Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
“Inverse” thermoresponse: heat-induced double-helix formation of an ethynylhelicene oligomer with tri(ethylene glycol) termini†
Nozomi
Saito
ab,
Higashi
Kobayashi
a and
Masahiko
Yamaguchi
*a
aDepartment of Organic Chemistry, Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, Japan. E-mail: yama@m.tohoku.ac.jp
bTohoku University Frontier Research Institute for Interdisciplinary Science, Sendai, Japan
First published on 12th February 2016
Abstract
An ethynylhelicene oligomer [(M)-D-4]-C12-TEG with six tri(ethylene glycol) (TEG) groups at the termini was synthesized, and double-helix formation was studied using CD, UV-Vis, vapor pressure osmometry, dynamic light scattering, and 1H NMR. [(M)-D-4]-C12-TEG reversibly changed its structure between a double helix and a random coil in response to heating and cooling in aromatic solvents, non-aromatic polar organic solvents, and aqueous solvent mixtures of acetone/water/triethylamine. Notably, [(M)-D-4]-C12-TEG in acetone/water/triethylamine (1/2/1) formed a double helix upon heating and disaggregated into random coils upon cooling. The double helix/random coil ratio sharply changed in response to temperature changes. This is an unprecedented “inverse” thermoresponse, which is opposite to the “ordinary” thermoresponse in molecular dimeric aggregate formation. This phenomenon was explained by the dehydration of the terminal TEG groups and the formation of condensed triethylamine domains upon heating.
Introduction
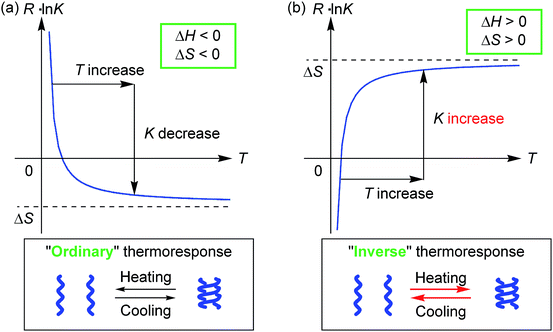
Molecules form dimeric aggregates in solution upon cooling and disaggregate upon heating,1–5 and such a thermoresponse is termed an “ordinary” thermoresponse in this study. DNA is a typical example of a biological molecule that exhibits the “ordinary” thermoresponse and forms double helices upon cooling and random coils upon heating.6 A dimeric molecular aggregation A + A → A2 is generally an exothermic process with a negative enthalpy change ΔH < 0, because A2 is a structure with less internal energy or enthalpy than 2A. Entropy also decreases in dimeric aggregation, which shows a negative entropy change ΔS < 0, because the freedom in molecular motion is decreased in A2 compared with 2A. The Gibbs free energy ΔG = ΔH − TΔS increases with an increase in temperature T, because of ΔH > 0. Consequently, the concentration of A2 decreases upon heating, and the equilibrium moves toward dissociation to give 2A according to the equations ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and R
K and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = −ΔH/T + ΔS (Fig. 1a), where K and R are the equilibrium constant and gas constant, respectively. The “ordinary” thermoresponse is widely observed for molecular dimeric aggregates.
K = −ΔH/T + ΔS (Fig. 1a), where K and R are the equilibrium constant and gas constant, respectively. The “ordinary” thermoresponse is widely observed for molecular dimeric aggregates.
 | ||
| Fig. 1 Graphical representation of the relationship between thermodynamic parameters and the (a) “ordinary” or (b) “inverse” thermoresponse. | ||
In contrast, molecules that exhibit the “inverse” thermoresponse in dimeric aggregation are also conceivable, for which heating induces aggregation and cooling induces disaggregation. Hypothetically, this phenomenon can occur in an endothermic process with a positive enthalpy change ΔH > 0, and the process is accompanied by an increase in freedom in molecular mobility with a positive entropy change ΔS > 0. Consequently, the equilibrium moves toward aggregation to form A2 upon heating according to the equations ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and R
K and R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = −ΔH/T + ΔS (Fig. 1b), because of −ΔH/T < 0. However, such a thermoresponse is counter-intuitive. The dimeric aggregation of synthetic molecules exhibiting the “inverse” thermoresponse, being a molecular-level phenomenon, has essentially not been observed.7
K = −ΔH/T + ΔS (Fig. 1b), because of −ΔH/T < 0. However, such a thermoresponse is counter-intuitive. The dimeric aggregation of synthetic molecules exhibiting the “inverse” thermoresponse, being a molecular-level phenomenon, has essentially not been observed.7
The “ordinary” and “inverse” thermoresponses are complementary, and the development of the latter will largely broaden the use of thermoresponsive materials. Here, we report the synthesis of [(M)-D-4]-C12-TEG, which is an ethynylhelicene tetramer with tri(ethylene glycol) (TEG) groups at its termini (Scheme 1), and it has a notable “inverse” thermoresponse: [(M)-D-4]-C12-TEG aggregated to form a double helix upon heating and disaggregated to give a random coil upon cooling in an aqueous solvent mixture of acetone/water/triethylamine. A sharp transition between the double helix and the random coil occurred due to temperature changes.
