Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
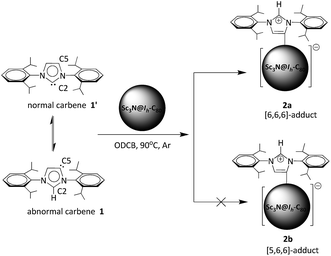
Sc3N@Ih-C80 as a novel Lewis acid to trap abnormal N-heterocyclic carbenes: the unprecedented formation of a singly bonded [6,6,6]-adduct†
Muqing
Chen‡
ab,
Lipiao
Bao‡
a,
Min
Ai
a,
Wangqiang
Shen
a and
Xing
Lu
*a
aState Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, 430074 China. E-mail: lux@hust.edu.cn
bSchool of Physics and Mechanical & Electronical Engineering, Hubei University of Education, Wuhan 430205, China
First published on 2nd December 2015
Abstract
The reaction between an N-heterocyclic carbene (NHC), namely 1,3-bis(diisopropylphenyl)-imidazol-2-ylene (1), and Sc3N@Ih-C80 successfully affords a Lewis acid–base pair (2a). Single crystal X-ray crystallographic results unambiguously reveal the unexpected structure of 2a where the abnormal carbene center of the NHC is connected to a triple-hexagon-junction (THJ) carbon atom of Sc3N@Ih-C80via a single bond. Theoretical calculations reveal that selective entrapment of the abnormal carbene 1 is caused by the steric hindrance between the normal NHC moiety and the fullerene cage, which precludes the formation of normal carbene adducts. Furthermore, the analysis of the electronic density distribution on the cage of Sc3N@Ih-C80 indicates that THJ carbons bear relatively low negative charge densities and, accordingly, are easily attacked by the electron-rich NHC 1 to form the singly bonded [6,6,6]-adduct 2a instead of the corresponding [5,6,6]-adduct 2b. It is thus confirmed that the regioselective formation of 2a is a synergistic effect of both cage size and electron density distribution. Sc3N@Ih-C80, although with a highly charged cage, is proven to show excellent Lewis acidity, opening a wide avenue toward carbon-based Lewis acids taking into account the diversity of endohedral metallofullerenes.
Introduction
“Frustrated Lewis pairs” are promising metal-free catalysts to activate small molecules such as H2, CO2, alkenes and alkynes.1–3 Carbon-based Lewis bases are naturally diverse, such as ylides, isonitriles, enamines and N-heterocyclic carbenes (NHCs).4 Among them, NHCs as stable carbene compounds featuring a neutral divalent carbon atom with two non-bonding electrons are considered as prototypical reactive intermediates and have attracted intensive interest.5 Usually, NHCs use the normal carbene center (e.g. C2 of 1′ in Scheme 1) to form η1 complexes.6 However, recent experimental and theoretical results show that abnormal carbenes with C5 as the active center (e.g.1 in Scheme 1) have a stronger electron-donating ability and, accordingly, their complexes show better catalytic properties than the normal ones.7,8 As a direct result, great efforts have been devoted to the exploration of abnormal carbene compounds.9In contrast, carbon-based Lewis acids are limited merely to trityl cations and some electron-poor allenes.4 Recently, Bazan and coworkers reported that fullerenes such as C60 and C70 can behave as all-carbon Lewis acids to form the corresponding Lewis acid–base pairs with a normal NHC structure (1′).10 This work opens a new perspective on the research of carbon-based Lewis acids. Meanwhile, as a novel class of metal–carbon hybrid molecules, endohedral metallofullerenes (EMFs) feature electron transfer from the internal metallic species to the carbon cage, forming zwitterionic compounds.11 Accordingly, it is of special interest to study whether the highly charged carbon cages of EMFs are willing to accept additional electrons to act as Lewis acids or not.
Herein, taking Sc3N@Ih-C80 as a representative, we show that EMFs also exhibit excellent Lewis acidity to form Lewis acid–base pairs with NHCs. Surprisingly, our unambiguous X-ray results reveal that the abnormal NHC 1 is bonded to Sc3N@Ih-C80, instead of the normal one 1′ (Scheme 1). More interestingly, a singly bonded [6,6,6]-adduct (2a) of Sc3N@Ih-C80 is formed during the reaction, which has never been observed or even predicted, in contrast to the commonly observed [5,6,6]-adduct (2b). Our theoretical results reveal that the regioselective formation of the unprecedented [6,6,6]-adduct with an abnormal carbene moiety (2a) is synergistically affected by the cage size of Sc3N@Ih-C80 and the electronic density distribution on the cage.
