Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Analysis of transition state mimicry by tight binding aminothiazoline inhibitors provides insight into catalysis by human O-GlcNAcase†
N.
Cekic‡
a,
J. E.
Heinonen‡
a,
K. A.
Stubbs
ab,
C.
Roth
 c,
Y.
He
c,
A. J.
Bennet
a,
E. J.
McEachern§
a,
G. J.
Davies
c,
Y.
He
c,
A. J.
Bennet
a,
E. J.
McEachern§
a,
G. J.
Davies
 c and
D. J.
Vocadlo
c and
D. J.
Vocadlo
 *ad
*ad
aDepartment of Chemistry, Simon Fraser University, Burnaby, British Columbia V5A 1S6, Canada. E-mail: dvocadlo@sfu.ca
bSchool of Chemistry and Biochemistry, The University of Western Australia (M313), 35 Stirling Highway, Crawley, WA 6009, Australia
cYork Structural Biology Laboratory, Department of Chemistry, The University of York, YO10 5DD, UK
dDepartment of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, British Columbia V5A 1S6, Canada
First published on 15th February 2016
Abstract
The modification of nucleocytoplasmic proteins with O-linked N-acetylglucosamine (O-GlcNAc) plays diverse roles in multicellular organisms. Inhibitors of O-GlcNAc hydrolase (OGA), the enzyme that removes O-GlcNAc from proteins, lead to increased O-GlcNAc levels in cells and are seeing widespread adoption in the field as a research tool used in cells and in vivo. Here we synthesize and study a series of tight binding carbohydrate-based inhibitors of human OGA (hOGA). The most potent of these 2′-aminothiazolines binds with a sub-nanomolar Ki value to hOGA (510 ± 50 pM) and the most selective has greater than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold selectivity for hOGA over mechanistically related human lysosomal β-hexosaminidase. Structural data of inhibitors in complex with an hOGA homologue reveals the basis for variation in binding among these compounds. Using linear free energy analyses, we show binding of these 2′-aminothiazoline inhibitors depends on the pKa of the aminothiazoline ring system, revealing the protonation state of the inhibitor is a key driver of binding. Using series of inhibitors and synthetic substrates, we show that 2′-aminothiazoline inhibitors are transition state analogues of hOGA that bind to the enzyme up to 1-million fold more tightly than the substrate. These collective data support an oxazoline, rather than a protonated oxazolinium ion, intermediate being formed along the reaction pathway. Inhibitors from this series will prove generally useful tools for the study of O-GlcNAc. The new insights gained here, into the catalytic mechanism of hOGA and the fundamental drivers of potency and selectivity of OGA inhibitors, should enable tuning of hOGA inhibitors with desirable properties.
000-fold selectivity for hOGA over mechanistically related human lysosomal β-hexosaminidase. Structural data of inhibitors in complex with an hOGA homologue reveals the basis for variation in binding among these compounds. Using linear free energy analyses, we show binding of these 2′-aminothiazoline inhibitors depends on the pKa of the aminothiazoline ring system, revealing the protonation state of the inhibitor is a key driver of binding. Using series of inhibitors and synthetic substrates, we show that 2′-aminothiazoline inhibitors are transition state analogues of hOGA that bind to the enzyme up to 1-million fold more tightly than the substrate. These collective data support an oxazoline, rather than a protonated oxazolinium ion, intermediate being formed along the reaction pathway. Inhibitors from this series will prove generally useful tools for the study of O-GlcNAc. The new insights gained here, into the catalytic mechanism of hOGA and the fundamental drivers of potency and selectivity of OGA inhibitors, should enable tuning of hOGA inhibitors with desirable properties.
Introduction
The modification of serine and threonine residues of nuclear and cytoplasmic proteins with terminal O-linked β-N-acetylglucosamine (O-GlcNAc)1 has been found on hundreds of proteins.2–4O-GlcNAc is present in all multi-cellular eukaryotes studied and occurs in a dynamic and reversible manner.5 Global O-GlcNAc levels have been shown to vary in response to cellular nutrient availability and stress and in some cases has been found to influence protein phosphorylation.6 These observations have stimulated interest in the physiological roles played by O-GlcNAc and research has implicated this modification in controlling various cellular processes including, for example, proteosomal degradation of proteins7–9 and transcriptional regulation.10,11 Additionally, a growing body of literature has implicated O-GlcNAcylation in chronic diseases such as neurodegeneration12–15 and cancer.9,16,17 Given the mounting potential in targeting protein O-GlcNAcylation for therapeutic benefit, there has been rising interest in understanding the molecular basis for inhibition of modulators of the O-GlcNAc pathway and the creation of small molecule modulators of this pathway for use in tissues.18–20The glycosyltransferase uridine diphospho-N-acetylglucosamine:peptide β-N-acetylglucosaminyl transferase (OGT) installs O-GlcNAc residues using uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as the sugar substrate donor.21,22 The enzyme responsible for removing O-GlcNAc from proteins is O-GlcNAcase (OGA),23 which is a member of glycoside hydrolase family 84 (GH84) of the CAZy classification system.24 Consistent with the reversible nature of protein O-GlcNAcylation, inhibitors of these enzymes have been shown to induce time-dependent changes in cellular O-GlcNAc levels.19,25–27 Small molecule inhibitors of OGA, in particular, have emerged as commonly used research tools for evaluating the phenotypic effects of increased O-GlcNAc levels in cultured cells, as well as in vivo.