Results and discussion
Our previous studies showed that (M)-D-n(ref. 5) exhibited the “ordinary” thermoresponse. [(M)-D-4]-C12-TEG, with six TEG groups at both termini, was designed in this study to examine aggregation behavior in aqueous solvents. Compound 4 with a TEG moiety was obtained from benzoic acid 1 (ref. 8) in 2 steps, which was then connected to the termini of the ethynylhelicene tetramer (M)-D-4H5e by Sonogashira coupling (Scheme 1).[(M)-D-4]-C12-TEG showed the “ordinary” thermoresponse in organic solvents; it formed double helices upon cooling and disaggregated into random coils upon heating. Solutions of [(M)-D-4]-C12-TEG were heated at 60 °C or 40 °C for disaggregation then cooled, and their circular dichroism (CD) and UV-Vis spectra were obtained. The CD spectra in chloroform (5 × 10−4 M) at 40, 25, and 5 °C showed weak Cotton effects mirror-imaged to the typical random-coil state of (P)-ethynylhelicene tetramers5a (ESI Fig. S1a†). On the other hand, [(M)-D-4]-C12-TEG showed an intense CD as well as a hypochromic shift in the UV-Vis spectrum in trifluoromethylbenzene (1 × 10−3 M) upon cooling to 5 and −10 °C (ESI Fig. S1b†). The apparent molecular weight of the double helix obtained by vapor pressure osmometry (VPO) studies (trifluoromethylbenzene, 40 °C) above 1 × 10−3 M was twice as large as the calculated molecular weight of [(M)-D-4]-C12-TEG (3726.7) (ESI Fig. S2 and Table S2†). The results indicated the formation of double helices of [(M)-D-4]-C12-TEG in trifluoromethylbenzene. The reversible structural change between random coils and double helices was examined for Δε at 360 nm (trifluoromethylbenzene, 1 × 10−3 M) by repeating the cycle of heating to 60 °C and cooling to 5 °C (ESI Fig. S1c†). Δε360 increased upon heating and decreased upon cooling: the “ordinary” thermoresponse was observed in the aromatic solvent.
[(M)-D-4]-C12-TEG was soluble in non-aromatic polar solvents such as acetone and ethyl acetate even at a concentration of 1 × 10−3 M, at which conventional ethynylhelicene oligomers5 without TEG moieties were not soluble. The “ordinary” thermoresponse was observed in the polar solvents as well as in the aromatic solvent. A solution of [(M)-D-4]-C12-TEG in acetone (1 × 10−5 M) showed an increase in the CD intensity and a hypochromic shift in the UV-Vis spectrum upon cooling from 40 to −10 °C (Fig. 2), which indicated the formation of double helices upon cooling. Dimeric aggregate formation in acetone was confirmed by VPO studies (acetone, 45 °C, above 4 × 10−3 M) (ESI Fig. S3 and Table S3†). The CD analysis at a low concentration of 1 × 10−5 M (−10 °C) and at high concentrations of 5 × 10−4 M and 1 × 10−3 M (5 °C) converged on the same spectrum with the Δε of −1.1 × 103 cm−1 M−1 at a wavelength of 360 nm (ESI Fig. S4†). It indicated that the spectrum was that of the equilibrium-shifted state to double-helices containing practically no random coils in the solution, namely S-double-helix state. The average diameter determined by dynamic light scattering (DLS) in acetone (1 × 10−3 M) at 5 °C was 5.2 nm (ESI Fig. S5a†), which was consistent with the VPO result showing dimeric aggregate formation, not polymolecular aggregate formation. At a lower concentration (1 × 10−4 M), particles of 3.8 nm and 0.76 nm in diameter were observed at 5 °C, which corresponded to double helices and random coils, respectively (ESI Fig. S5b†). The increase of larger particles and the decrease of smaller particles upon cooling from 25 and 40 °C are consistent with the formation of bimolecular double helices from monomeric random coils. In ethyl acetate, the “ordinary” thermoresponse was also observed in the CD and UV-Vis spectra (ethyl acetate, 1.0 × 10−3 M) (ESI Fig. S6†).
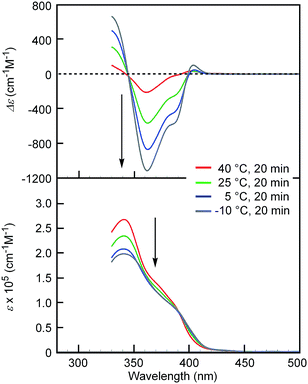 | ||
| Fig. 2 CD (top) and UV-Vis (bottom) spectra of [(M)-D-4]-C12-TEG in acetone (1 × 10−5 M) at different temperatures. Arrows show the changes upon cooling. | ||
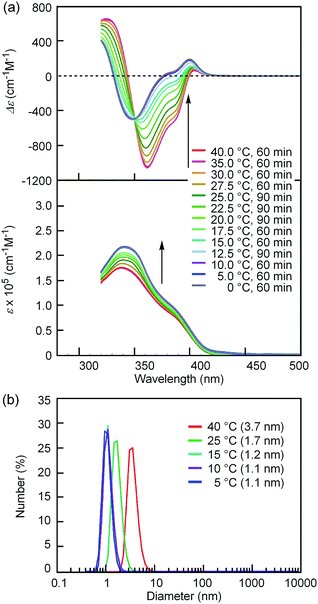
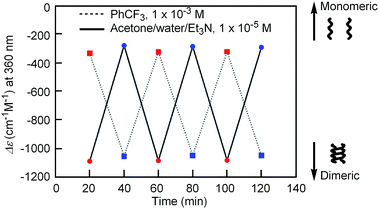
Notably, the “inverse” thermoresponse of [(M)-D-4]-C12-TEG was observed in aqueous solvents. In a mixed solvent of acetone/water/triethylamine (1/2/1, v/v/v), intense Cotton effects (1 × 10−5 M) were observed at 40 °C (Fig. 3a), which coincided with that for the S-double-helix state in acetone (ESI Fig. S7†). Upon cooling, UV-Vis absorption increased and CD intensity decreased, which indicated disaggregation (Fig. 3a). An isosbestic point at 350 nm indicated an equilibrium between two states, the double helix and the random coil. The average diameters obtained by DLS in acetone/water/triethylamine (1/2/1, 1 × 10−5 M) were 3.7 nm, 1.7 nm, and 1.1 nm at 40 °C, 25 °C, and 5 °C, respectively (Fig. 3b).‡The diameter at 40 °C coincided with that of the double helix in acetone (ESI Fig. S5a†), for which dimeric aggregate formation was confirmed by VPO (ESI Fig. S3†). The decrease in the diameter upon cooling is consistent with the disaggregation from a bimolecular double helix to a monomeric state. Thus, the “inverse” thermoresponse was observed for [(M)-D-4]-C12-TEG in acetone/water/triethylamine (1/2/1): the double helix was formed at 40 °C and the random coil at 5 °C. In addition, the “inverse” thermoresponse in this system was confirmed to be a molecular-level phenomenon in the dispersed state by DLS analysis; it was not caused by polymolecular aggregation. The spectra reversibly changed in response to changing temperature in a manner opposite to the “ordinary” thermoresponse (trifluoromethylbenzene, 1 × 10−3 M) (Fig. 4).
The “inverse” thermoresponse was also observed at different concentrations. Temperature-dependent changes in CD and UV-Vis spectra similar to those at 1 × 10−5 M were observed at 5 × 10−6 M and 1.5 × 10−5 M (Fig. S8a and b†). It is notable that dimeric aggregation occurred at these low concentrations. DLS analyses at these concentrations showed that the size of the aggregates were similar to those at 1 × 10−5 M (Fig. S9a and b†), and the “inverse” thermoresponse at the molecular level was confirmed. The “inverse” thermoresponse was observed at a higher concentration such as 3 × 10−5 M as well, although polymolecular aggregates partially formed below 20 °C, as indicated by CD and DLS (ESI Fig S8c and S9c†).
The CD spectra in acetone/water/triethylamine (1/2/1, 1 × 10−5 M) at 10 °C and 5 °C coincide, which show a convergence to a spectrum with a Δε of −3.1 × 102 cm−1 M−1 at 360 nm. The CD spectra at different concentrations, 5 × 10−6 M and 1.5 × 10−5 M, also converge to the same spectra at 5 °C (ESI Fig. S8a and b†). In the following discussions, the spectrum in acetone/water/triethylamine (1/2/1, 1 × 10−5 M, 5 °C) with a Δε of −3.1 × 102 cm−1 M−1 at 360 nm is defined as the Saq-random-coil state, which is the equilibrium-shifted state to random coils in aqueous media practically containing no double helices. It was noted that the CD spectra of the random-coil state in organic solvents (ESI Fig. S1a†) and the Saq-random-coil state in aqueous solvents (Fig. 3a) are slightly different, which may be due to a specific conformation of TEG moieties in aqueous media.9
The “inverse” thermoresponse also occurred when the amount of acetone was changed to 0.6/2/1 and 0.8/2/1, keeping the concentration of [(M)-D-4]-C12-TEG at 1 × 10−5 M (ESI Fig. S10†).
Note that [(M)-D-4]-C12-TEG provided an unprecedented example of dimeric aggregation with the “inverse” thermoresponse, which is in contrast to the other known synthetic double helices that exhibit the “ordinary” thermoresponse.4,5
An examination of the solvents revealed the critical roles of water and triethylamine. In a mixed solvent of acetone/triethylamine (3/1, 1 × 10−5 M), the “ordinary” thermoresponse was observed: CD and UV-Vis spectra of the random-coil state were obtained at 40 °C and 25 °C. The spectra changed at 5 °C, and those of partial double helices were obtained upon cooling to −10 °C (ESI Fig. S11a†). The presence of molecular-level aggregates but not polymolecular aggregates was confirmed by DLS, which showed average diameters from 0.84 to 0.88 nm at 25 °C (ESI Fig. S12†). In acetone/water (3/1, 1 × 10−5 M), the system was opaque, spectra of the random-coil state were obtained by CD at 40, 25, 5, and −10 °C (Fig. S11b),§ which may be due to the formation of polymolecular aggregates of random coils. The results indicated that both water and triethylamine are necessary for the “inverse” thermoresponse of [(M)-D-4]-C12-TEG.
Variable-temperature 1H NMR studies (acetone-d6/D2O/triethylamine-d15, 1/2/1, 2 × 10−4 M) were conducted to obtain insight into the thermoresponse of [(M)-D-4]-C12-TEG in aqueous solvents. Broad proton signals of the terminal TEG groups were observed between δ 2.4–3.5 at 25 °C (ESI Fig. S13†). When the temperature was increased to 40 °C, the signals of the TEG groups became sharper and increased in intensity. An upfield shift of the chemical shifts of HDO signals was also observed. These results are consistent with the reported hydration/dehydration of poly(ethylene glycol) (PEG) groups.10,11
Thermodynamic parameters of the double-helix formation with the “inverse” thermoresponse were experimentally determined using equilibrium constants K (Table S4†) obtained from the CD Δε values at 360 nm in acetone/water/triethylamine (1/2/1, 1 × 10−5 M), ΔH = +2.4 × 102 kJ mol−1 and ΔS = +9.2 × 102 J mol−1 K−1 (ESI Fig. S14†). Note that both ΔH and ΔS are positive and large. This contrasted with the “ordinary” thermoresponse of the dimeric aggregation of molecules giving negative ΔH and ΔS values,1,2b,3,4c,d including conventional ethynylhelicene oligomers in organic solvents.5b The positive ΔH and ΔS consequently induced an increase in the dimerization constant K upon heating according to the equation R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = −ΔH/T + ΔS, which appeared as the “inverse” thermoresponse (Fig. 1b). The result validated the hypothesized discussion in the introduction.
K = −ΔH/T + ΔS, which appeared as the “inverse” thermoresponse (Fig. 1b). The result validated the hypothesized discussion in the introduction.
A significant change in the double helix/random coil ratio was observed in response to small temperature changes (Table S4†). For example, the double helix/random coil ratios in acetone/water/triethylamine (1/2/1, 1 × 10−5 M) at 40 °C and 10 °C were estimated to be 90%/10% and 3%/97%, respectively. The large ΔH resulted in a substantial “inverse” thermoresponse.
The positive ΔH and ΔS of the “inverse” thermoresponse are counter intuitive in the dimeric molecular aggregation but can be explained by the hydration/dehydration of the PEG moiety. Dehydration upon heating makes the PEG moiety hydrophobic, which reduces the thermodynamic stability of random coils in aqueous solvents (ΔH > 0) and promotes dimeric aggregation. When the PEG moieties are dehydrated, ΔS increases and overcomes the decrease in ΔS resulting from dimeric aggregation. It is known that PEG and oligo(ethylene glycol) (OEG) are hydrated in water below the temperature defined as the lower critical solution temperature (LSCT), and that dehydration upon heating enhances hydrophobic interactions. Then, polymolecular aggregation and precipitation occur to reduce the molecular surface area exposed to water.11–14 Similar heat-induced aggregation15 and self-assembly16 related to the hydration/dehydration of biological peptide and protein molecules have also been reported.
It should be noted here that [(M)-D-4]-C12-TEG formed dimeric aggregates upon heating, not polymolecular aggregates, which is another unusual aspect of the “inverse” thermoresponse phenomenon in this system. Such dimeric aggregate formation of synthetic molecules has not been reported. The result reminds us of peptides and enzymes,15,17 which control their activities by forming dimeric aggregates upon heating and disaggregating to monomers upon cooling. In our system, triethylamine is considered to play a crucial role in the formation of dimeric aggregates of [(M)-D-4]-C12-TEG. Triethylamine and water are known to form hydrogen-bonds at low temperatures, and microscopic phase separation occurs upon heating.18 The resulting triethylamine domains incorporate dehydrated [(M)-D-4]-C12-TEG molecules (Fig. 5). Fewer polar environments made by organic solvents can promote the dimeric aggregate formation of ethynylhelicene oligomer moieties, which is enthalpically driven by π–π interactions, and does not induce polymolecular aggregation as a result of the hydrophobic interactions. It should be emphasized again that the “inverse” thermoresponse in dimeric aggregate formation shown in this study is a molecular-level phenomenon in the dispersed solution state and is different from the phenomenon in which polymolecular aggregates are formed by hydrophobic interactions above the LCST.
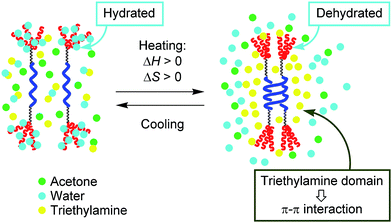 | ||
| Fig. 5 Schematic representation of the explanation for the “inverse” thermoresponse of [(M)-D-4]-C12-TEG in acetone/water/triethylamine (1/2/1). | ||
Materials and methods
Synthesis of [(M)-D-4]-C12-TEG
Under an argon atmosphere, a mixture of 4 (45.0 mg, 0.0451 mmol), tris(dibenzylideneacetone)dipalladium(0) chloroform adduct (0.82 mg, 0.753 μmol), cuprous iodide (1.72 mg, 9.03 μmol), tris(2,4,6-trimethylphenyl)phosphine (1.75 mg, 4.52 μmol), triphenylphosphine (1.18 mg, 4.52 μmol), tetrabutylammonium iodide (22.2 mg, 0.0602 mmol), triethylamine (0.1 mL) and N,N-dimethylformamide (1.0 mL) was freeze-evacuated four times in flask A. In flask B, a mixture of ethynylhelicene tetramer (M)-D-4H5e (30.0 mg, 0.0151 mmol) in THF (1.0 mL) was freeze-evacuated four times, and the mixture was slowly added to flask A. The mixture was stirred at 45 °C for 20 min. The reaction was quenched by adding saturated aqueous ammonium chloride, and the organic materials were extracted with ethyl acetate. The organic layer was washed with brine, and dried over sodium sulfate. The solvents were evaporated under reduced pressure, and separation by silica gel chromatography and recycling GPC gave [(M)-D-4]-C12-TEG as a yellow amber solid (42.7 mg, 0.0115 mmol, 72%). Mp: 59–61 °C (chloroform); [α]27D = −1631 (c 0.37, trifluoromethylbenzene); 1H NMR (400 MHz, CDCl3): δ 0.86 (9H, t, J = 6.8 Hz), 1.25–1.52 (74H, m), 1.72–1.89 (14H, m), 1.97 (12H, s), 2.00 (12H, s), 3.37 (18H, s), 3.52–3.55 (12H, m), 3.62–3.68 (24H, m), 3.70–3.75 (12H, m), 3.80 (4H, t, J = 5.2 Hz), 3.87 (8H, t, J = 5.0 Hz), 4.01 (4H, t, J = 6.6 Hz), 4.18–4.23 (12H, m), 4.28 (4H, t, J = 6.8 Hz), 4.41–4.45 (6H, m), 6.94 (4H, dt, J = 8.8, 1.8 Hz), 7.29 (4H, s), 7.46–7.52 (8H, m), 7.64 (4H, dt, J = 8.8, 1.8 Hz), 7.66–7.77 (8H, m), 8.06 (2H, s), 8.12 (2H, s), 8.16 (4H, s), 8.21 (2H, t, J = 1.6 Hz) 8.22 (1H, t, J = 1.6 Hz), 8.36–8.38 (6H, m), 8.52–8.58 (8H, m); 13C NMR (100 MHz, CDCl3): δ 14.1, 22.6, 23.2, 25.96, 26.02, 28.7, 29.2, 29.3, 29.4, 29.5, 29.6, 31.8, 58.97, 59.00, 65.2, 65.8, 68.1, 68.8, 69.6, 70.49, 70.52, 70.6, 70.8, 71.9, 72.4, 86.2, 89.2, 89.3, 89.4, 92.8, 92.97, 92.99, 95.1, 109.0, 114.6, 115.1, 119.6, 119.816, 119.818, 120.9, 123.5, 123.6, 123.7, 124.2, 124.28, 124.33, 125.3, 126.2, 126.7, 126.8, 127.0, 128.82, 128.84, 129.1, 129.2, 129.3, 129.8, 129.88, 129.92, 130.9, 131.01, 131.03, 131.2, 131.4, 132.0, 132.2, 132.4, 133.2, 136.76, 136.80, 136.9, 138.3, 142.5, 152.2, 159.4, 165.4, 166.1; IR (KBr): 2924, 1717, 1244, 1111 cm−1; UV-Vis (S-random-coil state: CHCl3, 5 × 10−4 M, 40 °C): λmax (ε) 344 nm (3.1 × 105 cm−1 M−1); UV-Vis (S-double-helix state: acetone, 1 × 10−3 M, 5 °C): λmax (ε) 340 nm (2.0 × 105 cm−1 M−1); CD (S-random-coil state: CHCl3, 5 × 10−4 M, 40 °C): λ (Δε) 296 nm (+51 cm−1 M−1), 341 nm (−76 cm−1 M−1), 389 nm (+189 cm−1 M−1); CD (S-double-helix state: acetone, 1 × 10−3 M, 5 °C): λ (Δε) 325 nm (+679 cm−1 M−1), 362 nm (−1133 cm−1 M−1); MALDI-TOF MS (m/z): [M + Na]+ calcd for C239H278O36Na, 3747.0; found, 3746.3; [M + K]+ calcd for C239H278O36K, 3763.0; found, 3763.0; analysis (calcd, found for C239H278O36): C (77.03, 76.90), H (7.52, 7.54).Conclusions
In summary, [(M)-D-4]-C12-TEG, an ethynylhelicene oligomer with six tri(ethylene glycol) moieties at its termini, was synthesized. [(M)-D-4]-C12-TEG formed double helices in aromatic solvents, polar non-aromatic solvents, and the aqueous solution of acetone/water/triethylamine. [(M)-D-4]-C12-TEG exhibited the “inverse” thermoresponse in acetone/water/triethylamine (1/2/1): [(M)-D-4]-C12-TEG aggregated and formed double helices upon heating and disaggregated to random coils upon cooling. The double helix/random coil ratio sharply and reversibly changed in response to thermal stimuli. This is an unprecedented molecular-level “inverse” thermoresponse, in which dimeric aggregates but not polymolecular aggregates are formed. Positive and large ΔH and ΔS values in the aggregation process were determined, which were explained by the dehydration of terminal TEG groups upon heating and the formation of triethylamine domains that promoted double-helix formation by π–π interactions.Note added after first publication
This article replaces the version published on 22nd February 2016, which contained errors in the grant numbers reported in the acknowledgements section.Acknowledgements
This work was supported by JSPS KAKENHI Grants (No. 25860003 and 15K18825). We also thank the Uehara Foundation and the Tohoku University Frontier Research Institute for Interdisciplinary Sciences (FRIS) for financial support.Notes and references
- Examples of macrocyclic compounds: (a) N. Saito, R. Terakawa and M. Yamaguchi, Chem.–Eur. J., 2014, 20, 5601–5607 CrossRef CAS PubMed; (b) H. Enozawa, M. Hasegawa, D. Takamatsu, K. Fukui and M. Iyoda, Org. Lett., 2006, 8, 1917–1920 CrossRef CAS PubMed; (c) Y. Tobe, N. Utsumi, K. Kawabata, A. Nagano, K. Adachi, S. Araki, M. Sonoda, K. Hirose and K. Naemura, J. Am. Chem. Soc., 2002, 124, 5350–5364 CrossRef CAS PubMed; (d) A. S. Shetty, J. Zhang and J. S. Moore, J. Am. Chem. Soc., 1996, 118, 1019–1027 CrossRef CAS; (e) D. J. Cram, H.-J. Choi, J. A. Bryant and C. B. Knobler, J. Am. Chem. Soc., 1992, 114, 7748–7765 CrossRef CAS.
- Examples of hydrogen-bonding compounds: (a) J. Catalán, J. Phys. Chem. A, 2010, 114, 811–816 CrossRef PubMed; (b) N. A. Prokopenko, I. A. Bethea, C. J. Clemens 4th, A. Klimek, K. Wargo, C. Spivey, K. Waziri and A. Grushow, Phys. Chem. Chem. Phys., 2002, 4, 490–495 RSC.
- Examples of thermoresponsive dimer formation by other compounds: (a) H. Sun, K. Ye, C. Wang, H. Qi, F. Li and Y. Wang, J. Phys. Chem. A, 2006, 110, 10750–10756 CrossRef CAS PubMed; (b) H. Grajek, G. Z. Zurkowaska, R. Drabent and C. Bojarski, Asian J. Spectrosc., 2001, 2, 49–55 Search PubMed; (c) B. R. Peterson, P. Wallimann, D. R. Carcanague and F. Diederich, Tetrahedron, 1995, 51, 401–421 CrossRef CAS.
