Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asymmetric synthesis of allylic amines via hydroamination of allenes with benzophenone imine†
Kun
Xu
,
Yu-Hsuan
Wang
,
Vahid
Khakyzadeh
and
Bernhard
Breit
*
Institut für Organische, Chemie Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg, Germany. E-mail: bernhard.breit@chemie.uni-freiburg.de; Fax: +49-761-203-8715
First published on 9th February 2016
Abstract
Rhodium-catalyzed highly regio- and enantioselective hydroamination of allenes is reported. Exclusive branched selectivities and excellent enantioselectivities were achieved applying a rhodium(I)/Josiphos catalyst. This method permits the practical synthesis of valuable α-chiral allylic amines using benzophenone imine as ammonia carrier.
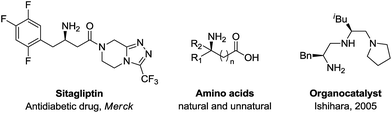
α-Chiral amines are of wide interest in organic synthesis1a due to their broad application in pharmaceutical research,1b,c catalysis1d,e and natural product synthesis1f (Scheme 1). Among them, the synthesis of α-chiral allylic amines is particularly important because of the versatility of the allylic moiety for further structural elaboration.2 In the past few decades, significant efforts have advanced the efficiency towards their synthesis. Many elegant approaches including allylic substitution,3 Overman rearrangements,4 allylic C–H amination5 and imine vinylation6 have been reported. However, these methods need pre-installation of a leaving group or stoichiometric amounts of an oxidant/metal-containing reagent. In this regard, efficient synthetic methods for the synthesis of α-chiral allylic amines are highly desirable.
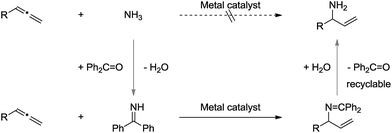
The catalytic and enantioselective addition of simple ammonia (NH3) to allenes would represent one of the most atom-economic transformation towards the synthesis of α-chiral allylic amines.7–9 However, initial experiments revealed this transformation to be very challenging, presumably due to the following reasons: (1) the volatility and toxicity of gaseous NH3 makes it less interesting in terms of practicality; (2) the catalytic systems were inactive in the presence of ammonia, possibly due to the high basicity of sp3 hybridized nitrogen atom, and its difficulty to undergo oxidative addition to a transition metal center.10
To address these issues, we assumed that the easy-to-use and N-sp2-hybridized benzophenone imine11 could serve as an ammonia carrier: (1) benzophenone imine is commercially available and can be easily prepared via condensation of benzophenone with ammonia;12 (2) the sp2 hybridized imine nitrogen is more reactive towards allenes in the presence of a suitable transition metal catalyst; (3) the final α-chiral primary allylic amines can be obtained via simple hydrolysis, and the benzophenone can be recycled (Scheme 2).
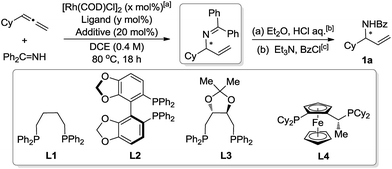
The initial assessment was performed by coupling cyclohexylallene and benzophenone imine using [{Rh(COD)Cl}2] (2.5 mol%) and racemic ligand 1,4-bis(diphenylphosphino)butane (L1, 10 mol%) in 1,2-dichloroethane (DCE) at 80 °C (Table 1, entry 1). The reaction afforded the desired product with 10% NMR yield. We wondered whether addition of acid would facilitate the reaction by promoting the catalytic cycle. Indeed, both trifluoroacetic acid (TFA) and pyridinium p-toluenesulfonate (PPTS) could significantly improve the yield, while the addition of p-toluenesulfonic acid (PTSA) had no effect (Table 1, entry 2–4). These promising results encouraged us to test the feasibility of its asymmetric variant. The (S)-Segphos ligand (L2) only led to 10% of the desired product. The (R,R)-DIOP (L3) gave 76% of the isolated amide product 1a, while only moderate ee was obtained. Further screening led to the discovery of Josiphos (L4), which afforded 1a with 72% yield and 92% ee.13