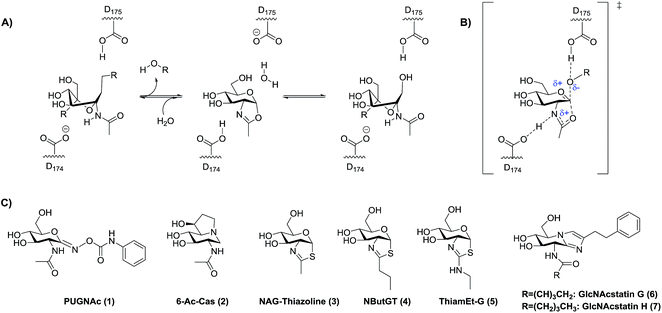
Interest in inhibitors of OGA has gained increasing attention due to growing recognition of the physiological roles of O-GlcNAc. Among the first reported inhibitors of OGA is O-(2-acetamido-2-deoxy-D-gluco-pyranosylidene)amino-N-phenylcarbamate (PUGNAc, Fig. 1, 1)28 (hOGA Ki = 46 nM). This inhibitor, however, has well-described off target effects29,30 including the inhibition of the functionally related lysosomal β-hexosaminidases HEXA and HEXB from family GH20.26 These two lysosomal enzymes cleave β-linked terminal N-acetylhexosamine residues from various glycoconjugates including gangliosides. Genetic deficiency of these hexosaminidases results in Tay–Sachs and Sandhoff's disease, which stem from the accumulation of gangliosides within lysosomes. More recently identified inhibitors such as 6-acetamido-6-deoxy-castanospermine (6-Ac-Cas)29 (hOGA Ki = 300 nM) (Fig. 1, 2), and 1,2-dideoxy-2′-methyl-α-D-glucopyranoso-[2,1-d]-Δ2′-thiazoline (NAG-thiazoline)26 (hOGA Ki = 70 nM) (Fig. 1, 3) while fairly potent, are also non-selective. Given that gangliosides play varied roles in cellular processes ranging from cell membrane structure to cell signaling, the selectivity of OGA inhibitors has emerged as being important for the creation of useful probe molecules for use in vivo.29,31,32
Rationally designed OGA inhibitors have been pursued based on knowledge of the catalytic mechanism of hOGA. Detailed mechanistic studies26,33 coupled with structural studies of bacterial homologues of hOGA34,35 have provided clear support for a catalytic mechanism involving substrate-assisted catalysis in which the 2-acetamido group of the substrate serves as a catalytic nucleophile to generate a transient enzyme-bound oxazoline or oxazolinium intermediate (Fig. 1A) and stabilizing an oxocarbenium ion-like transition state (Fig. 1B). In this two-step catalytic mechanism, two aspartates (Asp174 and Asp175) play key roles as general acid/base catalytic residues.33 Asp174 serves to orient and polarize the 2-acetamido group to aid its attack at the anomeric center, accepting a proton during formation of the oxazoline ring. Asp175 acts as a general acid, donating a proton to the glycosidic oxygen during cleavage of the glycosidic bond (Fig. 1A and B). Given the clear resemblance of NAG-thiazoline to the oxazoline intermediate, analogues of this molecule in which the 2′-position of the thiazoline ring is modified showed fair selectivities coupled with moderate nanomolar potencies as exemplified by NButGT (hOGA Ki = 230 nM, Ki(HEX)/Ki(hOGA) = 1,500, Fig. 1C, 4).26
More selective hOGA inhibitors have since been generated including the GlcNAcstatins27,36 such as GlcNAcstatin C (hOGA Ki = 3.2 nM, Ki(HEX)/Ki(hOGA) = 190) and a bioisostere of NButGT, 1,2-dideoxy-2′-ethylamino-α-D-glucopyranoso-[2,1-d]-Δ2′-thiazoline (ThiamEt-G)(hOGA Ki = 21 nM, Ki(HEX)/Ki(hOGA) = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)19 (Fig. 1C, 5), which is more synthetically accessible. ThiamEt-G is orally available and increases brain O-GlcNAc levels in mammals and has been used by several groups to show chronic OGA inhibition and increased O-GlcNAcylation over several months has no apparent deleterious effects and also protects in various mouse models of AD against both tau14,37,38 and amyloid pathologies.39,40 Given the great interest in OGA inhibitors as research tools, we aimed to explore the basis for the inhibition of hOGA by ThiamEt-G. Here we report on the synthesis and characterization of aminothiazoline inhibitors with hOGA and human HexB (hHexB), demonstrate the strongly pKa dependent inhibition of hOGA by such inhibitors, illuminate the molecular basis for observed selectivity and potency using structural biology, and reveal these inhibitors are genuine TS analogues – which reveals new insight into the nature of the catalytic mechanism as well as explaining the picomolar binding of the best representative from this inhibitor family.
000)19 (Fig. 1C, 5), which is more synthetically accessible. ThiamEt-G is orally available and increases brain O-GlcNAc levels in mammals and has been used by several groups to show chronic OGA inhibition and increased O-GlcNAcylation over several months has no apparent deleterious effects and also protects in various mouse models of AD against both tau14,37,38 and amyloid pathologies.39,40 Given the great interest in OGA inhibitors as research tools, we aimed to explore the basis for the inhibition of hOGA by ThiamEt-G. Here we report on the synthesis and characterization of aminothiazoline inhibitors with hOGA and human HexB (hHexB), demonstrate the strongly pKa dependent inhibition of hOGA by such inhibitors, illuminate the molecular basis for observed selectivity and potency using structural biology, and reveal these inhibitors are genuine TS analogues – which reveals new insight into the nature of the catalytic mechanism as well as explaining the picomolar binding of the best representative from this inhibitor family.
Results and discussion
Early studies showed that varying the 2′-alkyl substituent of NAG-thiazoline resulted in increased selectivity for OGA over the lysosomal hexosaminidases at the slight expense of potency.26 This trend does not hold for PUGNAc analogues which show only modest selectivity.41,42 Exploiting a similar approach of increasing steric bulk of the acetamido group, however, cell-penetrant glucoimidazole inhibitors including the hOGA inhibitors GlcNAcstatin G (hOGA = 4.1 nM, Ki(HEX)/Ki(hOGA) = 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (Fig. 1C, 6) and GlcNAcstatin H (hOGA = 2.6 nM, Ki(HEX)/Ki(hOGA) = 35
000) (Fig. 1C, 6) and GlcNAcstatin H (hOGA = 2.6 nM, Ki(HEX)/Ki(hOGA) = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (Fig. 1C, 7), have been uncovered as some of the most selective hOGA inhibitors to date.20 Structural studies have suggested that these OGA inhibitors derive their selectivity from structural differences between the active sites of OGA and the lysosomal β-hexosaminidases in the region that serves to position the 2-acetamido group of the substrate.34,43 We therefore first set out to gain an understanding of the detailed relationships between the size of substituents at the 2′-position of a series of 2′-aminothiazoline inhibitors and the influence on binding of altered electronic properties of the 2′-aminothiazoline system.
000) (Fig. 1C, 7), have been uncovered as some of the most selective hOGA inhibitors to date.20 Structural studies have suggested that these OGA inhibitors derive their selectivity from structural differences between the active sites of OGA and the lysosomal β-hexosaminidases in the region that serves to position the 2-acetamido group of the substrate.34,43 We therefore first set out to gain an understanding of the detailed relationships between the size of substituents at the 2′-position of a series of 2′-aminothiazoline inhibitors and the influence on binding of altered electronic properties of the 2′-aminothiazoline system.
