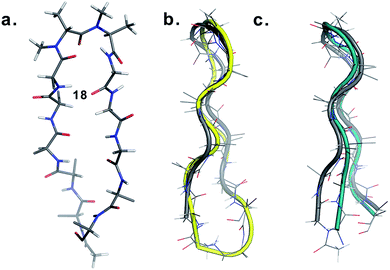 | ||
| Fig. 6 (a) Structure of 18 in aqueous solution and backbone overlay (in grey) with (b) a cyclic β-sheet peptide TS+ (yellow) (RMSD 1.0 Å) and (c) an engineered GB1 peptide (teal) (RMSD 0.75 Å). | ||
Conclusions
In conclusion, we show that the N-methyl groups in conjunction with the Cβ at the i + 1 and i + 2 amino acid side chains provide sufficient steric locking to fold a linear peptide into a β-hairpin. The introduction of heterochirality in linear peptides alone is not enough to induce the formation of β-sheets, unlike in cyclic peptides. Our engineering strategy is quite modular in terms of decorating the reverse turn with different functional groups to alter the physicochemical properties of the designed β-sheets and attach various probes for biophysical studies. Furthermore, branched amino acids at the reverse turn add another dimension to its modularity by introducing a varying extent of twist that could be utilized to probe the structure activity relationship of designed β-sheets and the modulate folding of proteins. N-methylation has found tremendous utility in cyclic peptides, however, its use in linear peptides was limited. With this report we strongly believe that this simple strategy will not only find enormous utility in foldamer design26 and protein engineering but also in the development of novel materials27 and peptide based catalysts.28Acknowledgements
The authors gratefully acknowledge the Science and Engineering Research Board (SERB) through the project SB/YS/LS-220/2013 to JC and the Department of Biotechnology, Ministry of Science and Technology through the DBT-IISc partnership program for providing funding for this work. We also thank the DBT & DST funded NMR facility and the DBT funded Proteomics facility at IISc. We also wish to thank Prof. Siddhartha P. Sarma for assistance with the NMR experiments and Prof. Debnath Pal for the computing facility.References
- (a) M. Pelay-Gimeno, A. Glas, O. Koch and T. N. Grossmann, Angew. Chem., Int. Ed., 2015, 54, 8896 CrossRef CAS PubMed; (b) A. M. Watkins and P. S. Arora, ACS Chem. Biol., 2014, 9, 1747 CrossRef CAS PubMed.
- (a) M. D. Struthers, R. P. Cheng and B. Imperiali, Science, 1996, 271, 342 CAS; (b) Z. E. Reinert, G. A. Lengyel and S. W. Horne, J. Am. Chem. Soc., 2013, 135, 12528 CrossRef CAS PubMed.
- (a) C. M. Nair, M. Vijayan, Y. V. Venkatachalapathi and P. Balaram, J. Chem. Soc., Chem. Commun., 1979, 1183 RSC; (b) T. S. Haque, J. C. Little and S. H. Gellman, J. Am. Chem. Soc., 1996, 118, 6975 CrossRef CAS; (c) J. A. Robinson, Acc. Chem. Res., 2008, 41, 1278 CrossRef CAS PubMed.
- (a) M. Ramírez-Alvarado, F. J. Blanco and L. Serrano, Nat. Struct. Biol., 1996, 3, 604 CrossRef; (b) C. D. Tatko and M. L. Waters, J. Am. Chem. Soc., 2002, 124, 9372 CrossRef CAS PubMed; (c) S. R. Griffiths-Jones, A. J. Maynard and M. S. Searle, J. Mol. Biol., 1999, 292, 1051 CrossRef CAS PubMed; (d) A. G. Cochran, N. J. Skelton and M. A. Starovasnik, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5578 CrossRef CAS PubMed.
- W. J. Cooper and M. L. Waters, Org. Lett., 2005, 7, 3825 CrossRef CAS PubMed.
- (a) J. Xiao, B. Weisblum and P. Wipf, Org. Lett., 2006, 8, 4731 CrossRef CAS PubMed; (b) G.-B. Liang and S. H. Gellman, J. Am. Chem. Soc., 1999, 121, 1806 CrossRef; (c) Y. J. Chung, B. R. Huck, L. A. Christianson, H. E. Stanger, S. Krauthäuser, D. R. Powell and S. H. Gellman, J. Am. Chem. Soc., 2000, 122, 3995 CrossRef CAS; (d) G. Jeannotte and W. D. Lubell, J. Am. Chem. Soc., 2004, 126, 14334 CrossRef CAS PubMed; (e) R. V. Nair, S. B. Baravkar, T. S. Ingole and G. J. Sanjayan, Chem. Commun., 2014, 50, 13874 RSC; (f) S. Chowdhury, G. Schatte and H. B. Kraatz, Angew. Chem., Int. Ed., 2008, 47, 7056 CrossRef CAS PubMed; (g) J. S. Nowick and J. O. Brower, J. Am. Chem. Soc., 2003, 125, 876 CrossRef CAS PubMed; (h) J. S. Nowick, K. S. Lam, T. V. Khasanova, W. E. Kemnitzer, S. Maitra, H. T. Mee and R. Liu, J. Am. Chem. Soc., 2002, 124, 4972 CrossRef CAS PubMed.
- G. A. Lengyel, Z. E. Reinert, B. D. Griffith and S. W. Horne, Org. Biomol. Chem., 2014, 12, 5375 CAS.
- (a) D. K. Chalmers and G. R. Marshall, J. Am. Chem. Soc., 1995, 117, 5927 CrossRef CAS; (b) Y. Takeuchi and G. R. Marshall, J. Am. Chem. Soc., 1998, 120, 5363 CrossRef CAS; (c) A. C. Gibbs, T. C. Bjorndahl, R. S. Hodges and D. S. Wishart, J. Am. Chem. Soc., 2002, 124, 1203 CrossRef CAS PubMed.
- (a) J. Chatterjee, D. Mierke and H. Kessler, J. Am. Chem. Soc., 2006, 128, 15164 CrossRef CAS PubMed; (b) J. G. Beck, J. Chatterjee, B. Laufer, M. U. Kiran, A. O. Frank, S. Neubauer, O. Ovadia, S. Greenberg, C. Gilon, A. Hoffman and H. Kessler, J. Am. Chem. Soc., 2012, 134, 12125 CrossRef CAS PubMed.
- N. El Tayar, A. E. Mark, P. Vallat, R. M. Brunne, B. Testa and W. F. van Gunsteren, J. Med. Chem., 1993, 36, 3757 CrossRef CAS PubMed.
- R. Rai, S. Raghothama and P. Balaram, J. Am. Chem. Soc., 2006, 128, 2675 CrossRef CAS PubMed.
- A. E. Wakefield, W. M. Wuest and V. A. Voelz, J. Chem. Inf. Model., 2015, 55, 806 CrossRef CAS PubMed.
- P. Y. Chou and G. D. Fasman, J. Mol. Biol., 1977, 115, 135 CrossRef CAS PubMed.
- J. Chatterjee, B. Laufer and H. Kessler, Nat. Protoc., 2012, 7, 432 CrossRef CAS PubMed.
- R. Mahalakshmi, S. Raghothama and P. Balaram, J. Am. Chem. Soc., 2006, 128, 1125 CrossRef CAS PubMed.
- H. Kessler, Angew. Chem., Int. Ed. Engl., 1970, 9, 219 CrossRef CAS.
- H. Kessler, U. Anders and M. Schudok, J. Am. Chem. Soc., 1990, 112, 5908 CrossRef CAS.
- See ESI for details†.
- I. L. Karle, S. K. Awasthi and P. Balaram, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 8189 CrossRef CAS.
- C. Zhao, P. L. Polavarapu, C. Das and P. Balaram, J. Am. Chem. Soc., 2000, 122, 8228 CrossRef CAS.
- R. M. Culik, H. Jo, W. F. DeGrado and F. Gai, J. Am. Chem. Soc., 2012, 134, 8026 CrossRef CAS PubMed.
- F. A. Syud, J. F. Espinosa and S. H. Gellman, J. Am. Chem. Soc., 1999, 121, 11577 CrossRef CAS.
- M. Körling and A. Geyer, Eur. J. Org. Chem., 2015, 2382 CrossRef.
- B. Eckhardt, W. Grosse, L. O. Essen and A. Geyer, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18336 CrossRef CAS PubMed.
- (a) S. J. Maynard, A. M. Almeida, Y. Yoshimi and S. H. Gellman, J. Am. Chem. Soc., 2014, 136, 16683 CrossRef CAS PubMed; (b) G. A. Lengyel, R. C. Frank and W. S. Horne, J. Am. Chem. Soc., 2011, 133, 4246 CrossRef CAS PubMed.
- (a) C. M. Goodman, S. Choi, S. Shandler and W. F. DeGrado, Nat. Chem. Biol., 2007, 3, 252 CrossRef CAS PubMed; (b) S. W. Horne and S. H. Gellman, Acc. Chem. Res., 2008, 41, 1399 CrossRef PubMed.
- C. M. Micklitsch, P. J. Knerr, M. C. Branco, R. Nagarkar, D. J. Pochan and J. P. Schneider, Angew. Chem., Int. Ed., 2011, 50, 1577 CrossRef CAS PubMed.
- (a) J. T. Blank, D. J. Guerin and S. J. Miller, Org. Lett., 2000, 2, 1247 CrossRef CAS PubMed; (b) M. E. Diener, A. J. Metrano, S. Kusano and S. J. Miller, J. Am. Chem. Soc., 2015, 137, 12369 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, HPLC and MALDI traces, NMR spectra, DMSO-d6 titrations, coupling constants, chemical shifts, NMR structure calculations and overlay of structures. See DOI: 10.1039/c6sc00518g |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2016 |