| Entry | x | Ligand (y) | Additive | Yieldd/% | eee/% |
|---|---|---|---|---|---|
| a Benzophenone imine (1.0 equiv.), cyclohexylallene (1.5 equiv.). b Et2O (2.0 ml), HCl aq. (2.0 ml, 2.0 M, 4.0 mmol), room temperature, 24 hours. c CH2Cl2 (2.0 ml), Et3N (223 μl, 1.6 mmol, 4.0 equiv.), benzoyl chloride (84.3 mg, 0.6 mmol, 1.5 equiv.). d 1H NMR yield of the coupling product (hydroamination step) in the crude reaction mixture using 1,3,5-trimethoxybenzene as internal standard. e ee of 1a was determined by chiral HPLC. f Yield is that of the isolated product of 1a. g Acetonitrile was used as solvent. | |||||
| 1 | 2.5 | L1 (10) | — | (10) | — |
| 2 | 2.5 | L1 (10) | PTSA | (11) | — |
| 3 | 2.5 | L1 (10) | TFA | (50) | — |
| 4 | 2.5 | L1 (10) | PPTS | (65) | — |
| 5 | 2.0 | L1 (4.0) | PPTS | (59) | — |
| 6 | 2.0 | L2 (4.0) | PPTS | (10) | — |
| 7 | 2.0 | L3 (4.0) | PPTS | 76f | 54g |
| 8 | 2.0 | L4 (4.0) | PPTS | 72f | 92 |
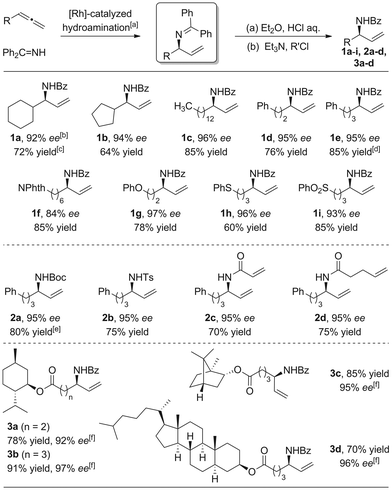
With the optimized conditions in hand, we then investigated the feasibility of various allene substrates (Scheme 3, 1a–i). Interestingly, in basically all cases perfect regioselectivities and excellent enantioselectivities were observed. Allenes containing alkyl substituents as well as ether, thio ether, phthalimide and sulfone functional groups were well tolerated.
 | ||
| Scheme 4 Large scale synthesis of primary allylic amine. [a] Scope conditions. [b] Determined by its amide derivative 1evia chiral HPLC. | ||
Direct synthesis of branched allylic amides using simple amides and allenes was difficult. However, a one-pot synthesis of branched allylic amides or carbamate can be achieved easily upon acylation or sulfonylation of the crude allylic amine with the corresponding acyl/sulfonyl chlorides or anhydride, respectively (Scheme 3, 2a–d).
Hydroamination with bioactive moieties containing substrates using the scope conditions resulted in the desired branched allylic amines with high yields and excellent enantioselectivities (Scheme 3, 3a–c).
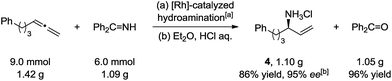
To test the practicality of this method for primary amine synthesis, chiral allylic amine HCl salt 4 was synthesized under scope conditions in a 1.1 gram scale with 86% yield and 95% ee. The released ammonia carrier (benzophenone) can be recycled after hydrolysis with 96% yield (Scheme 4).
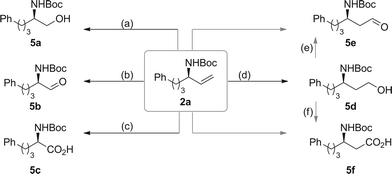
To exemplify the utility of chiral allylic amines, derivatization of compound 2a was performed (Scheme 5). Ozonolysis of 2a followed by treatment with triphenylphosphine and NaBH4 gave β-amino alcohol 5a and α-amino aldehyde 5b, respectively. Direct oxidation of 2a could afford α-amino acid 5c. Hydroboration of 2a using 9-borabicyclo(3.3.1)nonane (9-BBN), then oxidation with H2O2 led to the formation of γ-amino alcohol 5d, which could be further oxidized to the corresponding β-amino aldehyde 5e and β-amino acid 5f.
Isotopic labeling experiments using deuterated benzophenone imine (Ph2C = ND) and deuterated PPTS (D-PPTS) were conducted under scope conditions.13 Deuterium incorporation was only observed at the internal position of the allylic double bond. Hence, we suggest that the mechanism follows a similar pathway as for previously reported coupling reactions.9e Oxidative addition of the benzophenone imine N–H bond to Rh(I) generates Rh(III) complex. Hydrometalation of the less substituted double bond could generate σ-allyl-Rh complex, which is in equilibrium with the π-allyl-Rh complex. Reductive elimination of the allyl-Rh complexes generates the branched N-allylic amine.
