Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure, spectroscopy and reactivity of new heterotrinuclear water oxidation catalysts†
Lorenzo
Mognon
a,
Sukanta
Mandal
b,
Carmen E.
Castillo
cd,
Jérôme
Fortage
cd,
Florian
Molton
cd,
Guillem
Aromí
e,
Jordi
Benet-Buchhlolz
a,
Marie-Noëlle
Collomb
cd and
Antoni
Llobet
*af
aInstitute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Avinguda Països Catalans 16, 43007 Tarragona, Spain. E-mail: allobet@iciq.cat
bDepartment of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur-721302, West Bengal, India
cUniv. Grenoble Alpes, DCM, F-38000 Grenoble, France
dCNRS, DCM, F-38000 Grenoble, France
eDepartament de Química Inorgànica, Universitat de Barcelona, Diagonal 645, 08028 Barcelona, Spain
fDepartament de Química, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, 08193 Barcelona, Spain
First published on 2nd February 2016
Abstract
Four heterotrinuclear complexes containing the ligands 3,5-bis(2-pyridyl)pyrazolate (bpp−) and 2,2′:6′,2′′-terpyridine (trpy) of the general formula {[RuII(trpy)]2(μ-[M(X)2(bpp)2])}(PF6)2, where M = CoII, MnII and X = Cl−, AcO− (M = CoII, X = Cl−: Ru2Co–Cl2; M = MnII, X = Cl−: Ru2Mn–Cl2; M = CoII, X = AcO−: Ru2Co–OAc2; M = MnII, X = AcO−: Ru2Mn–OAc2), have been prepared for the first time. The complexes have been characterized using different spectroscopic techniques such as UV-vis, IR, and mass spectrometry. X-Ray diffraction analyses have been used to characterize the Ru2Mn–Cl2 and Ru2Mn–OAc2 complexes. The cyclic voltammograms (CV) for all four complexes in organic solvent (CH3CN or CH2Cl2) display three successive reversible oxidative waves corresponding to one-electron oxidations of each of the three metal centers. The oxidized forms of the complexes Ru2Co–OAc2 and Ru2Mn–OAc2 are further characterized by EPR and UV-vis spectroscopy. The magnetic susceptibility measurements of all complexes in the temperature range of 2–300 K reveal paramagnetic properties due to the presence of high spin Co(II) and Mn(II) centers. The complexes Ru2Co–OAc2 and Ru2Mn–OAc2 act as precatalysts for the water oxidation reaction, since the acetato groups are easily replaced by water at pH = 7 generating the active catalysts, {[Ru(H2O)(trpy)]2(μ-[M(H2O)2(bpp)2])}4+ (M = CoII: Ru2Co–(H2O)4; M = MnII: Ru2Mn–(H2O)4). The photochemical water oxidation reaction is studied using [Ru(bpy)3]2+ as the photosensitizer and Na2S2O8 as a sacrificial electron acceptor at pH = 7. The Co containing complex generates a TON of 50 in about 10 minutes (TOFi = 0.21 s−1), whereas the Mn containing complex only generates a TON of 8. The water oxidation reaction of Ru2Co–(H2O)4 is further investigated using oxone as a sacrificial chemical oxidant at pH = 7. Labelled water oxidation experiments suggest that a nucleophilic attack mechanism is occurring at the Co site of the trinuclear complex with cooperative involvement of the two Ru sites, via electronic coupling through the bpp− bridging ligand and via neighboring hydrogen bonding.
Introduction
Water oxidation catalysis is a field that has expanded enormously over the last few years.1–10 This development has been fuelled by interest in the topic for the achievement of new energy conversion schemes based on water and sunlight to obtain so-called solar fuel (such as H2), mimicking natural photosynthesis. Water oxidation is the main reaction that occurs at the OEC-PSII,11 yielding molecular oxygen, four protons and four electrons, which can later react further. The development of new water oxidation catalysts (WOCs) has been based mainly on mononuclear12 and dinuclear13,14 Ru complexes containing polypyridilic ligands. However, recently, a number of Ir and first row transition metal complexes have also been reported to be able to oxidize water.3,15,16While mononuclear complexes are in general easier to prepare, targeted polynuclear complexes can present significant synthetic challenges. Nevertheless, polynuclear complexes containing bridging ligands that can electronically couple the metal centers can attain important benefits from the perspective of a WOC. For example, multiple electronically coupled redox active metal centers can cooperate during the four electron transfers needed for the water oxidation reaction. On the other hand, non-redox active centers can be of interest to exert electronic perturbation over the redox active center.17,18 They can also provide aqua/hydroxo ligands which, when strategically situated, can help to lower activation energies by hydrogen bonding with key active species bonded to the active metal, or even participate in O–O bond formation, as has been proposed for the Ca–OH2 moiety of the OEC-PSII on a number of occasions.19,20
With these considerations in mind, we undertook the preparation of heterotrinuclear complexes where all of the metal centers are redox active and possess aqua ligands, to favor the achievement of high oxidation states via Proton Coupled Electron Transfer (PCET). Herein, we report the synthesis, structural, spectroscopic and electrochemical characterization of a new family of heterotrinuclear complexes containing Ru and Co or Mn as metal centers, together with their capacity to oxidize water to dioxygen.
Results and discussion
Synthesis and solid state structure
The well-known Hbpp ligand is an asymmetric molecule which, once deprotonated, presents two equivalent coordination environments. The protonated ligand thus allows the preparation of mononuclear complexes that can be used as starting materials for the preparation of homo-21 or heterodinuclear, or heterotrinuclear22 complexes. In the present report we use out-[Ru(Cl)(Hbpp)(trpy)]+, out-0, as the starting material for the preparation of heterotrinuclear complexes containing redox active metals, as displayed in Scheme 1.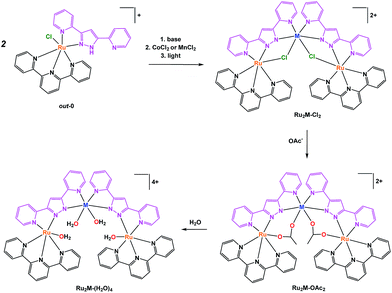 | ||
| Scheme 1 Synthetic strategy followed for the preparation of trinuclear complexes and their labelling. | ||
The pyrazolato proton of out-0 is removed using NaOMe as a base, and then MnCl2 or CoCl2 salts are used to react with the vacant coordination sites of the bpp− ligand, as shown in eqn (1) for the case of cobalt.