Results and discussion
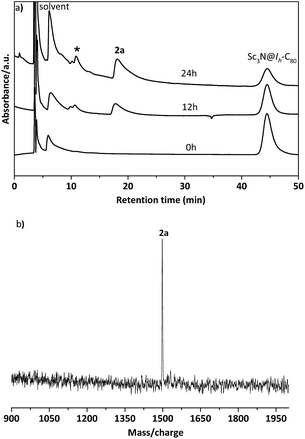
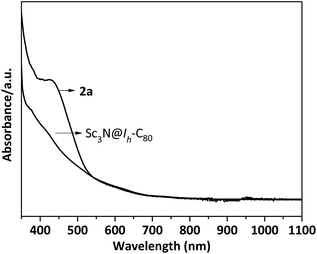
In a typical reaction, an ortho-dichlorobenzene (ODCB) solution of Sc3N@Ih-C80 and an excess amount (ca. 50-fold) of 1,3-bis(diisopropylphenyl)-imidazol-2-ylene (1) was heated to 90 °C under an argon atmosphere (Scheme 1). The reaction progress was monitored via high performance liquid chromatography (HPLC). After the solution was heated for 12 hours, a new peak appeared at 18.3 min, which is ascribed to the adduct 2a as identified using mass spectrometry (Fig. 1). The reaction was terminated after 24 hours, and 2a was isolated with preparative HPLC in ∼80% conversion yield based on consumed Sc3N@Ih-C80. The matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum of 2a displays a single peak at m/z 1498.2, firmly confirming the successful attachment of the NHC moiety onto the fullerene cage (Fig. 1b).The electronic configuration of 2a was investigated using UV-Vis-NIR spectroscopy in toluene (Fig. 2). Although the spectrum of 2a resembles that of pristine Sc3N@Ih-C80 in the wavelength range between 540 nm and 1100 nm, their curves at 350–540 nm differ significantly from one another, confirming that the electronic structure of Sc3N@Ih-C80 has been altered by the modification.
The structure of 2a is unequivocally established via single-crystal X-ray crystallography. The entire system is fully ordered, including the functionalized cage, the internal cluster and even the three CS2 solvent molecules.12 It is evident that a single bond is formed between the addend and the cage with a bond length of 1.515 Å (C5–C6), confirming unambiguously the formation of a Lewis acid–base complex (Fig. 3a).13,14 More surprisingly, the addition site on the cage of Sc3N@Ih-C80 involves a triple hexagon junction (THJ) which is generally less reactive than the carbon atoms of other kinds on a fullerene cage. Such an addition pattern has never been observed or even expected for Sc3N@Ih-C80 because previously reported singly bonded derivatives contained at least two substituents that are exclusively linked to the pentagon–hexagon–hexagon junction (PHHJ) carbon atoms unless the cage is severely functionalized.15–17 Because of the substitution, the carbon atom at the site of addition (C6) is slightly pulled out from the cage sphere which causes a ‘Y-shaped’ displacement of the internal Sc3N cluster with the Sc3–N1 bond nearly collinear with the new bond C5–C6, whereas the Sc3N-plane is perpendicular to the N-heterocyclic ring of the addend (Fig. 3b).
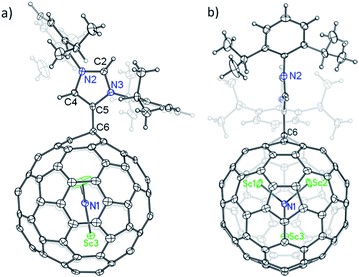 | ||
| Fig. 3 Single-crystal X-ray structure of 2a: (a) side view, and (b) front view. Thermal ellipsoids are shown at the 50% probability level. Solvent molecules are omitted for clarity. | ||
Surprisingly, the NHC moiety is linked to the cage of Sc3N@Ih-C80 with its abnormal carbene center C5 instead of the normal site C2, which is completely different from the corresponding Lewis pairs of C60 and C70.10 The bond length of C4–C5 (1.367 Å) falls into the range of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, confirming the existence of imidazol-2-ylene. The lengths of the other four C–N bonds forming the N-heterocyclic ring are similar: 1.384 Å (C4–N2), 1.326 Å (C2–N2), 1.333 Å (C2–N3) and 1.386 Å (C5–N3), excluding the existence of C
C double bond, confirming the existence of imidazol-2-ylene. The lengths of the other four C–N bonds forming the N-heterocyclic ring are similar: 1.384 Å (C4–N2), 1.326 Å (C2–N2), 1.333 Å (C2–N3) and 1.386 Å (C5–N3), excluding the existence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds.
N double bonds.