To conclude, we have developed the first highly regio- and enantioselective hydroamination of allenes using benzophenone imine as an ammonia carrier via a rhodium/Josiphos catalyst system. The reaction gave valuable α-chiral primary allylic amines and α-chiral allylic amides in a practical manner. Recycle of the ammonia carrier with high yield maximized the atom-economy of this protocol. Applications of this method in target oriented synthesis and using more challenging terminal alkyne as the coupling partner for the enantioselective synthesis of branched allylic amines are currently under way in our laboratories and will be reported in due course.
Acknowledgements
This work was supported by the DFG, the International Research Training Group “Catalysts and Catalytic Reactions for Organic Synthesis” (IRTG 1038) and the Krupp Foundation. We thank Umicore, BASF and Wacker for generous gifts of chemicals.Notes and references
- (a) T. C. Nugent, Chiral Amine Synthesis, Wiley, 2010 Search PubMed; (b) D. J. Drucker, Lancet, 2006, 368, 1696–1705 CrossRef CAS PubMed; (c) D. J. Heal, S. C. Cheetham and S. L. Smith, Neuropharmacology, 2009, 57, 608–618 CrossRef CAS PubMed; (d) V. A. Ostrovskii, R. E. Trifonov and E. A. Popova, Russ. Chem. Bull., Int. Ed., 2012, 61, 768–780 CrossRef CAS; (e) L. Xu, J. Luo and Y. Lu, Chem. Commun., 2009, 1807–1821 RSC; (f) K. Ishihara and K. Nakano, J. Am. Chem. Soc., 2005, 127, 10504–10505 CrossRef CAS PubMed; (g) G. Cardillo and C. Tomasini, Chem. Soc. Rev., 1996, 25, 117–128 RSC.
- (a) E. M. Skoda, G. C. Davis and P. Wipf, Org. Process Res. Dev., 2012, 16, 26–34 CrossRef CAS PubMed; (b) B. M. Trost, T. Zhang and J. D. Sieber, Chem. Sci., 2010, 1, 427–440 RSC; (c) B. M. Trost and Z. Shi, J. Am. Chem. Soc., 1996, 118, 3037–3038 CrossRef CAS; (d) B. M. Trost and G. Dong, J. Am. Chem. Soc., 2006, 128, 6054–6055 CrossRef CAS PubMed.
- Selected reviews on transition-metal-catalyzed allylic substitution: (a) B. M. Trost, Chem. Rev., 1996, 96, 395–422 CrossRef CAS PubMed; (b) M. Johannsen and K. A. Jørgensen, Chem. Rev., 1998, 98, 1689–1708 CrossRef CAS; (c) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921–2943 CrossRef CAS PubMed; (d) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258–297 CrossRef CAS PubMed.
- (a) L. E. Overman, J. Am. Chem. Soc., 1976, 98, 2901–2910 CrossRef CAS; (b) J. M. Bauer and R. Peters, Catal. Sci. Technol., 2015, 5, 2340–2346 RSC; (c) D. F. Fischer, A. Barakat, Z.-Q. Xin, M. E. Weiss and R. Peters, Chem.–Eur. J., 2009, 15, 8722–8741 CrossRef CAS PubMed; (d) D. F. Fischer, Z.-Q. Xin and R. Peters, Angew. Chem., Int. Ed., 2007, 46, 7704–7707 CrossRef CAS PubMed.
- Selected review on allylic C–H amination: G. Liu and Y. Wu, Top. Curr. Chem., 2010, 292, 195–209 CrossRef CAS PubMed.
- Selected example on imine vinylation which avoids stoichiometric organometallics: A. Barchuk, M. Ngai and M. J. Krische, J. Am. Chem. Soc., 2007, 129, 8432–8433 CrossRef CAS PubMed.
- Selected examples on the asymmetric synthesis of primary allylic amines using ammonia equivalents: (a) R. Weihofen, O. Tverskoy and G. Helmchen, Angew. Chem., Int. Ed., 2006, 45, 5546–5549 CrossRef CAS PubMed; (b) T. Nagano and S. Kobayashi, J. Am. Chem. Soc., 2009, 131, 4200–4201 CrossRef CAS PubMed; (c) P. A. Evans and E. A. Clizbe, J. Am. Chem. Soc., 2009, 131, 8722–8723 CrossRef CAS PubMed; (d) M. J. Pouy, L. M. Stanley and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 11312–11313 CrossRef CAS PubMed; (e) M. Lafrance, M. Roggen and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3470–3473 CrossRef CAS PubMed.