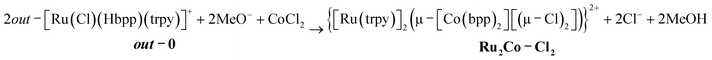 | (1) |
Complex Ru2Co–Cl2 is obtained in 58% yield, whereas a 70% yield is obtained for Ru2Mn–Cl2 following a similar synthetic procedure.22 Treatment of these trinuclear complexes with sodium acetate at 75 °C in an acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (5
water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution replaces the chlorido bridging ligands by the more labile acetato ligands, as indicated in the following equation.
1) solution replaces the chlorido bridging ligands by the more labile acetato ligands, as indicated in the following equation.
 | (2) |
Finally, substitution of the acetato bridges by monodentate aqua ligands leads to the formation of the corresponding tetra-aqua complex in neutral pH (eqn (3)).
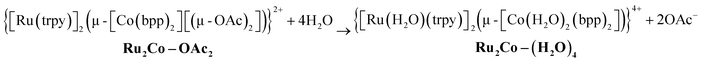 | (3) |
In contrast, under acidic conditions, the trinuclear complexes decompose to the corresponding mononuclear in-[Ru(Hbpp)(trpy)(H2O)]2+, in-1, and free Co(II) (Fig. S27†).
The synthetic strategies used for the preparation of Ru2Mn–OAc2and Ru2Mn–(H2O)4 are analogous to those for the cobalt counterparts.
All of these new complexes have been characterized by analytic, spectroscopic or electrochemical techniques. Further, X-ray diffraction analysis has been carried out for the Mn complexes, Ru2Mn–Cl2 and Ru2Mn–OAc2, and ORTEP views of their cationic moiety are presented in Fig. 1.
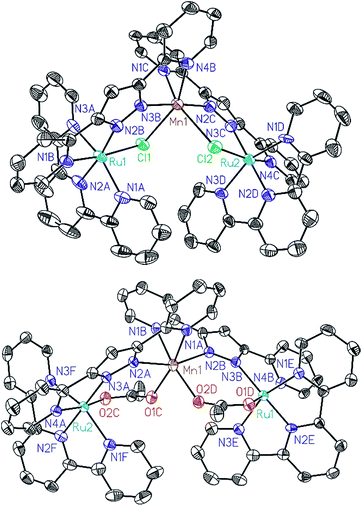 | ||
| Fig. 1 ORTEP plots (50% probability) for the cationic structures of complexes Ru2Mn–Cl2 (top) and Ru2Mn–(OAc)2 (bottom). Hydrogen atoms have been omitted. | ||
All the metal centers present pseudo-octahedral symmetry around the first coordination sphere. In the Ru2Mn–Cl2 case, the ruthenium atoms are coordinated by five N-atoms, three from a meridional trpy ligand and two from bpp−, while the sixth coordination position is occupied by a chlorido ligand. The manganese center is coordinated by the same two bridging chlorido moieties, in a cis fashion, and by the two chelating bpp− ligands. The “Mn(bpp)2” moiety can thus be considered as a bridge between the two Ru centers (see Fig. 1 and Scheme 1). A very similar structure is obtained for Ru2Mn–OAc2 where the bridging chlorido ligands have been substituted by bridging acetato ligands. For both structures, the bonding distances and angles presented by the Ru(II) and Mn(II) centers are unremarkable.23,24
Redox properties and UV-vis spectroscopy in organic solvents for Ru2M–OAc2
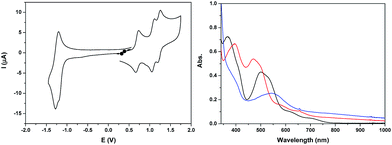
The cyclic voltammogram of Ru2Co–OAc2 in CH3CN (Fig. 2) displays three successive reversible oxidation waves at E1/2 = 0.70 (ΔE = 75 mV), 1.08 (ΔE = 60 mV), and 1.22 V (ΔE = 60 mV), and one reversible reduction wave at E1/2 = −1.23 V (ΔE = 80 mV). All the redox potentials in this work are reported vs. NHE. Each of the three oxidation processes corresponds to the exchange of one electron per molecule of complex, as evidenced by rotating disk electrode experiments (Fig. S19†). The first process is assigned to the oxidation of the cobalt Co(III)/Co(II) and the last two to the oxidation of the two Ru sites, Ru2(II,III)/Ru2(II,II) and Ru2(III,III)/Ru2(II,III). The presence of two distinct one-electron redox processes, close in potential (ΔE1/2 = 140 mV), instead of a two-electron single wave, is in agreement with there being two identical electroactive centers in the molecule that can electronically communicate.24 This is the case of the two Ru sites interacting through the conjugation of the bridging bpp− ligands and the acetato bridge via the central Co3+ core. The two electron reduction wave at −1.23 V is assigned to the reduction of the terpyridine units of the Ru(II) centers. The shoulder observed at −1.18 V indicates that the two terpyridine ligands are not fully equivalent, which is in accordance with there being weak electronic coupling between the two Ru subunits.The two oxidized forms of the complex, Ru2(II)Co(III) and Ru2(III)Co(III) are stable in CH3CN, as tested by successive electrolysis at E = 0.85 V and E = 1.40 V, which consume, respectively, one and two electrons (Fig. S18†). Rotating disk electrode experiments confirm the quantitative formation of these species (Fig. S19†). The electrogenerated solutions have also been analyzed by UV-visible (Fig. 2) and EPR spectroscopy (Fig. 4, see below).
The three stable oxidation states, Ru2(II)Co(II), Ru2(II)Co(III) and Ru2(III)Co(III), have distinct UV-visible signatures. The initial orange Ru2(II)Co(II) solution exhibits two intense visible bands at 366 and 500 nm (with a shoulder at 530 nm) and two less intense shoulders at 620 and 710 nm. The oxidation of the central Co(II) unit into Co(III) leads to a shift of the intense visible bands to 396 and 473 nm (shoulder at 500 nm) and of the two shoulders to 580 and 655 nm. A more pronounced color change of the solution is observed when the two Ru(II) species are oxidized into Ru(III), as indicated by the replacement of all the previous visible bands by new ones at 390 (shoulder) and 545 nm. An increase in absorption is also observed between 600 and 1000 nm. As the more evident changes occur after the oxidation from Ru2(II)Co(III) to Ru2(III)Co(III), the intense visible absorption bands originate from the ruthenium units.
Back electrolysis of the final solution conducted at 0.35 V (three electrons exchanged) restores the initial complex, Ru2(II)Co(II), quantitatively (Fig. S20†). This demonstrates the perfect stability of the different oxidation states of the trinuclear compound, and the reversibility of the processes.
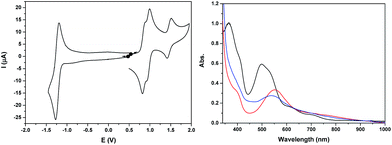
The cyclic voltammogram of Ru2Mn–OAc2 in CH3CN (Fig. 3 and S21†) also displays three successive one-electron reversible oxidation waves at E1/2 = 0.85 V (ΔE = 60 mV), 0.96 V (ΔE = 70 mV) and 1.47 V (ΔE = 100 mV) at a scan rate of 50 mV s−1 and a reversible two-electron terpyridine-centered reduction wave at E1/2 = −1.23 V (ΔE = 80 mV). The two first oxidation waves, very close in potential (ΔE1/2 = 110 mV), are thus assigned to the oxidation of the Ru sites, Ru2(II,III)/Ru2(II,II) and Ru2(III,III)/Ru2(II,III) and the last one to the Mn central unit, Mn(III)/Mn(II).
The stability of the two oxidized states, Ru2(III)Mn(II) and Ru2(III)Mn(III), has been evaluated by two successive electrolyses at E = 1.11 V and 1.69 V. The first electrolysis at 1.11 V consumed two electrons per molecule of initial complex and lead to the quantitative formation of the Ru2(III)Mn(II) species. An additional one electron oxidation carried out at 1.69 V leads to the formation of the fully oxidized form, Ru2(III)Mn(III). At this stage, the presence of an additional reversible process at E1/2 = 1.23 V with very small intensity should be pointed out, which is probably related to minor decomposition of the complex by decoordination of the Mn ion (less than 5%, Fig. S21†). Indeed, the potential of this new process is similar to that of a RuN6 mononuclear complex such as in-[Ru(Hbpp)(trpy)(CH3CN)]2+. Both electrolysis processes have been monitored by UV-vis and X-band EPR spectroscopy (Fig. 3 and 4, respectively).
The UV-vis absorption spectrum of Ru2Mn–OAc2, with two intense visible bands at 365 and 497 nm (with a shoulder at 530 nm) and two less intense shoulders at 620 and 710 nm, is nearly identical to that of Ru2Co–OAc2 (see Fig. S9† for a comparison of the spectra). These observations confirm that the visible absorption bands originate mainly from the Ru units. Once the two Ru(II) units have been oxidized, formation of the Ru2(III)Mn(II) species leads to significant changes with the replacement of the initial visible bands by new ones at 400 (shoulder) and 553 nm and two shoulders at 710 and 764 nm. For the fully oxidized solution, the oxidation of the central Mn(II) unit into Mn(III) leads to minor changes, with a shift of the band at 553 nm to 538 nm and a small increase of the absorption around 450 nm (Fig. 3).
The initial reduced state of the complex Ru2Mn–OAc2 is restored almost quantitatively by a back electrolysis of the final solution at 0.35 V (Fig. S22†).
EPR properties
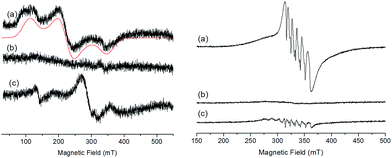
The X-band EPR spectra of the initial and electrochemically oxidized solutions of Ru2Co–OAc2 have been recorded at low temperature (13 K) (Fig. 4). The initial solution of Ru2Co–OAc2 shows an EPR signal characteristic of a Co(II) ion (d7) in the high spin state (S = 3/2),25 arising from the central Co(II) unit, as both Ru units (Ru(II), d6) are diamagnetic and thus EPR silent. The analysis of high spin Co(II) centers is difficult because the zero field splitting (ZFS) energy is usually greater than the Zeeman interaction, leading to spectra insensitive to the magnitude of the axial term (D) of the ZFS. Consequently, only the real g-values (greal) and the rhombicity (E/D ratio) of the system can be extracted from the simulation of the EPR spectra. Simulation of the experimental data provides the following spin-Hamiltonian parameters for the Ms = |±1/2〉: greal(x, y) = 2.42, greal(z) = 2.31, and E/D = 0.23.After the one-electron oxidation into Ru2(II)Co(III), the solution becomes EPR silent, in accordance with the formation of the low-spin Co(III) species (d6) (S = 0).26
The EPR spectrum of the fully oxidized solution of Ru2(III)Co(III) displays two features ascribable to an effective S = 1 spin state which results from a magnetic interaction between two S = 1/2 spin states located on the Ru(III) metals. The characteristic rhombic signature of the low spin Ru(III) S = 1/2 spin state (d5) between 210 and 360 mT is retained in the spectrum.27,28 A weak half-field EPR line is also observed at 139 mT, which is consistent with the S = 1 spin state induced by the magnetic interaction of two Ru(III) ions. EPR spectra with similar features have been previously reported for magnetically coupled dinuclear Ru(III)–Ru(III) complexes.28
The EPR signal of the initial Ru2Mn–OAc2 solution at 100 K exhibits a 6-line signal characteristic of a high-spin Mn(II) ion (3d5, S = 5/2), related to the central Mn(II) unit of the complex (Fig. 4).27 This signal fully disappears after the oxidation of the two diamagnetic Ru(II) units into Ru(III). Although an EPR signal is expected for a magnetically coupled system involving two Ru(III) (S = 1/2) ions and one Mn(II) (S = 5/2), no EPR signal was detected regardless of temperature (from 13 K to 200 K), presumably due to fast relaxation. The fully oxidized solution of Ru2(III)Mn(III) is also EPR silent, which is in agreement with there being magnetic coupling between the two Ru(III) (S = 1/2) and the Mn(III) (d4, S = 2) ions, which must produce a integer spin ground state. The low-intensity signals observed around g = 2 are attributed to some decomposition of the trinuclear Ru2(III)Mn(III) (OAc)2 complex (less than 5%, as shown by electrochemistry) into “free” Mn(II) (six line feature centered at g = 2.0, 339 mT) and the mononuclear [RuIII(bpp)(trpy)(CH3CN)]3+ complex (S = 1/2) (the two features at a low magnetic field compared to the six line feature).
Magnetic properties
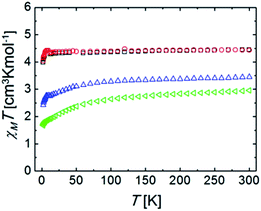
Magnetic susceptibility measurements were performed under a constant magnetic field of 5000 Oe, in the temperature range of 2–300 K. Variable field reduced magnetization at 2 K was performed for all complexes. Isofield variable temperature reduced magnetization measurements were also performed under various fields. These experiments served as criteria of purity, since this chemistry often leads to the formation of small impurities of Ru(0) particles that become very visible when the magnetic properties are examined.The constant field and variable temperature magnetic susceptibility results are represented in the form of χT vs. T curves (where χ is the molar paramagnetic susceptibility) (Fig. 5).
The χT products for the complexes Ru2Co–Cl2 and Ru2Co–OAc2 are, at 300 K, 3.44 and 2.96 cm3 K mol−1, respectively, which are significantly higher than the expected values for spin-only Co(II) ions (calculated as 1.875 cm3 K mol−1 for g = 2.0 and S = 3/2). This product decreases with an increasing rate as the temperature declines. Both observations are accounted for by the fact that octahedral high spin Co(II) exhibits an orbital angular momentum (L = 1) in addition to the spin state (S = 3/2). This causes the observation of an effective g value that is much higher than expected for a spin only system (here calculated as 2.51 and 2.71, respectively). The differences between both ions are caused by their slightly differing coordination geometries, as well as the varying ligand field strength of some of the donors.29 The depopulation of the J multiplets arising from the spin–orbit coupling explains the decline of χT upon cooling. The reduced magnetization curves measured at 2 K (Fig. S25†) saturate for both compounds at values lower than expected for an S = 3/2 system. This is because at such a low temperature, only the lowest J multiplet is populated. Isofield magnetization curves (at temperatures below 7 K and under several fields) produce the same saturation values and show quasi-superposition of the various curves (Fig. S26†). This shows that the anisotropy of these low energy states is very small. These results are consistent with the above characterization and with the interpretation of the EPR results.
At 300 K, the χT products for the complexes Ru2Mn–Cl2 and Ru2Mn–OAc2 are almost identical; 4.43 and 4.45 cm3 K mol−1, respectively, which correspond to the expected spin-only value for an isolated Mn(II) center with S = 5/2 spin state and g = 2.01 and 2.02, respectively, consistent with the EPR and electrochemical results. This value stays nearly constant until around 5 K, where a decline down to 3.98 and 4.04 cm3 K mol−1 at 2 K, respectively, is observed. The low temperature decline can be due to a small value of ZFS, or weak antiferromagnetic intermolecular interactions. The variable field reduced magnetization curves, measured at 2 K (Fig. S23†), and the isofield magnetization curves (Fig. S24†) are the result of Mn(II) saturating near 5 Bohr magneton, and this is consistent with the above results. The fact that the isofield lines superimpose with each other for the various fields employed confirms that the amount of ZFS is very small for both complexes.
Redox properties in aqueous solutions
The redox properties of the trinuclear complexes Ru2Co–(H2O)4 and Ru2Mn–(H2O)4 were investigated by means of CV and DPV in a mixture of a pH = 7.0 phosphate buffer (50 mM) and CF3CH2OH (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the use of the latter forced by the poor solubility of the complexes in water. While very nicely defined waves were observed in organic solvents for their acetato counterparts as shown previously, in aqueous solution, the waves are very wide (Fig. S28†). Nevertheless, the DPVs in Fig. 6 allow the observation of three faradaic processes situated at approximately 0.6, 0.7 and 1.0 V for both Ru2Co–(H2O)4 and Ru2Mn–(H2O)4, which are plotted together with the mononuclear aqua complex in-1 for comparative purposes. However, the most important feature is the large electrocatalytic wave displayed by these complexes, starting at 1.3 V for Ru2Co–(H2O)4 and at around 1.5 V for Ru2Mn–(H2O)4 and in-1, which is associated to water oxidation catalysis.
1), the use of the latter forced by the poor solubility of the complexes in water. While very nicely defined waves were observed in organic solvents for their acetato counterparts as shown previously, in aqueous solution, the waves are very wide (Fig. S28†). Nevertheless, the DPVs in Fig. 6 allow the observation of three faradaic processes situated at approximately 0.6, 0.7 and 1.0 V for both Ru2Co–(H2O)4 and Ru2Mn–(H2O)4, which are plotted together with the mononuclear aqua complex in-1 for comparative purposes. However, the most important feature is the large electrocatalytic wave displayed by these complexes, starting at 1.3 V for Ru2Co–(H2O)4 and at around 1.5 V for Ru2Mn–(H2O)4 and in-1, which is associated to water oxidation catalysis.