Preparation, potency, and selectivity of 2′-alkylaminothiazoline OGA inhibitors
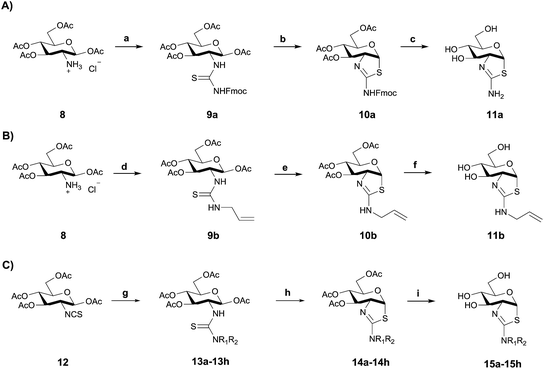
Using three different approaches (Scheme 1) we synthesized a series of 2′-alkylaminothiazoline derivatives. Using the common intermediate hydrochloride salt of 1,3,4,6-tetra-O-acetyl-2-amino-2-deoxy-β-D-glucopyranose 8 (Scheme 1A and B), which was conveniently accessed in three steps,44 we prepared compounds 11a and 11b by reacting either N-fluorenylmethyloxycarbonyl (Fmoc)-protected isothiocyanate or N-allyl isothiocyanate with 8 in the presence of triethylamine to generate the respective thiourea intermediates 9a and 9b. Subsequent cyclization with excess SnCl4 followed by a two step deprotection using catalytic NaOMe in anhydrous methanol, and piperidine catalyzed removal of the Fmoc group, afforded analogue 11a. Cyclization of thiourea 9b with excess TFA, followed by a one step deprotection using catalytic K2CO3 in anhydrous methanol afforded inhibitor 11b.Inhibitors 15a–h were synthesized using an alternate route from the common isothiocyanate intermediate 1,3,4,6-tetra-O-acetyl-2-deoxy-2-isothiocyanato-β-D-glucopyranose (Scheme 1C, 12), which was prepared from 8via a biphasic reaction in H2O/DCM with thiophosgene.44 Reaction of isothiocyanate 12 with a series of alkylamines and dialkylamines, or their respective hydrochloride salts, yielded thioureas 13a–h. Acid catalyzed cyclization of these thiourea-containing compounds using TFA provided protected aminothiazolines 14a–h, which after deprotection with K2CO3 afforded 15a–h.
We then determined the Ki values for inhibition of hOGA by compounds 11a–b and 15a–h. Using Michael–Menten kinetics we obtained the Ki values for the less potent inhibitors 15e–h, and as expected, double-reciprocal Lineweaver–Burk plots revealing a competitive mode of inhibition (ESI Fig. S1†). For the increasingly tight binding inhibitors we used the non-linear fitting method described by Morrison,45 which can be used to determine Ki values which are comparable to the concentration of the enzyme being studied. Refinement of the Morrison approach by Copeland enables defining the Ki value for an inhibitor through the use of relative rates using a quadratic equation (eqn (1)).46 This approach depends on knowing the initial free enzyme and inhibitor concentrations without the assumption that the free inhibitor concentration is equal to the total inhibitor concentration.
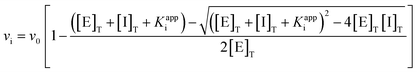 | (1) |
Further, Ki values can be accurately determined over a wide range of enzyme concentrations using this method,47 which enabled us to use hOGA concentrations that permit accurate initial rate determinations. We followed existing guidance48 to select inhibitor concentrations for our Ki value determinations and confirmed the accuracy of this method by showing the Ki values for inhibitor 11b were in reasonable accord when using either the Michaelis–Menten or Morrison method (ESI Fig. S2†).
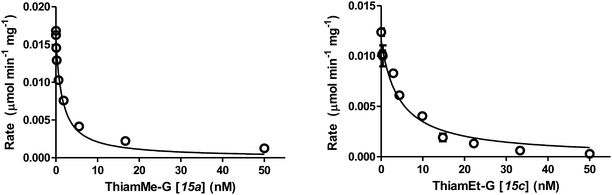
Using these methods we found the Ki values for inhibition of hOGA by compounds 11a–b and 15a–h ranged from the high sub-nanomolar to low nanomolar range (Table 1). Notably, we find that the Ki value for ThiamEt-G (Fig. 1 and 2) was 10-fold lower (Ki = 2.1 nM) than that previously determined19 using the Michaelis–Menten method (Ki = 21 nM). This makes Thiamet-G over 100-fold more potent than the isosteric NButGT (Ki = 230 nM). The most tight-binding compound with a Ki of 510 ± 50 pM (Fig. 2) is 15a (ThiamMe-G, Scheme 1), which ranks this compound among the most potent glycoside hydrolase inhibitors known, as well as the most potent selective hOGA inhibitor reported. Interestingly, we note that there is only a slight decrease in potency for hOGA upon increasing the volume of the 2′-aminoalkyl substituent to the point where the alkyl group is a propyl (15d, Ki = 2 nM). A butyl chain, however, leads to a greater than 100-fold loss of potency (15e, Ki = 350 nM). Structures of bacterial OGA homologues, in which the active site residues are completely conserved with hOGA,34,35 show a discretely sized pocket having a volume that nicely accommodates the propyl substituent of 15d.
| Inhibitor | hOGA Kia (nM) | hHexB Kib (μM) | (hHexB/hOGA)c | pKae | Fraction protonated at pH 7.4 |
|---|---|---|---|---|---|
| a Determined using the Morrison Ki fit if the values are below 5 mM. b Determined using Dixon plot analysis. c Selectivity ratios representing the favored selectivity for hOGA compared to hHexB. d Determined using Michaelis–Menten inhibition analysis. e See ESI for full details, pKa (NButGT) = 4.65. | |||||
| 11a: R1 = R2 = H | 4.7 ± 0.3 | 5.0 ± 0.6d | 1100 | ||
| 15a: R1 = H, R2 = CH3 | 0.51 ± 0.05 | 1.7 ± 0.19d | 3300 | ||
| 15b: R1 = R2 = CH3 | 2.4 ± 0.2 | 13.0 ± 3.8 | 5400 | ||
| 15c: R1 = H, R2 = CH2CH3 | 2.1 ± 0.3 | 740 ± 60 (ref. 19) | 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.68 | 0.66 |
| 11b: R1 = H, R2 = CH2CHCH2 | 3.2 ± 0.4 | 2850 ± 570 | 950![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| 15d: R1 = H, R2= (CH2)2CH3 | 2.0 ± 0.2 | 3700 ± 670 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| 15e: R1 = H, R2= (CH2)3CH3 | 350 ± 90d | 4800 ± 763 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
||
| 15f: R1 = H, R2= (CH2)2F | 15 ± 5d | 180 ± 44 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.92 | 0.25 |
| 15g: R1 = H, R2 = CH2CHF2 | 60 ± 10d | 150 ± 50 | 2500 | 6.18 | 0.06 |
| 15h: R1 = H, R2 = CH2CF3 | 1000 ± 200d | 4200 ± 1525 | 4200 | 5.33 | 0.01 |
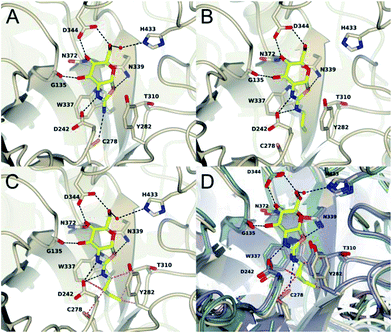
To assess the structural basis for this stepped decrease in potency observed on going to the 2′-aminobutylthiazoline 15e, we determined the structure of Bacteroides thetaiotaomicron, a bacterial homolog (BtGH84) of hOGA, in complex with the parent 2′-aminothiazoline 11a, the tighter binding 2′-aminopropylenethiazoline 11b, and the butyl derivative 15e, which shows greatly diminished binding. In all three structures, the respective inhibitor binds in the active site in a conserved mode with an invariant hydrogen bond pattern for the aminothiazoline moiety (Fig. 3). The alkyl chain of the modified aminothiazolines points into the conserved hydrophobic pocket as shown for ThiamEt-G.19 An inspection of the binding pocket shows no further hydrophobic interactions in the case of the unsubstituted aminothiazoline 11a, except a possible weak electrostatic interaction with C278 at the bottom of the pocket (Fig. 3A). Propylene derivative 11b matches the size of the pocket, requiring only a minor adjustment of the C278 rotamer and showing favorable hydrophobic interactions with W337, T310, and Y282 (Fig. 3B). Even a small further extension of the alkyl chain, as in the butyl derivative 15e, leads to steric clashes with multiple residues in the cavity, driving the side chain of C278 to adopt a different orientation. Furthermore, we observe the polypeptide main chain surrounding C278 is shifted away from the inhibitor. Thus, the size of the pocket disfavors substituents longer than three carbon units in chain length, and those inhibitors having such larger groups induce unfavorable conformational changes within the active site (Fig. 3C and D).
We next set out to assess the selectivity of this series of inhibitors for hOGA over the lysosomal β-hexosaminidases, which are comprised of combinations of α and β subunits that are products of the highly homologous HEXA and HEXB genes. Using purified human hexosaminidase B (hHexB) we determined the approximate Ki values for compounds 11a to 15h using Dixon plot analysis and found remarkably high inhibitor selectivities ranging from 1100- to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850
850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold preference for hOGA (Table 1). We confirmed these Dixon plot analyses for the two most potent compounds, 11a and 15a, by determining full Ki values for their inhibition of hHexB (ESI Fig. S4†). Remarkably, inhibitors 11a and 15a still retain 1100 and 3300-fold selectivity for hOGA despite their similarity in size to NAG-thiazoline, which itself demonstrated no selectivity.26 Accordingly, the presence of the 2′-amino substituent, on its own, confers at least 1000-fold selectivity for hOGA over hHexB.
000-fold preference for hOGA (Table 1). We confirmed these Dixon plot analyses for the two most potent compounds, 11a and 15a, by determining full Ki values for their inhibition of hHexB (ESI Fig. S4†). Remarkably, inhibitors 11a and 15a still retain 1100 and 3300-fold selectivity for hOGA despite their similarity in size to NAG-thiazoline, which itself demonstrated no selectivity.26 Accordingly, the presence of the 2′-amino substituent, on its own, confers at least 1000-fold selectivity for hOGA over hHexB.
We also noted that the selectivity ratio for hOGA increases as the 2′-aminoalkyl chain length increases to the three-carbon propyl (15d) and propylene (11b) derivatives, but this trend reverses once the chain length increases further, as seen for the 2′-aminobutyl analogue (15e). Since the active site pocket for hHexB is more constrained in the vicinity of the acetamido group, these observations are consistent with structural observations of both bacterial hOGA homologues34 and hHexB.43 This suggests that once the 2′-substituent passes the volume that can be accommodated in the active site of hOGA, increases in bulk are even slightly more deleterious for hOGA as compared to hHexB.
Preparation of 2′-alkylaminothiazoline OGA inhibitors for evaluating electronic effects in OGA inhibition
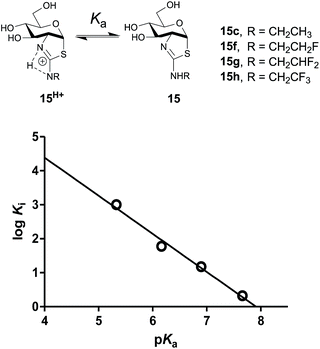
Notably, ThiamEt-G (Ki = 2.1 nM) binds over 100-fold more tightly than NButGT (Ki = 230 nM) and is 25-fold more selective for hOGA. Detailed mechanistic studies in combination with pH-rate profiles of wild-type and mutant hOGA revealed the key catalytic residue Asp174 in the OGA catalytic site (Asp242 in BtGH84) acts as a general base to assist the attack of the substrate 2-acetamido group onto the anomeric center. The kinetic pKa of this residue was determined to be 5.2 so that at physiological pH this residue is expected to be in its carboxylate form33 and therefore suitably ionized in the resting enzyme to facilitate catalysis. Given that aminothiazolines are known to be more basic than thiazolines, it was speculated that installation of the 2′-alkylamino group would increase the basicity of ThiamEt-G as compared to NButGT and thereby contribute to its enhanced potency relative to inhibitors bearing 2′-alkyl groups at physiological pH. Structural data of ThiamEt-G bound within the active site of BtGH84 is consistent with this proposal, revealing that both the endo and exocyclic amines engaged Asp242 of BtGH84.19We set out to evaluate this proposal and assess the importance of the inhibitor pKa on potency by studying a series of 2′-aminoethylthiazoline inhibitors with increasing fluorine substitution at the terminal methyl group (15f–h). Evaluation of the potency of these compounds revealed a progressive increase in Ki value upon increasing substitution with fluorine [Ki = 2.1 (CH3, 15c) 15 (CH2F,15f), 60 (CHF2, 15g) and 1000 (CF3, 15h) nM] (Table 1). To clearly understand the relationship between inhibitor basicity and potency we used 13C NMR titration to determine the pKa values of conjugate acids of these inhibitors (15c, 15f–h). NMR methods are highly accurate and can be used to determine relative pKa values between one or more compounds having an unknown pKa and a reference compound having a well established pKa value.49
Practically, two advantages of this relative measurement approach is that it does not require repeated pH measurements throughout the titration and it can be used to evaluate small changes in pKa values. In this way, a non-linear plot of the difference between NMR resonance frequencies for a compound of interest and those for a standard, such as 3-nitrophenol, can be used to determine the ratio (R) of the acid dissociation constants between these two materials. A typical plot for the difference in 13C chemical shifts is shown in Fig. 4 (see ESI† methods for a full discussion).