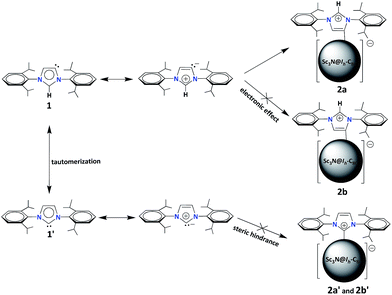
The unprecedented structure of 2a with an abnormal NHC moiety singly bonded to a THJ carbon atom is of great interest. First, we try to understand why a [6,6,6]-adduct (2a) is formed instead of the corresponding [5,6,6]-adduct (2b). It is well-known that THJ carbons are less pyramidal and accordingly are less reactive than the carbon atoms of other kinds of fullerenes.18 Indeed, our theoretical results suggest that the [5,6,6]-adduct 2b, if formed, is 0.49 kcal mol−1 more stable than the [6,6,6]-adduct 2a (Fig. 4a), indicating that the preferential formation of 2a is not a thermodynamically controlled process. We then consider that the electron density distribution on the cage of Sc3N@Ih-C80 should be a critical factor. It is widely accepted that pentagons are the sites of the negative charges of highly charged fulleride species such as EMFs.19 Accordingly, [5,6,6]-junction carbon atoms always accumulate more negative charges than THJ carbons do. As a direct result, NHCs as electron-rich Lewis bases tend to attack the [6,6,6]-junction carbon atoms which have lower electron densities, revealing the Lewis acidic property of Sc3N@Ih-C80. In summary, the [6,6,6]-addition pattern of 2a is a consequence of the electron density distribution on the cage of Sc3N@Ih-C80.
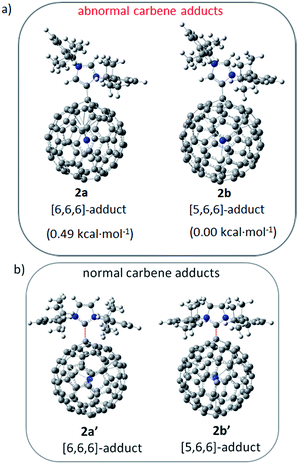 | ||
| Fig. 4 Possible structures of NHC–Sc3N@Ih-C80 complexes and their relative energies calculated at the B3LYP/6-31G*/LANL2DZ (Sc) level. | ||
We then try to find a reasonable explanation for the unexpected formation of the abnormal carbene structure of 2a. Usually NHCs use the normal carbene center (C2 in 1′, Scheme 1) to bind metals. However, abnormal NHC carbene species (1, Scheme 1) can stably exist and can even be isolated.20 Several examples of abnormal carbene complexes have been reported, which show enhanced catalytic properties for the activation of unreactive bonds.7,8 Furthermore, Dagorne and coworkers reported that a normal but sterically congested NHC–AlMe3 Lewis acid–base pair can isomerize to its abnormal NHC–AlMe3 species.20 This result inspires us to speculate that the abnormal carbene structure of 2a is also caused by a steric effect. Our computational results showed that neither the normal [6,6,6]-adduct (2a′) nor the normal [5,6,6]-adduct (2b′) can exist as stable compounds: during the optimization processes the single bond connecting the normal NHC moiety and Sc3N@Ih-C80 is broken (Fig. 4b), which can be attributed to the steric hindrance between the congested diisopropylphenyl groups of the normal NHC (1′) and the large cage of Sc3N@Ih-C80.
Finally, we propose a plausible mechanism to rationalize the unexpected formation of 2a (Scheme 2). According to the literature, the normal carbene 1′, where C2 is the carbene center, can tautomerize into the abnormal one 1 with C5 as the active site.21 Then, the tautomers (1 and 1′) turn into the corresponding mesoionic compounds.9 Since the mesoionic species of 1′ can not form stable adducts (2a′and 2b′) with Sc3N@Ih-C80 because of the high steric hindrance between the addend and the cage, only the abnormal carbene structure is possible. As discussed above, the electron-rich NHC 1 (or the corresponding mesoionic compound) tends to attack one of the THJ carbon atoms with low electron densities, forming the [6,6,6]-adduct 2a in a highly regioselective manner.
Conclusions
In summary, Sc3N@Ih-C80 is confirmed to be an excellent carbon-based Lewis acid although its cage is negatively charged, representing the first example of EMFs that readily undergo Lewis acid–base complexation reactions with NHCs. The regioselective formation of the unusual singly bonded [6,6,6]-adduct 2a is reasonably interpreted by analyzing the charge density distribution on the cage because the electron-rich NHC is prone to attack one of the THJ carbons with low electron densities. More interestingly, Sc3N@Ih-C80 here is found to selectively trap the rare abnormal NHC 1 as a consequence of the steric hindrance between the normal NHC moiety and Sc3N@Ih-C80. Hence, we conclude that the regioselective and unprecedented formation of 2a is a synergistic effect of both the cage size and electron density distribution of Sc3N@Ih-C80. This synthetic strategy can be easily extended to create various EMF-based Lewis acid–base pairs with different metallic cores and/or cage structures, which may show unique catalytic properties in organic synthesis, taking into account their “frustrated” characteristics.Acknowledgements
Professors M. M. Olmstead and A. L. Balch in UC Davis are gratefully acknowledged for their assistance in single-crystal X-ray measurement. Financial support from the National Thousand Talents Program of China, NSFC (21171061, 51472095), Program for Changjiang Scholars and Innovative Research Team in University (IRT1014) and Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education are gratefully acknowledged. We thank the Analytical and Testing Center in Huazhong University of Science and Technology for all related measurements.Notes and references
- D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 46 CrossRef CAS PubMed.