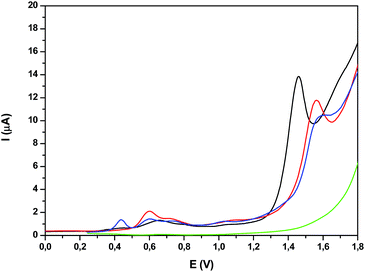 | ||
Fig. 6 DPV of (black) Ru2Co–(H2O)4, (red) Ru2Mn–(H2O)4, (blue) in-1 and (green) blank in a pH = 7.0 (50 mM) phosphate buffer solution and CF3CH2OH mixture (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Water oxidation catalysis
Photochemically driven water oxidation catalysis has been carried out through the photogeneration of [Ru(bpy)3]3+ as a chemical oxidant using persulfate as a sacrificial electron acceptor (eqn (4)–(6)).| [RuII(bpy)3]2+ + hν → [RuII(bpy)3]2+* | (4) |
| [RuII(bpy)3]2+* + S2O82− → [RuIII(bpy)3]3+ + SO42− + SO4−˙ | (5) |
| [RuII(bpy)3]2+ + SO4−˙ → [RuIII(bpy)3]3+ + SO42− | (6) |
The oxygen generation profile as a function of time is presented in Fig. 7 for the systems Cat 50 μM/[Ru(bpy)3]2+ 0.5 mM/Na2S2O8 20 mM/pH = 7.0 (50 mM phosphate buffer), with a total volume of 2.0 mL (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CF3CH2OH = 19
CF3CH2OH = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
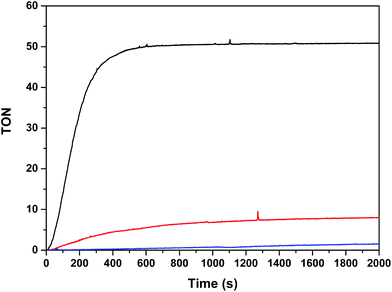
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using a 300 W xenon lamp with a band pass filter of 440 nm, thermostated at 298 K. Under these conditions, Ru2Co–(H2O)4 generates a TON of 50 in about 10 minutes (TOFi = 0.21 s−1) whereas Ru2Mn–(H2O)4 only generates a TON of 8 and the mononuclear complex in-1 does not generate any molecular oxygen within this time frame. These results are consistent with the electrochemical analysis, as the potentials for catalysis for Ru2Mn–(H2O)4 and in-1 are too high for efficient catalysis, given the fact that the Ru(III)/Ru(II) potential for [Ru(bpy)3]2+/3+ is E1/2 = 1.26 V.
1) using a 300 W xenon lamp with a band pass filter of 440 nm, thermostated at 298 K. Under these conditions, Ru2Co–(H2O)4 generates a TON of 50 in about 10 minutes (TOFi = 0.21 s−1) whereas Ru2Mn–(H2O)4 only generates a TON of 8 and the mononuclear complex in-1 does not generate any molecular oxygen within this time frame. These results are consistent with the electrochemical analysis, as the potentials for catalysis for Ru2Mn–(H2O)4 and in-1 are too high for efficient catalysis, given the fact that the Ru(III)/Ru(II) potential for [Ru(bpy)3]2+/3+ is E1/2 = 1.26 V.
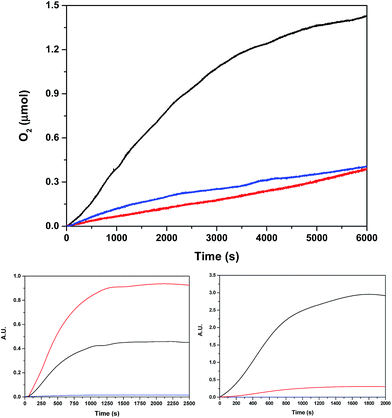
In light of the good results obtained in the photoactivated experiment, water oxidation catalysis with Ru2Co–(H2O)4, and with the mononuclear in-1 for comparison, was also investigated using oxone (KHSO5) as a chemical oxidant. The oxygen evolution profiles as a function of time are presented in Fig. 8. The Co containing complex generates 1.0 μmol of oxygen (0.41 μmol are subtracted due to the activity of the blank under the same conditions) that correspond to a TON of about 13.
Labelling experiments using H218O were also carried out in order to extract mechanistic information regarding the O–O bond formation event when using oxone as a chemical oxidant. The O2 36/34/32 isotope ratio was followed by on-line Mass spectrometry. Two different degrees of H2O labelling were employed, and the results obtained are shown in Fig. 8.
For discussion of the mechanism, we consider first the experiment with 97% H218O, and then test our hypothesis on the data obtained from the experiment with 15% H218O. However, before making a hypothesis on the mechanism of oxygen evolution, it is paramount to obtain information on the labeling state of the system at the moment of the oxygen evolving event.
(a). Raman experiments carried out under the same conditions as the water oxidation labelling experiments showed that no 18O exchange occurs at all between water and oxone for at least 10 h (Fig. S30†).30
(b). Substitution reactions of aqua ligands at low oxidation states (II) for Ru and Co complexes occur rapidly. As the precatalyst presents all acetate bridge ligands, we can assume that all the aqua ligands are initially present as H218O.
(c). Peroxide oxidations of Ru-aqua polypyridyl complexes occur through dehydrogenation pathways.31 Thus, oxone will react with low oxidation states of Ru-aqua as follows (auxiliary ligands not shown):
RuII–18OH2 + [HO–S(O)2(OO)]− → RuIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 18O + H2O + HSO4− 18O + H2O + HSO4− | (7) |
(d). Oxidation of first row transition metals, particularly iron and cobalt polyridylic complexes, with peroxides occurs primarily via nucleophilic substitution.32–34
| CoII–18OH2 + [HO–S(O)2(OO)]− → CoII–OO–S(O)2(OH) + H2O18 | (8) |
CoII–OO–S(O)2(OH) → CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + HSO4− O + HSO4− | (9) |
(e). Tautomeric equilibrium between the oxo-hydroxo complexes, as described in eqn (10), would produce labelling scrambling, as has been earlier proposed for related complexes.35
| CoIV(18O)(OH) ⇌ CoIV(O)(18OH) | (10) |
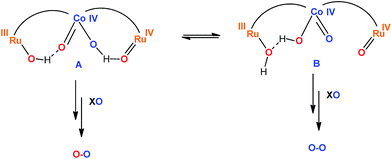
For the Ru2Co–(H2O)4 complex, this situation at the oxygen evolving event is described in the top section of Scheme 2. This assumes that for 100% H218O labeling at oxidation state II, all the aqua groups are exchanged. For the Ru center reaching high oxidation states with non-labeled oxone, [Ru(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 18O] will be produced with 100% 18O labeling. However, for the cobalt center, a mixed labeled [Co(IV)(16O)(18OH)] will be generated because of the nucleophilic attack mechanism mentioned above.
18O] will be produced with 100% 18O labeling. However, for the cobalt center, a mixed labeled [Co(IV)(16O)(18OH)] will be generated because of the nucleophilic attack mechanism mentioned above.
At this point, the following conclusions are extrapolated from the oxygen data shown in Fig. 8.
(1). The practically negligible amount of 36O2 in the 97% H218O experiment indicates the inexistence of intramolecular pathways between the Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Co
O and Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties. In addition, it also precludes the existence of bimolecular pathways involving only the Ru
O moieties. In addition, it also precludes the existence of bimolecular pathways involving only the Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. On the other hand, potential bimolecular pathways involving only Co centers are sterically highly disfavorable.
O groups. On the other hand, potential bimolecular pathways involving only Co centers are sterically highly disfavorable.
(2). The mononuclear complex in-1 does not generate sufficient oxygen to be significant under the applied conditions and thus the inexistence of O2 coming independently from Ru-aqua/Ru-oxo moieties is ruled out.
(3). Cis O–O coupling within the same Co metal center would be compatible with the ratios of isotopic labelling obtained, although this mechanism has been discarded based on DFT in a number of examples.36,37
(4). The 97% labelling experiment (Fig. 8 and S31†) is consistent with the existence of a very fast tautomeric equilibrium where species A and B exist in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio respectively (see Scheme 2). The origin of the higher stabilization of species A is proposed to come from the higher hydrogen bond capacity of isomer A with regard to that of B. Oxidation states of Ru and Co are tentatively assigned from the apparent removal of 4/5 electrons from the oxidation state II,II,II, as judged from the electrocatalytic wave displayed by the complex. Assuming this equilibrium, the reaction of oxone (XO) with A and B would generate molecular oxygen with a ratio of isotopes of 34O2/32O2 = 2/1, which is what is found experimentally.
1 ratio respectively (see Scheme 2). The origin of the higher stabilization of species A is proposed to come from the higher hydrogen bond capacity of isomer A with regard to that of B. Oxidation states of Ru and Co are tentatively assigned from the apparent removal of 4/5 electrons from the oxidation state II,II,II, as judged from the electrocatalytic wave displayed by the complex. Assuming this equilibrium, the reaction of oxone (XO) with A and B would generate molecular oxygen with a ratio of isotopes of 34O2/32O2 = 2/1, which is what is found experimentally.
(5). Finally, changing the ratio of labelled water to 15% 18O (Fig. 8 and S31†), will generate molecular oxygen with a ratio of 34O2/32O2 = 1/9. Experimentally, we obtained a ratio of 1/10, which is in very good agreement with the proposed mechanism.
Overall, the labelling experiments carried out together with the rest of the electrochemical and spectroscopic properties are in agreement with the presence of a water nucleophilic attack (WNA) mechanism occurring at the Co site of the trinuclear complex, with cooperative interaction of the two Ru sites via electronic coupling through the bpp− bridging ligand and via neighboring hydrogen bonding.
It is worth mentioning that in our previous work with related dinuclear Ru21 and Co3 complexes containing the Hbpp ligand, namely {[M(H2O)(trpy)]2μ-[(bpp)}3+ (M = Ru or Co), the O–O bond formation occurred via an intramolecular mechanism (I2M).38 However, with the new trinuclear complexes described here, given the new substantially different geometries and electronic interactions between the metal centers, the mechanism changes to a WNA.
Conclusions
We have prepared and isolated a family of trinuclear complexes, Ru2M–X2 (M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Co or Mn; X = Cl− or OAc−), where the central Co or Mn atom, together with two bpp− ligands, acts as a bridge between the two external Ru moieties. These complexes have been characterized in the solid state by X-ray diffraction analysis and by magnetic measurements. In solution they have been characterized by spectroscopic (EPR, UV-vis) and by electrochemical (CV, DPV) techniques. Overall, all these experiments for the chlorido or acetato bridge complexes show the presence of a relatively weak electronic coupling between the metal centers, transmitted through the bridging ligands. In addition, it is very interesting to see how the redox pattern radically changes from the Mn complexes with regards to the Co complexes manifesting the intrinsically different electronic properties of the two transition metals. In aqueous acidic media the trinuclear complexes revert to their Ru mononuclear counterparts and free Co(II) or Mn(II). However at pH = 7.0 the integrity of the complex is fully retained. Of particularly interest is the heterotrinuclear Ru2Co–(H2O)4 complex, that is capable at this pH of catalytically oxidizing water to molecular dioxygen chemically, electrochemically and photochemically. Finally, while a few detailed H218O labelling experiments have been described for Ru complexes13,39–51 that allow the tracing of the O–O bond formation step, this type of information is lacking for first row transition water oxidation catalysts. Our H218O labelling experiments constitute the first example where the O–O bond formation step has been elucidated based on labeling experiments.
Co or Mn; X = Cl− or OAc−), where the central Co or Mn atom, together with two bpp− ligands, acts as a bridge between the two external Ru moieties. These complexes have been characterized in the solid state by X-ray diffraction analysis and by magnetic measurements. In solution they have been characterized by spectroscopic (EPR, UV-vis) and by electrochemical (CV, DPV) techniques. Overall, all these experiments for the chlorido or acetato bridge complexes show the presence of a relatively weak electronic coupling between the metal centers, transmitted through the bridging ligands. In addition, it is very interesting to see how the redox pattern radically changes from the Mn complexes with regards to the Co complexes manifesting the intrinsically different electronic properties of the two transition metals. In aqueous acidic media the trinuclear complexes revert to their Ru mononuclear counterparts and free Co(II) or Mn(II). However at pH = 7.0 the integrity of the complex is fully retained. Of particularly interest is the heterotrinuclear Ru2Co–(H2O)4 complex, that is capable at this pH of catalytically oxidizing water to molecular dioxygen chemically, electrochemically and photochemically. Finally, while a few detailed H218O labelling experiments have been described for Ru complexes13,39–51 that allow the tracing of the O–O bond formation step, this type of information is lacking for first row transition water oxidation catalysts. Our H218O labelling experiments constitute the first example where the O–O bond formation step has been elucidated based on labeling experiments.
Acknowledgements
We thank MINECO (CTQ-2013–49075-R, SEV-2013-0319, CTQ2014-52974-REDC, ENE2014-52280-REDT, CTQ2012-32247), the Feder funds and the EU COST actions CM1202 and CM1205. L. M. thanks “ICIQ-Foundation” for a Ph.D. grant. S. M. gratefully acknowledges “INSPIRE Faculty Award” by Government of India (No. DST/INSPIRE Faculty Award/2012/CH-72), and ISIRD research grant from IIT Kharagpur (project code: SMR). C. E. C.'s acknowledges the MICINN for a postdoctoral grant. The authors wish to thank (for financial support) the LABEX ARCANE (ANR-11-LABX-0003-01) for the project H2Photocat including the C. E. C.'s post-doctoral fellowship. This work was also supported by ICMG FR 2067 and COST CM1202 program (PERSPECT H2O). The Reseau National de RPE interdisciplinaire TGE CNRS is also thanked for EPR measurements and facilities.Notes and references
- S. Berardi, S. Drouet, L. Francas, C. Gimbert-Surinach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501–7519 RSC
.
- I. López, M. Z. Ertem, S. Maji, J. Benet-Buchholz, A. Keidel, U. Kuhlmann, P. Hildebrandt, C. J. Cramer, V. S. Batista and A. Llobet, Angew. Chem., Int. Ed., 2014, 53, 205–209 CrossRef PubMed
.
- M. L. Rigsby, S. Mandal, W. Nam, L. C. Spencer, A. Llobet and S. S. Stahl, Chem. Sci., 2012, 3, 3058–3062 RSC
.
- M. D. Kärkäs, O. Verho, E. V. Johnston and B. Åkermark, Chem. Rev., 2014, 114, 11863–12001 CrossRef PubMed
.
- J. T. Muckerman, M. Kowalczyk, Y. M. Badiei, D. E. Polyansky, J. J. Concepcion, R. Zong, R. P. Thummel and E. Fujita, Inorg. Chem., 2014, 53, 6904–6913 CrossRef CAS PubMed
.
- M. Wiechen, M. M. Najafpour, S. I. Allakhverdiev and L. Spiccia, Energy Environ. Sci., 2014, 7, 2203–2212 CAS
.
- M. V. Sheridan, B. D. Sherman, Z. Fang, K.-R. Wee, M. K. Coggins and T. J. Meyer, ACS Catal., 2015, 5, 4404–4409 CrossRef CAS
.
- L. Tong, R. Zong, R. Zhou, N. Kaveevivitchai, G. Zhang and R. P. Thummel, Faraday Discuss., 2015, 185, 87–104 RSC
.
- J. D. Blakemore, R. H. Crabtree and G. W. Brudvig, Chem. Rev., 2015, 115, 12974–13005 CrossRef CAS PubMed
.
- R. L. House, N. Y. M. Iha, R. L. Coppo, L. Alibabaei, B. D. Sherman, P. Kang, M. K. Brennaman, P. G. Hoertz and T. J. Meyer, J. Photochem. Photobiol., C, 2015, 25, 32–45 CrossRef CAS
.
- S. Karlsson, J. Boixel, Y. Pellegrin, E. Blart, H.-C. Becker, F. Odobel and L. Hammarstrom, Faraday Discuss., 2012, 155, 233–252 RSC
.
- L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet and L. Sun, Nat. Chem., 2012, 4, 418–423 CrossRef CAS PubMed
.
- S. Neudeck, S. Maji, I. López, S. Meyer, F. Meyer and A. Llobet, J. Am. Chem. Soc., 2013, 136, 24–27 CrossRef PubMed
.
- A. C. Sander, S. Maji, L. Francàs, T. Böhnisch, S. Dechert, A. Llobet and F. Meyer, ChemSusChem, 2015, 8, 1697–1702 CrossRef CAS PubMed
.
- P. Garrido-Barros, I. Funes-Ardoiz, S. Drouet, J. Benet-Buchholz, F. Maseras and A. Llobet, J. Am. Chem. Soc., 2015, 137, 6758–6761 CrossRef CAS PubMed
.
- E. A. Karlsson, B.-L. Lee, T. Åkermark, E. V. Johnston, M. D. Kärkäs, J. Sun, Ö. Hansson, J.-E. Bäckvall and B. Åkermark, Angew. Chem., Int. Ed., 2011, 50, 11715–11718 CrossRef CAS PubMed
.
- H. Yoon, Y.-M. Lee, X. Wu, K.-B. Cho, R. Sarangi, W. Nam and S. Fukuzumi, J. Am. Chem. Soc., 2013, 135, 9186–9194 CrossRef CAS PubMed
.
- E. Y. Tsui, R. Tran, J. Yano and T. Agapie, Nat. Chem., 2013, 5, 293–299 CrossRef PubMed
.
- K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831–1838 CrossRef CAS PubMed
.
- H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan and P. Strasser, ChemCatChem, 2010, 2, 724–761 CrossRef CAS
.
- C. Sens, I. Romero, M. Rodríguez, A. Llobet, T. Parella and J. Benet-Buchholz, J. Am. Chem. Soc., 2004, 126, 7798–7799 CrossRef CAS PubMed
.