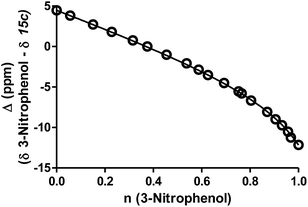 | ||
| Fig. 4 A representative example for 13C-NMR determination of the pKa value for ThiamEt-G (15c) with chemical shifts of the 2′-C of the thiazoline ring resonances (Δ ppm) for compounds 15c as a function of the fractional protonation (n) of the reference compound, 3-nitrophenol (pKa of 8.42). The solid line is the best non-linear least squares fit to eqn (S1) (ESI†). | ||
To assess the extent to which inhibitor potency depends on its pKa value, we plotted the pKa value of inhibitors (15c, 15f–h and NButGT, 4) with the corresponding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki, which are both free energy terms. The resulting linear free energy relationship (LFER) shows a linear correlation (R2 = 0.9876) with a slope of −1.12 ± 0.09 (Fig. 5), which is consistent with the pKa value of inhibitors mostly dominating the effect of binding as compared to steric effects associated with increasing fluorine substitution. Notably, we also find that NButGT, which is isosteric to ThiamEt-G (15c), matches reasonably well within this correlation, supporting the electronic effects dominating this correlation. While the affinities of each protonation state of these inhibitors for hOGA (Fig. 5) cannot be readily determined because the enzyme itself has various ionization states, these data collectively suggest that the pKa value of the inhibitor, either by favoring the protonated inhibitor form or by optimizing hydrogen bonding strength, plays a key role in binding of these 2′-aminothiazoline inhibitors.
Ki, which are both free energy terms. The resulting linear free energy relationship (LFER) shows a linear correlation (R2 = 0.9876) with a slope of −1.12 ± 0.09 (Fig. 5), which is consistent with the pKa value of inhibitors mostly dominating the effect of binding as compared to steric effects associated with increasing fluorine substitution. Notably, we also find that NButGT, which is isosteric to ThiamEt-G (15c), matches reasonably well within this correlation, supporting the electronic effects dominating this correlation. While the affinities of each protonation state of these inhibitors for hOGA (Fig. 5) cannot be readily determined because the enzyme itself has various ionization states, these data collectively suggest that the pKa value of the inhibitor, either by favoring the protonated inhibitor form or by optimizing hydrogen bonding strength, plays a key role in binding of these 2′-aminothiazoline inhibitors.
Assessment of 2′-aminothiazoline inhibitors as transition state analogs
The tight binding of these 2′-aminothiazoline inhibitors prompted us to consider their potency in the context of their size. One widely used parameter to understand the efficiency of binding as a function of molecular weight is to consider the ligand efficiency (LE) of a ligand. This measure provides the binding affinity of the compound as a measure of the number of heavy atoms. We calculate a remarkably high LE of 0.88 kcal per mol per heavy atom for compound 15a. Such a LE is comparable to some of the highest ever observed LEs observed for compounds in the size range of between 10–50 heavy atoms50 and suggests to us that these compounds could well be TS analogues, as had been observed for the related thiazoline inhibitors51. Tight-binding inhibitors that bear resemblance to enzyme substrates or intermediates have often been considered to be TS analogues simply by virtue of their potency. However, because enzymes are thought to catalyze reactions by tightly binding the TS, for genuine TS analogues changes in free energies of binding of a series of TS analogues (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki) should parallel changes in the free energies of a series of related transition states TS (log
Ki) should parallel changes in the free energies of a series of related transition states TS (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kcat/Km). Bartlett has formalized these concepts and methods to quantitatively assess whether compounds are TS analogues using LFERs.52,53 Using this method, genuine TS analogues yield plots of log
kcat/Km). Bartlett has formalized these concepts and methods to quantitatively assess whether compounds are TS analogues using LFERs.52,53 Using this method, genuine TS analogues yield plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km/kcat values, for a series of substrates having defined structural differences, versus log
Km/kcat values, for a series of substrates having defined structural differences, versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki values, for a series of inhibitors having the analogous structural changes, which show linear correlations having a slope of unity. Furthermore, log
Ki values, for a series of inhibitors having the analogous structural changes, which show linear correlations having a slope of unity. Furthermore, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kmversus log
Kmversus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki are not correlated for TS analogues but do correlate for substrate analogues.52
Ki are not correlated for TS analogues but do correlate for substrate analogues.52
Previous studies showed that NAG-thiazoline analogues are TS analogues despite their obvious resemblance to the oxazoline intermediate, perhaps due to the longer C–S bonds altering the thiazoline ring to resemble a late TS.51 Given the greater than 100-fold increase in potency we observe for the 2′-aminothiazoline inhibitors over their thiazoline counterparts and their structural resemblance to the oxazoline intermediate found along the reaction coordinate of hOGA, we wanted to assess whether incorporation of the 2′-amino group benefitted binding through serendipitous interactions, or whether the presence of the charge included in this class of inhibitors also made them TS analogues. We therefore turned to using the Bartlett LFER approach. With the series of inhibitors in hand we synthesized a series of fluorogenic 4-methylumbelliferyl 2-deoxy-2-urea-β-D-glucopyranoside substrates (18a–e, Scheme 2) bearing N-alkyl substituents on the terminal urea nitrogen that correspond to those alkyl groups present on the series of 2′-aminothiazoline inhibitors (11a, 15a, 15c–e). We started from 4-methylumbelliferyl 2-amino-2-deoxy-β-D-glucopyranoside hydrochloride (16) as a common intermediate.54 Per-O-acetylated urea substrates 17a–e were prepared by reacting 16 with the appropriate alkylisocyanate in the presence of triethylamine, followed by Zemplen de-O-acetylation. With this series of substrates (18a–e) in hand we determined the kcat/Km values governing their hOGA catalyzed hydrolysis (ESI Table S1†). Plotting these data to assess TS analogy, we observe (Fig. 6) a clear correlation (R2 = 0.9950) with a slope of 1.08 ± 0.04 between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki values for the inhibitors versus the log
Ki values for the inhibitors versus the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km/kcat values for the corresponding series of urea substrates (Fig. 6A). In contrast, we find no correlation between log
Km/kcat values for the corresponding series of urea substrates (Fig. 6A). In contrast, we find no correlation between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki values for the inhibitors and log
Ki values for the inhibitors and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km values for the series of substrates (Fig. 6B). These results indicate the 2′-aminothiazoline inhibitors are TS analogues for the hOGA catalyzed hydrolysis of urea substrates.
Km values for the series of substrates (Fig. 6B). These results indicate the 2′-aminothiazoline inhibitors are TS analogues for the hOGA catalyzed hydrolysis of urea substrates.