- Selected examples on the coupling of pronucleophiles with allenes: (a) R. Zimmer, C. Dinesh, E. Nandanan and F. A. Khan, Chem. Rev., 2000, 100, 3067–3125 CrossRef CAS PubMed; (b) J. Johnson and R. G. Bergman, J. Am. Chem. Soc., 2001, 123, 2923–2924 CrossRef CAS PubMed; (c) S. S. Kinderman, R. Gelder, J. H. Maarseveen, H. E. Schoemaker, H. Hiemstra and F. P. J. T. Rutjes, J. Am. Chem. Soc., 2004, 126, 4100–4101 CrossRef CAS PubMed; (d) R. L. Lalonde, B. D. Sherry, E. J. Kang and F. D. Toste, J. Am. Chem. Soc., 2007, 129, 2452–2453 CrossRef CAS PubMed; (e) I. S. Kim and M. J. Krische, Org. Lett., 2008, 10, 513–515 CrossRef CAS PubMed; (f) T. Kawamoto, S. Hirabayashi, X. Guo, T. Nishimura and T. Hayashi, Chem. Commun., 2009, 3528–3530 RSC; (g) S. B. Han, I. S. Kim, H. Han and M. J. Krische, J. Am. Chem. Soc., 2009, 131, 6916–6917 CrossRef CAS PubMed; (h) J. Moran, A. Preetz, R. A. Mesch and M. J. Krische, Nat. Chem., 2011, 3, 287–290 CrossRef CAS PubMed; (i) P. Koschker, A. Lumbroso and B. Breit, J. Am. Chem. Soc., 2011, 133, 20746–20749 CrossRef CAS PubMed; (j) C. Li and B. Breit, J. Am. Chem. Soc., 2014, 136, 862–865 CrossRef CAS PubMed; (k) B. M. Trost, C. Jäkel and B. Plietker, J. Am. Chem. Soc., 2003, 125, 4438–4439 CrossRef CAS PubMed; (l) K. Xu, N. Thieme and B. Breit, Angew. Chem., Int. Ed., 2014, 53, 7268–7271 CrossRef CAS PubMed.
- Selected examples on the asymmetric synthesis N-allylic amines via intermolecular hydroamination of allenes: (a) N. Nishina and Y. Yamamoto, Angew. Chem., Int. Ed., 2006, 45, 3314–3317 CrossRef CAS; (b) K. L. Butler, M. Tragni and R. A. Widenhoefer, Angew. Chem., Int. Ed., 2012, 51, 5175–5178 CrossRef CAS PubMed; (c) Y. Fang, P. M. Tadross and E. N. Jacobsen, J. Am. Chem. Soc., 2014, 136, 17966–17968 CrossRef CAS PubMed; (d) K. Xu, N. Thieme and B. Breit, Angew. Chem., Int. Ed., 2014, 53, 2162–2165 CrossRef CAS PubMed; (e) C. Li, M. Kähny and B. Breit, Angew. Chem., Int. Ed., 2014, 53, 13780–13784 CrossRef CAS PubMed; (f) K. Xu, T. Gilles and B. Breit, Nat. Commun., 2015, 6, 7616–7623 CrossRef PubMed; (g) A. M. Haydl, K. Xu and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 7149–7153 CrossRef CAS PubMed.
- (a) Oxidative addition of N–H to Rhodium: O. V. Ozerov, C. Guo, V. A. Papkov and B. M. Foxman, J. Am. Chem. Soc., 2004, 126, 4792–4793 CrossRef CAS PubMed; (b) Although it is beyond the scope of this article, other catalytic systems might work for the coupling of allenes with ammonia. For example the copper catalyzed hydroaminations: M. T. Pirnot, Y. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 48–57 CrossRef CAS PubMed.
- Selected example on using benzophenone imine as ammonia carrier: J. P. Wolfe, J. Åhman, J. P. Sadighi, R. A. Singer and S. L. Buchwald, Tetrahedron Lett., 1997, 38, 6367–6370 CrossRef CAS.
- G. Verardo, A. G. Giumanini, P. Strazzolini and M. Poiana, Synth. Commun., 1988, 18, 1501–1511 CrossRef CAS.
- See ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and detailed characterization data of all new compounds. See DOI: 10.1039/c5sc04984a |
| This journal is © The Royal Society of Chemistry 2016 |