- L. Mognon, J. Benet-Buchholz, S. M. W. Rahaman, C. Bo and A. Llobet, Inorg. Chem., 2014, 53, 12407–12415 CrossRef CAS PubMed
.
- S. Roeser, M. Z. Ertem, C. Cady, R. Lomoth, J. Benet-Buchholz, L. Hammarström, B. Sarkar, W. Kaim, C. J. Cramer and A. Llobet, Inorg. Chem., 2011, 51, 320–327 CrossRef PubMed
.
- S. Romain, J. Rich, C. Sens, T. Stoll, J. Benet-Buchholz, A. Llobet, M. Rodriguez, I. Romero, R. Clérac, C. Mathonière, C. Duboc, A. Deronzier and M. N. l. Collomb, Inorg. Chem., 2011, 50, 8427–8436 CrossRef CAS PubMed
.
- P. Pietrzyk, M. Srebro, M. Radoń, Z. Sojka and A. Michalak, J. Phys. Chem. A, 2011, 115, 2316–2324 CrossRef CAS PubMed
.
- S. Varma, C. E. Castillo, T. Stoll, J. Fortage, A. G. Blackman, F. Molton, A. Deronzier and M.-N. Collomb, Phys. Chem. Chem. Phys., 2013, 15, 17544–17552 RSC
.
- S. Romain, J.-C. Leprêtre, J. Chauvin, A. Deronzier and M.-N. Collomb, Inorg. Chem., 2007, 46, 2735–2743 CrossRef CAS PubMed
.
- M. Fabre and J. Bonvoisin, J. Am. Chem. Soc., 2007, 129, 1434–1444 CrossRef CAS PubMed
.
- F. Lloret, M. Julve, J. Cano, R. Ruiz-García and E. Pardo, Inorg. Chim. Acta, 2008, 361, 3432–3445 CrossRef CAS
.
- J. Limburg, J. S. Vrettos, H. Chen, J. C. de Paula, R. H. Crabtree and G. W. Brudvig, J. Am. Chem. Soc., 2001, 123, 423–430 CrossRef CAS PubMed
.
- J. Gilbert, L. Roecker and T. J. Meyer, Inorg. Chem., 1987, 26, 1126–1132 CrossRef CAS
.
- D. T. Sawyer, A. Sobkowiak and T. Matsushita, Acc. Chem. Res., 1996, 29, 409–416 CrossRef CAS
.
- W. N. Oloo, A. J. Fielding and L. Que, J. Am. Chem. Soc., 2013, 135, 6438–6441 CrossRef CAS PubMed
.
- J. P. Hage, A. Llobet and D. T. Sawyer, Bioorg. Med. Chem., 1995, 3, 1383–1388 CrossRef CAS PubMed
.
- J. Bernadou, A.-S. Fabiano, A. Robert and B. Meunier, J. Am. Chem. Soc., 1994, 116, 9375–9376 CrossRef CAS
.
- L.-P. Wang and T. Van Voorhis, J. Phys. Chem. Lett., 2011, 2, 2200–2204 CrossRef CAS
.
- X. Sala, M. Z. Ertem, L. Vigara, T. K. Todorova, W. Chen, R. C. Rocha, F. Aquilante, C. J. Cramer, L. Gagliardi and A. Llobet, Angew. Chem., Int. Ed., 2010, 49, 7745–7747 CrossRef CAS PubMed
.
- X. Sala, S. Maji, R. Bofill, J. García-Antón, L. Escriche and A. Llobet, Acc. Chem. Res., 2014, 47, 504–516 CrossRef CAS PubMed
.
- D. Geselowitz and T. J. Meyer, Inorg. Chem., 1990, 29, 3894–3896 CrossRef CAS
.
- C. W. Chronister, R. A. Binstead, J. Ni and T. J. Meyer, Inorg. Chem., 1997, 36, 3814–3815 CrossRef CAS
.
- R. A. Binstead, C. W. Chronister, J. Ni, C. M. Hartshorn and T. J. Meyer, J. Am. Chem. Soc., 2000, 122, 8464–8473 CrossRef CAS
.
- J. K. Hurst, J. Zhou and Y. Lei, Inorg. Chem., 1992, 31, 1010–1017 CrossRef CAS
.
- Y. Lei and J. K. Hurst, Inorg. Chem., 1994, 33, 4460–4467 CrossRef CAS
.
- Y. Lei and J. K. Hurst, Inorg. Chim. Acta, 1994, 226, 179–185 CrossRef CAS
.
- H. Yamada and J. K. Hurst, J. Am. Chem. Soc., 2000, 122, 5303–5311 CrossRef CAS
.
- H. Yamada, T. Koike and J. K. Hurst, J. Am. Chem. Soc., 2001, 123, 12775–12780 CrossRef CAS PubMed
.
- H. Yamada, W. F. Siems, T. Koike and J. K. Hurst, J. Am. Chem. Soc., 2004, 126, 9786–9795 CrossRef CAS PubMed
.
- S. Romain, F. Bozoglian, X. Sala and A. Llobet, J. Am. Chem. Soc., 2009, 131, 2768–2769 CrossRef CAS PubMed
.
- F. Bozoglian, S. Romain, M. Z. Ertem, T. K. Todorova, C. Sens, J. Mola, M. Rodríguez, I. Romero, J. Benet-Buchholz, X. Fontrodona, C. J. Cramer, L. Gagliardi and A. Llobet, J. Am. Chem. Soc., 2009, 131, 15176–15187 CrossRef CAS PubMed
.
- L. Vigara, M. Z. Ertem, N. Planas, F. Bozoglian, N. Leidel, H. Dau, M. Haumann, L. Gagliardi, C. J. Cramer and A. Llobet, Chem. Sci., 2012, 3, 2576–2586 RSC
.
- A. M. Angeles-Boza, M. Z. Ertem, R. Sarma, C. H. Ibanez, S. Maji, A. Llobet, C. J. Cramer and J. P. Roth, Chem. Sci., 2014, 5, 1141–1152 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental procedures, compound characterization data and experimental data. CCDC 1440794 and 1440795. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04672f |
| This journal is © The Royal Society of Chemistry 2016 |