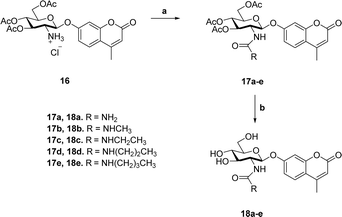 | ||
| Scheme 2 Synthesis of 4-methylumbelliferyl fluorogenic substrates 18a–e. (a) R-NCO, NEt3, CH3CN; (b) (i) NaOMe, MeOH; (ii) Dowex 50-H+. | ||
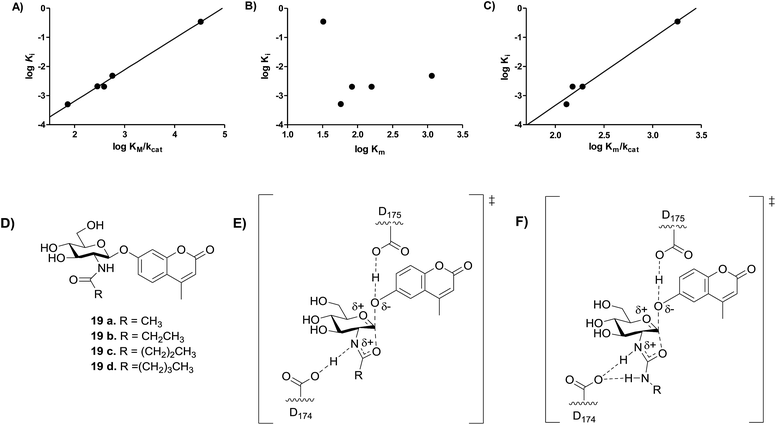 | ||
Fig. 6 Analysis of transition state analogy for hOGA 2′-aminothiazoline inhibitors (11a, 15a, 15c–e) and urea (18a–e) and acyl (19a–e) substrates. (A) The correlation between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km/kcat of substrates 18a–e and log Km/kcat of substrates 18a–e and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki of inhibitors 11a, 15a and 15c–e (m = 1.08 ± 0.04, R2 = 0.9950). (B) The correlation between the log Ki of inhibitors 11a, 15a and 15c–e (m = 1.08 ± 0.04, R2 = 0.9950). (B) The correlation between the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km of substrates 18a–e and log Km of substrates 18a–e and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki of inhibitors 11a, 15a, 15c–e. (C) The correlation between the log Ki of inhibitors 11a, 15a, 15c–e. (C) The correlation between the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km/kcat of substrates 19a–d and log Km/kcat of substrates 19a–d and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki of inhibitors 15a and 15c–e (m = 2.31 ± 0.25, R2 = 0.9768). (D) Substrates 19a–d synthesized by Whitworth et al.51 (E) Transition state for the hOGA catalyzed hydrolysis of substrates 19a–d likely involves transfer of the amidic proton. (F) Transition state for the hOGA catalyzed hydrolysis of substrates 18a–e likely involves no transfer of the amidic proton. Ki of inhibitors 15a and 15c–e (m = 2.31 ± 0.25, R2 = 0.9768). (D) Substrates 19a–d synthesized by Whitworth et al.51 (E) Transition state for the hOGA catalyzed hydrolysis of substrates 19a–d likely involves transfer of the amidic proton. (F) Transition state for the hOGA catalyzed hydrolysis of substrates 18a–e likely involves no transfer of the amidic proton. | ||
We recognized that our observations showed 2′-aminothiazoline inhibitors are TS analogues for the hOGA-catalyzed hydrolysis of unnatural urea substrates (18a–e), however, we were curious as to whether these inhibitors would also be TS analogues for the hOGA-catalyzed hydrolysis of the more natural N-acyl substrates. To address this question, we examined the correlation between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki values for the same series of 2′-aminothiazoline inhibitors and the series of N-acyl substrates (Fig. 6C) having the analogous structural changes for which kcat/Km values are reported (Fig. 6D).51 We find a fair correlation (R2 = 0.9768) with a slope of 2.3 ± 0.3. For this analysis we excluded the 2-aminothiazoline (11a) because of its unexpectedly poor inhibition of hOGA (Table 1).
Ki values for the same series of 2′-aminothiazoline inhibitors and the series of N-acyl substrates (Fig. 6C) having the analogous structural changes for which kcat/Km values are reported (Fig. 6D).51 We find a fair correlation (R2 = 0.9768) with a slope of 2.3 ± 0.3. For this analysis we excluded the 2-aminothiazoline (11a) because of its unexpectedly poor inhibition of hOGA (Table 1).
Notably, previous studies have shown kcat and kcat/Km values for the OGA-catalyzed hydrolysis of aryl 2-acetamido-2-deoxy-glucopyranosides, including 19a, vary in according to the pKa value of the phenolic leaving group.55 Additionally, such substrates bearing different sized N-acyl groups, including 19a–d (ref. 51) coupled with the large α–D(V)-KIE (kH/kD = 1.14 ± 0.02) value observed for the OGA-catalyzed hydrolysis of p-nitrophenol 2-acetamido-2-deoxy-glucopyranoside55 all support kcat and kcat/Km values reflect a chemical step being rate limiting for human OGA, which supports the validity of this TS analogy study.
The steep slope observed for this series of 2-acyl substrates in correlation with the aminothiazoline inhibitors is surprising. However, slopes other than unity in Bartlett LFER plots are precedented though not often rationalized.56 Here, we interpret this steep slope as indicating that the TS for the hOGA catalyzed hydrolysis of N-acyl substrates bears less positive charge in the forming oxazoline ring system. This may arise because the amide proton is in flight in the TS, as compared to the N-urea substrates (Fig. 6E and F), which are expected to be more basic and therefore likely lead to the formation of 2′-amino-oxazolinium ion intermediates that retain their proton. Accordingly, the TS leading to such aminooxazolinium ion intermediates is expected to have more positive charge than the corresponding transition state leading to the oxazoline intermediate (Fig. 6E and F). In keeping with this proposal, it is notable that site-directed deletion of the side chain of Asp174, which is the catalytic general acid/base catalytic residue that interacts with the acetamido group of the substrate, leads to a similar drop of between 150 to 750-fold in second order rate constant33 as seen on going from N-acyl to N-urea substrates (250-fold). Accordingly, these data indicate that 2′-aminothiazoline inhibitors are TS analogues for hOGA, by virtue of both their shape and general charge distribution. However, the steep slope observed for the LFER between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km/kcat values observed for N-acyl substrates and the log
Km/kcat values observed for N-acyl substrates and the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki values seen for the 2′-aminothiazoline inhibitors suggests that this feature lends improved binding over the corresponding partial charge that likely develops for the TS found for the hOGA catalyzed processing of natural N-acyl-containing substrates. These data support a catalytic mechanism in which residue D174 of hOGA acts as a general acid/base catalytic residue rather than simply stabilizing an oxazolinium ion intermediate as proposed for GH20 β-hexosaminidases.57
Ki values seen for the 2′-aminothiazoline inhibitors suggests that this feature lends improved binding over the corresponding partial charge that likely develops for the TS found for the hOGA catalyzed processing of natural N-acyl-containing substrates. These data support a catalytic mechanism in which residue D174 of hOGA acts as a general acid/base catalytic residue rather than simply stabilizing an oxazolinium ion intermediate as proposed for GH20 β-hexosaminidases.57
Conclusion
In summary, we describe a series of aminothiazoline inhibitors for human OGA having picomolar and low nanomolar Ki values. The great potency of this inhibitor family is in large part attributable to their pKa values since a clear correlation was observed between the pKa and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ki of a series of these compounds. Structures of these inhibitors in complex with BtOGA reveal the molecular basis for the trends in observed inhibitor potencies and selectivities. Using quantitative methods we find that these 2′-aminothiazoline inhibitors are tight-binding TS analogues for hOGA. These inhibitors benefit from their formal positive charge at physiological pH, harnessing favorable interactions that are only partly realized within the transition state for the natural 2-acyl-containing substrates. These observations should permit the design of more potent and selective inhibitors, not only in this class of inhibitor but also using other inhibitor scaffolds. Finally, the great potencies and selectivities of these inhibitors reveal a series of useful tool compounds that can be used to manipulate hOGA activity in vivo.
Ki of a series of these compounds. Structures of these inhibitors in complex with BtOGA reveal the molecular basis for the trends in observed inhibitor potencies and selectivities. Using quantitative methods we find that these 2′-aminothiazoline inhibitors are tight-binding TS analogues for hOGA. These inhibitors benefit from their formal positive charge at physiological pH, harnessing favorable interactions that are only partly realized within the transition state for the natural 2-acyl-containing substrates. These observations should permit the design of more potent and selective inhibitors, not only in this class of inhibitor but also using other inhibitor scaffolds. Finally, the great potencies and selectivities of these inhibitors reveal a series of useful tool compounds that can be used to manipulate hOGA activity in vivo.
Acknowledgements
The authors thank Diamond Light Source for beamtime (proposals mx-1221, mx-7864 and mx-9948) and the staff of beamlines I02, I03 and I24 for assistance with crystal testing and data collection. We are also grateful to Johan Turkenburg and Sam Hart for help during data collection. We thank Dr. Michael Tropak and Prof. Donald Mahuran, Sick Children's Hospital Toronto, for the samples of human hexosaminidase B. This research was supported by funding from Canadian Institutes of Health Research for support (MOP-123341) (DJV), Biotechnology and Biological Sciences Research Council (BB/K003836/1) (GJD and CR), and the Natural Sciences and Engineering Research Council (NSERC, DG-121348) of Canada (AJB). KAS thanks the Australian Research Council (FT100100291). DJV also thanks Brain Canada, Genome British Columbia, and the Michael Smith Foundation for Health Research for support of this research and the Canada Research Chairs program for support as a Tier I Canada Research Chair in Chemical Glycobiology.References
- C. R. Torres and G. W. Hart, J. Biol. Chem., 1984, 259, 3308–3317 CAS.
- N. Khidekel, S. B. Ficarro, E. C. Peters and L. C. Hsieh-Wilson, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 13132–13137 CrossRef CAS PubMed.
- S. A. Whelan and G. W. Hart, Circ. Res., 2003, 93, 1047–1058 CrossRef CAS PubMed.
- J. C. Trinidad, D. T. Barkan, B. F. Gulledge, A. Thalhammer, A. Sali, R. Schoepfer and A. L. Burlingame, Mol. Cell. Proteomics, 2012, 11, 215–229 Search PubMed.
- G. W. Hart, Front. Endocrinol., 2014, 5, 183 Search PubMed.
- Z. Wang, M. Gucek and G. W. Hart, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13793–13798 CrossRef CAS PubMed.
- H. B. Ruan, Y. Nie and X. Yang, Mol. Cell. Proteomics, 2013, 12, 3489–3497 Search PubMed.
- Y. Zhu, T. W. Liu, Z. Madden, S. A. Yuzwa, K. Murray, S. Cecioni, N. Zachara and D. J. Vocadlo, J. Mol. Cell Biol., 2016, 8, 2–16 CrossRef CAS PubMed.
- C. M. Ferrer, T. P. Lynch, V. L. Sodi, J. N. Falcone, L. P. Schwab, D. L. Peacock, D. J. Vocadlo, T. N. Seagroves and M. J. Reginato, Mol. Cell, 2014, 54, 820–831 CrossRef CAS PubMed.
- S. Ozcan, S. S. Andrali and J. E. Cantrell, Biochim. Biophys. Acta, 2010, 1799, 353–364 CrossRef PubMed.
- S. M. Ranuncolo, S. Ghosh, J. A. Hanover, G. W. Hart and B. A. Lewis, J. Biol. Chem., 2012, 287, 23549–23561 CrossRef CAS PubMed.
- F. Liu, J. Shi, H. Tanimukai, J. Gu, J. Gu, I. Grundke-Iqbal, K. Iqbal and C. X. Gong, Brain, 2009, 132, 1820–1832 CrossRef PubMed.
- F. Liu, K. Iqbal, I. Grundke-Iqbal, G. W. Hart and C. X. Gong, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 10804–10809 CrossRef CAS PubMed.
- S. A. Yuzwa, X. Shan, M. S. Macauley, T. Clark, Y. Skorobogatko, K. Vosseller and D. J. Vocadlo, Nat. Chem. Biol., 2012, 8, 393–399 CrossRef CAS PubMed.
- S. A. Yuzwa and D. J. Vocadlo, Chem. Soc. Rev., 2014, 43, 6839–6858 RSC.
- Z. Ma and K. Vosseller, Amino Acids, 2013, 45, 719–733 CrossRef CAS PubMed.
- W. Yi, P. M. Clark, D. E. Mason, M. C. Keenan, C. Hill, W. A. Goddard 3rd, E. C. Peters, E. M. Driggers and L. C. Hsieh-Wilson, Science, 2012, 337, 975–980 CrossRef CAS PubMed.
- R. F. Ortiz-Meoz, J. Jiang, M. B. Lazarus, M. Orman, J. Janetzko, C. Fan, D. Y. Duveau, Z. W. Tan, C. J. Thomas and S. Walker, ACS Chem. Biol., 2015, 10, 1392–1397 CrossRef CAS PubMed.
- S. A. Yuzwa, M. S. Macauley, J. E. Heinonen, X. Shan, R. J. Dennis, Y. He, G. E. Whitworth, K. A. Stubbs, E. J. McEachern, G. J. Davies and D. J. Vocadlo, Nat. Chem. Biol., 2008, 4, 483–490 CrossRef CAS PubMed.
- H. C. Dorfmueller, V. S. Borodkin, M. Schimpl, X. Zheng, R. Kime, K. D. Read and D. M. van Aalten, Chem. Biol., 2010, 17, 1250–1255 CrossRef CAS PubMed.
- W. A. Lubas and J. A. Hanover, J. Biol. Chem., 2000, 275, 10983–10988 CrossRef CAS PubMed.
- L. K. Kreppel, M. A. Blomberg and G. W. Hart, J. Biol. Chem., 1997, 272, 9308–9315 CrossRef CAS PubMed.
- Y. Gao, L. Wells, F. I. Comer, G. J. Parker and G. W. Hart, J. Biol. Chem., 2001, 276, 9838–9845 CrossRef CAS PubMed.
- V. Lombard, H. Golaconda Ramulu, E. Drula, P. M. Coutinho and B. Henrissat, Nucleic Acids Res., 2014, 42, D490–D495 CrossRef CAS PubMed.
- R. S. Haltiwanger, K. Grove and G. A. Philipsberg, J. Biol. Chem., 1998, 273, 3611–3617 CrossRef CAS PubMed.
- M. S. Macauley, G. E. Whitworth, A. W. Debowski, D. Chin and D. J. Vocadlo, J. Biol. Chem., 2005, 280, 25313–25322 CrossRef CAS PubMed.
- H. C. Dorfmueller, V. S. Borodkin, M. Schimpl, S. M. Shepherd, N. A. Shpiro and D. M. van Aalten, J. Am. Chem. Soc., 2006, 128, 16484–16485 CrossRef CAS PubMed.
- D. Beer, J.-L. Maloisel, D. M. Rast and A. Vasella, Helv. Chim. Acta, 1990, 73, 1918–1922 CrossRef CAS.
- M. S. Macauley, Y. He, T. M. Gloster, K. A. Stubbs, G. J. Davies and D. J. Vocadlo, Chem. Biol., 2010, 17, 937–948 CrossRef CAS PubMed.
- A. Mehdy, W. Morelle, C. Rosnoblet, D. Legrand, T. Lefebvre, S. Duvet and F. Foulquier, J. Biochem., 2012, 151, 439–446 CrossRef CAS PubMed.
- M. S. Macauley, X. Shan, S. A. Yuzwa, T. M. Gloster and D. J. Vocadlo, Chem. Biol., 2010, 17, 949–958 CrossRef CAS PubMed.
- M. S. Macauley, A. K. Bubb, C. Martinez-Fleites, G. J. Davies and D. J. Vocadlo, J. Biol. Chem., 2008, 283, 34687–34695 CrossRef CAS PubMed.
- N. Cetinbas, M. S. Macauley, K. A. Stubbs, R. Drapala and D. J. Vocadlo, Biochemistry, 2006, 45, 3835–3844 CrossRef CAS PubMed.
- R. J. Dennis, E. J. Taylor, M. S. Macauley, K. A. Stubbs, J. P. Turkenburg, S. J. Hart, G. N. Black, D. J. Vocadlo and G. J. Davies, Nat. Struct. Mol. Biol., 2006, 13, 365–371 Search PubMed.
- F. V. Rao, H. C. Dorfmueller, F. Villa, M. Allwood, I. M. Eggleston and D. M. van Aalten, EMBO J., 2006, 25, 1569–1578 CrossRef CAS PubMed.
- H. C. Dorfmueller, V. S. Borodkin, M. Schimpl and D. M. van Aalten, Biochem. J., 2009, 420, 221–227 CrossRef CAS PubMed.
- P. Borghgraef, C. Menuet, C. Theunis, J. V. Louis, H. Devijver, H. Maurin, C. Smet-Nocca, G. Lippens, G. Hilaire, H. Gijsen, D. Moechars and F. Van Leuven, PLoS One, 2013, 8, e84442 CrossRef PubMed.
- D. L. Graham, A. J. Gray, J. A. Joyce, D. Yu, J. O'Moore, G. A. Carlson, M. S. Shearman, T. L. Dellovade and H. Hering, Neuropharmacology, 2014, 79, 307–313 CrossRef CAS PubMed.
- S. A. Yuzwa, X. Shan, B. A. Jones, G. Zhao, M. L. Woodward, X. Li, Y. Zhu, E. J. McEachern, M. A. Silverman, N. V. Watson, C. X. Gong and D. J. Vocadlo, Mol. Neurodegener., 2014, 9, 42 CrossRef PubMed.
- C. Kim, D. W. Nam, S. Y. Park, H. Song, H. S. Hong, J. H. Boo, E. S. Jung, Y. Kim, J. Y. Baek, K. S. Kim, J. W. Cho and I. Mook-Jung, Neurobiol. Aging, 2013, 34, 275–285 CrossRef CAS PubMed.
- K. A. Stubbs, N. Zhang and D. J. Vocadlo, Org. Biomol. Chem., 2006, 4, 839–845 Search PubMed.
- E. J. Kim, M. Perreira, C. J. Thomas and J. A. Hanover, J. Am. Chem. Soc., 2006, 128, 4234–4235 CrossRef CAS PubMed.
- B. L. Mark, D. J. Mahuran, M. M. Cherney, D. Zhao, S. Knapp and M. N. G. James, J. Mol. Biol., 2003, 327, 1093–1109 CrossRef CAS PubMed.
- M. Avalos Gonzalez, J. Fuentes Mota, I. M. Gomez Monterrey, J. L. Jimenez Requejo, J. C. Palacios Albarran and M. C. Ortiz Mellet, Carbohydr. Res., 1986, 154, 49–62 CrossRef CAS.
- J. F. Morrison, Biochim. Biophys. Acta, 1969, 185, 269–286 CrossRef CAS.
- R. A. Copeland, Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists, John Wiley and Sons Inc., Hoboken, New Jersey, 2005 Search PubMed.
- P. Kuzmic, K. C. Elrod, L. M. Cregar, S. Sideris, R. Rai and J. W. Janc, Anal. Biochem., 2000, 286, 45–50 CrossRef CAS PubMed.
- D. J. Murphy, Anal. Biochem., 2004, 327, 61–67 CrossRef CAS PubMed.
- L. C. Perrin and M. A. Fabian, Anal. Chem., 1996, 68, 2127–2134 CrossRef PubMed.
- I. D. Kuntz, K. Chen, K. A. Sharp and P. A. Kollman, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9997–10002 CrossRef CAS.
- G. E. Whitworth, M. S. Macauley, K. A. Stubbs, R. J. Dennis, E. J. Taylor, G. J. Davies, I. R. Greig and D. J. Vocadlo, J. Am. Chem. Soc., 2007, 129, 635–644 CrossRef CAS PubMed.
- P. A. Bartlett and C. K. Marlowe, Biochemistry, 1983, 22, 4618–4624 CrossRef CAS PubMed.
- M. M. Mader and P. A. Bartlett, Chem. Rev., 1997, 97, 1281–1301 CrossRef CAS PubMed.
- K.-R. Roeser and G. Legler, Biochim. Biophys. Acta, 1981, 657, 321–333 CrossRef CAS.
- M. S. Macauley, K. A. Stubbs and D. J. Vocadlo, J. Am. Chem. Soc., 2006, 127, 17202–17203 CrossRef PubMed.
- R. Mosi, H. Sham, J. C. Uitdehaag, R. Ruiterkamp, B. W. Dijkstra and S. G. Withers, Biochemistry, 1998, 37, 17192–17198 CrossRef CAS PubMed.
- I. R. Greig, F. Zahariev and S. G. Withers, J. Am. Chem. Soc., 2008, 130, 17620–17628 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, NMR spectra and additional figures illustrating Ki graphs, kinetic data, pKa titration data and structural analysis data. See DOI: 10.1039/c6sc00370b |
| ‡ These authors contributed equally. |
| § Current address: Alectos Therapeutics, 8999 Nelson Way, Burnaby, British Columbia, V5A 4B5, Canada. |
| This journal is © The Royal Society of Chemistry 2016 |