- Examples of thermoresponsive double helix or helix-dimer formation: (a) H. Yamada, Z.-Q. Wu, Y. Furusho and E. Yashima, J. Am. Chem. Soc., 2012, 134, 9506–9520 CrossRef CAS PubMed; (b) B. Baptiste, J. Zhu, D. Haldar, B. Kauffmann, J.-M. Léger and I. Huc, Chem.–Asian J., 2010, 5, 1364–1375 CAS; (c) H. Goto, Y. Furusho, K. Miwa and E. Yashima, J. Am. Chem. Soc., 2009, 131, 4710–4719 CrossRef CAS PubMed; (d) J. Li, J. A. Wisner and M. C. Jennings, Org. Lett., 2007, 9, 3267–3269 CrossRef CAS PubMed; (e) V. Berl, I. Huc, R. G. Khoury, M. J. Krische and J.-M. Lehn, Nature, 2000, 407, 720–723 CrossRef CAS PubMed.
- (a) N. Saito, R. Terakawa, M. Shigeno, R. Amemiya and M. Yamaguchi, J. Org. Chem., 2011, 76, 4841–4858 CrossRef CAS PubMed; (b) H. Sugiura, R. Amemiya and M. Yamaguchi, Chem.–Asian J., 2008, 3, 244–260 CrossRef CAS PubMed; (c) R. Amemiya, N. Saito and M. Yamaguchi, J. Org. Chem., 2008, 73, 7137–7144 CrossRef CAS PubMed; (d) H. Sugiura and M. Yamaguchi, Chem. Lett., 2007, 36, 58–59 CrossRef CAS; (e) H. Sugiura, Y. Nigorikawa, Y. Saiki, K. Nakamura and M. Yamaguchi, J. Am. Chem. Soc., 2004, 126, 14858–14864 CrossRef CAS PubMed.
- (a) C. R. Cantor and P. R. Schmmel, Biophysical Chemistry Part III: The Behavior of Biological Macromolecules, W. H. Freeman and Co., San Francisco, 1980 Search PubMed; (b) G. D. Stormo, Introduction to Protein-DNA Interactions: Structure, Thermodynamics, and Bioinformations, Cold Spring Harbor Laboratory Press, New York, 2013, Chapter 2: The structure of DNA, pp. 13−26 Search PubMed.
- The “inverse” thermoresponse of a synthetic molecule was suggested by the shift of 1H NMR signals, but the ratio of monomer and dimer was not clarified: A. Brück and K. V. Kilway, Tetrahedron, 2001, 57, 7263–7268 CrossRef . In ref. 3a, only one molecule gave a positive ΔH in a very limited temperature range.
- C. Münzenberg, A. Rossi, K. Feldman, R. Fiolka, A. Stermmer, K. Kita-Tokarczyk, W. Meier, J. Sakamoto, O. Lukin and A. D. Schülter, Chem.–Eur. J., 2008, 14, 10797–10807 CrossRef PubMed.
- (a) T. Muraoka, K. Adachi, M. Ui, S. Kawasaki, N. Sadhukhan, H. Obara, H. Tochio, M. Shirakawa and K. Kinbara, Angew. Chem., Int. Ed., 2013, 52, 2430–2434 CrossRef CAS PubMed; (b) J. J. Shephard, P. J. Bremer and A. J. McQuillan, J. Phys. Chem. B, 2009, 113, 14229–14237 CrossRef CAS PubMed; (c) R. Begum and H. Matsuura, J. Chem. Soc., Faraday Trans., 1997, 93, 3839–3848 RSC; (d) K. Tasaki, J. Am. Chem. Soc., 1996, 118, 8459–8469 CrossRef CAS.
- The number of hydration water molecules per ethylene oxide group has been argued. Examples: (a) T. Shikata, M. Okuzono and N. Sugimoto, Macromolecules, 2013, 46, 1956–1961 CrossRef CAS; (b) T. Shikata, R. Takahashi and A. Sakamoto, J. Phys. Chem. B, 2006, 110, 8941–8945 CrossRef CAS PubMed; (c) S. Lüsse and K. Arnold, Macromolecules, 1996, 29, 4251–4257 CrossRef.
- Examples of temperature-dependent change of 1H NMR spectra of compounds with PEG or OEG moieties: (a) C. Zhang, H. Peng and A. K. Whittaker, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2375–2385 CrossRef CAS; (b) J. Y. Choi, J. Y. Kim, H. J. Moon, M. H. Park and B. Jeong, Macromol. Rapid Commun., 2014, 35, 66–70 CrossRef CAS PubMed; (c) G. Hu, W. Li, Y. Hu, A. Xu, J. Yan, L. Liu, X. Zhang, K. Liu and A. Zhang, Macromolecules, 2013, 46, 1124–1132 CrossRef CAS; (d) S. Sun and P. Wu, Macromolecules, 2013, 46, 236–246 CrossRef CAS; (e) X. Jiang, M. R. Smith III and G. L. Baker, Macromolecules, 2008, 41, 318–324 CrossRef CAS.
- Examples of polymers with PEG or OEG moieties which exhibit LCST behavior: (a) Y. Liao and C.-M. Dong, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1834–1843 CrossRef CAS; (b) J. Roeser, F. Moingeon, B. Heinrich, P. Masson, F. Arnaud-Neu, M. Rawiso and S. Méry, Macromolecules, 2011, 44, 8925–8935 CrossRef CAS; (c) P. Dimitrov, S. Rangelov, A. Dworak and C. B. Tsvetanov, Macromolecules, 2004, 37, 1000–1008 CrossRef CAS.
- Examples of small molecules with PEG or OEG moieties which exhibit LCST behavior: (a) X. Chi and M. Xue, Chem. Commun., 2014, 50, 13754–13756 RSC; (b) P. Rajdev, M. R. Molla and S. Ghosh, Langmuir, 2014, 30, 1969–1976 CrossRef CAS PubMed; (c) T. Ogoshi, K. Kida and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (d) T. Ogoshi, R. Shiga and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 4577–4580 CrossRef CAS PubMed; (e) F. Wang, A. Klaikherd and S. Thayumanavan, J. Am. Chem. Soc., 2011, 133, 13496–13503 CrossRef CAS PubMed.
- Examples of small molecules without PEG or OEG moieties which exhibit LCST behavior: (a) S. Dong, B. Zheng, Y. Yao, C. Han, J. Yuan, M. Antonietti and F. Huang, Adv. Mater., 2013, 25, 6864–6867 CrossRef CAS PubMed; (b) M. Noh, Y. Mok, S. Lee, H. Kim, S. H. Lee, G. Jin, J.-H. Seo, H. Koo, T. H. Park and Y. Lee, Chem. Commun., 2012, 48, 3845–3847 RSC; (c) J. E. Betancourt and J. M. Rivera, J. Am. Chem. Soc., 2009, 131, 16666–16668 CrossRef CAS PubMed; (d) K. Fukumoto and H. Ohno, Angew. Chem., Int. Ed., 2007, 46, 1852–1855 CrossRef CAS PubMed.
- Examples of heat-induced dimer formation of peptides and proteins related to hydration/dehydration: (a) K. Ruan and G. Weber, Biochemistry, 1988, 27, 3295–3301 CrossRef CAS PubMed; (b) P. Doty and G. E. Myers, J. Chem. Soc., Faraday Trans., 1953, 13, 51–58 Search PubMed.
- Examples of heat-induced self-assembly of proteins related to hydration/dehydration: (a) M. A. Lauffer, A. T. Ansevin, T. E. Cartwright and C. C. Brinton, Nature, 1958, 181, 1338–1339 CrossRef CAS PubMed; (b) S. Asakura, M. Kasai and F. Oosawa, J. Polym. Sci., 1960, 44, 35–49 CrossRef CAS; (c) J. C. Lee and S. N. Timasheff, Biochemistry, 1977, 16, 1754–1764 CrossRef CAS PubMed; (d) K. E. Kadler, Y. Hojima and D. J. Prockop, J. Biol. Chem, 1987, 260, 15696–15701 Search PubMed.
- Heat-induced dimer formation of enzymes: (a) H. Isohashi, Y. Nakanishi and Y. Sakamoto, Eur. J. Biochem., 1983, 134, 447–452 CrossRef PubMed; (b) S. Smith and S. Abraham, J. Biol. Chem, 1971, 246, 6428–6435 CAS; (c) H. N. Kirkman and E. M. Hendrickson, J. Biol. Chem, 1962, 237, 2371–2376 CAS.
- (a) S. Kajimoto, N. Yoshii, J. Hobley, H. Fukumura and S. Okazaki, Chem. Phys. Lett., 2007, 448, 70–74 CrossRef CAS; (b) A. Ikehara, C. Hashimoto, Y. Mikami and Y. Ozaki, Chem. Phys. Lett., 2004, 393, 403–408 CrossRef; (c) J. Hobley, S. Kajimoto, A. Takamizawa, K. Ohta, Q. Tran-Cong and H. Fukumura, J. Phys. Chem. B, 2003, 107, 11411–11418 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04959h |
| ‡ The solubility of [(M)-D-4]-C12-TEG in the aqueous media was poor, and the apparent molecular weight was not obtained by a VPO measurement. |
| § Acetone/water (3/1, 1 × 10−5 M) systems were opaque, and the formation of polymolecular assemblies were suggested. Studies of the assemblies are ongoing. |
| This journal is © The Royal Society of Chemistry 2016 |