- M. Légaré, M. Courtemanche, É. Rochette and F. Fontaine, Science, 2015, 349, 513 CrossRef PubMed.
- S. K. Bose and T. B. Marder, Science, 2015, 349, 473 CrossRef CAS PubMed.
- J. Iglesias-Siguenza and M. Alcarazo, Angew. Chem., Int. Ed., 2012, 51, 1523 CrossRef CAS PubMed.
- F. E. Ahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122 CrossRef PubMed.
- S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef PubMed.
- H. Lebel, M. K. Janes, A. B. Charette and S. P. Nolan, J. Am. Chem. Soc., 2004, 126, 5046 CrossRef CAS PubMed.
- M. Heckenroth, E. Kluser, A. Neels and M. Albrecht, Angew. Chem., Int. Ed., 2007, 46, 6293 CrossRef CAS PubMed.
- E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Science, 2009, 326, 556 CrossRef CAS PubMed.
- H. P. Li, C. Risko, J. H. Seo, C. Campbell, G. Wu, J. Bredas and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 12410 CrossRef CAS PubMed.
- X. Lu, L. Echegoyen, A. L. Balch, S. Nagase and T. Akasaka, Endohedral Metallofullerenes: Basics and Applications, CRC Press, New York, 2015 Search PubMed.
- Crystal data of 2a. Black block, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.9903(19) Å, b = 17.558(3) Å, c = 19.758(4) Å, α = 99.562(3)°, β = 90.938(3)°, γ = 107.217(3)°, V = 3376.2(10) Å3, Z = 1, Dcalc = 1.661 Mg m−3, μ = 0.514 mm−1, T = 90(2) K, 2θmax = 50.852°; 12355 reflections, 7675 with I > 2σ(I); R1 = 0.0784 [I > 2σ(I)], wR2 = 0.2401 (all data). The maximum residual electron density is 1.255 eÅ−3.
, a = 10.9903(19) Å, b = 17.558(3) Å, c = 19.758(4) Å, α = 99.562(3)°, β = 90.938(3)°, γ = 107.217(3)°, V = 3376.2(10) Å3, Z = 1, Dcalc = 1.661 Mg m−3, μ = 0.514 mm−1, T = 90(2) K, 2θmax = 50.852°; 12355 reflections, 7675 with I > 2σ(I); R1 = 0.0784 [I > 2σ(I)], wR2 = 0.2401 (all data). The maximum residual electron density is 1.255 eÅ−3. - C. Shu, W. Xu, C. Slebodnick, H. Champion, W. Fu, J. E. Reid, H. Azurmendi, C. Wang, K. Harich, H. C. Dorn and H. W. Gibson, Org. Lett., 2009, 11, 1753 CrossRef CAS PubMed.
- M. Izquierdo, M. R. Cerón, M. M. Olmstead, A. L. Balch and L. Echegoyen, Angew. Chem., Int. Ed., 2013, 125, 12042 CrossRef.
- N. B. Shustova, A. A. Popov, M. A. Mackey, C. E. Coumbe, J. P. Phillips, S. Stevenson, S. H. Strauss and O. V. Boltalina, J. Am. Chem. Soc., 2007, 129, 11676 CrossRef CAS PubMed.
- C. Shu, C. Slebodnick, L. Xu, H. Champion, T. Fuhrer, T. Cai, J. E. Reid, W. Fu, K. Harich, H. C. Dorn and H. W. Gibson, J. Am. Chem. Soc., 2008, 130, 17755 CrossRef CAS PubMed.
- N. B. Shustova, Y. S. Chen, M. A. Mackey, C. E. Coumbe, J. P. Phillips, S. Stevenson, A. A. Popov, O. V. Boltalina and S. H. Strauss, J. Am. Chem. Soc., 2009, 131, 17630 CrossRef CAS PubMed.
- Y. Z. Tan, S. Y. Xie, R. B. Huang and L. S. Zheng, Nat. Chem., 2009, 1, 450 CrossRef CAS PubMed.
- A. Rodríguez-Fortea, N. Alegret, A. L. Balch and J. M. Poblet, Nat. Chem., 2010, 2, 955 CrossRef PubMed.
- A. Schmitt, G. Schnee, R. Welter and S. Dagorne, Chem. Commun., 2010, 46, 2480 RSC.
- M. Albrecht, Chem. Commun., 2008, 3601 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1406974. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04070a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |